Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents
Abstract
1. Introduction
2. Results
2.1. Transgenerational Impact of Arecoline on the Timing of Lifespan and Development of C. elegans
2.2. Effect of Arecoline on Fertility of C. elegans
2.3. Neurotoxicity of Arecoline on C. elegans
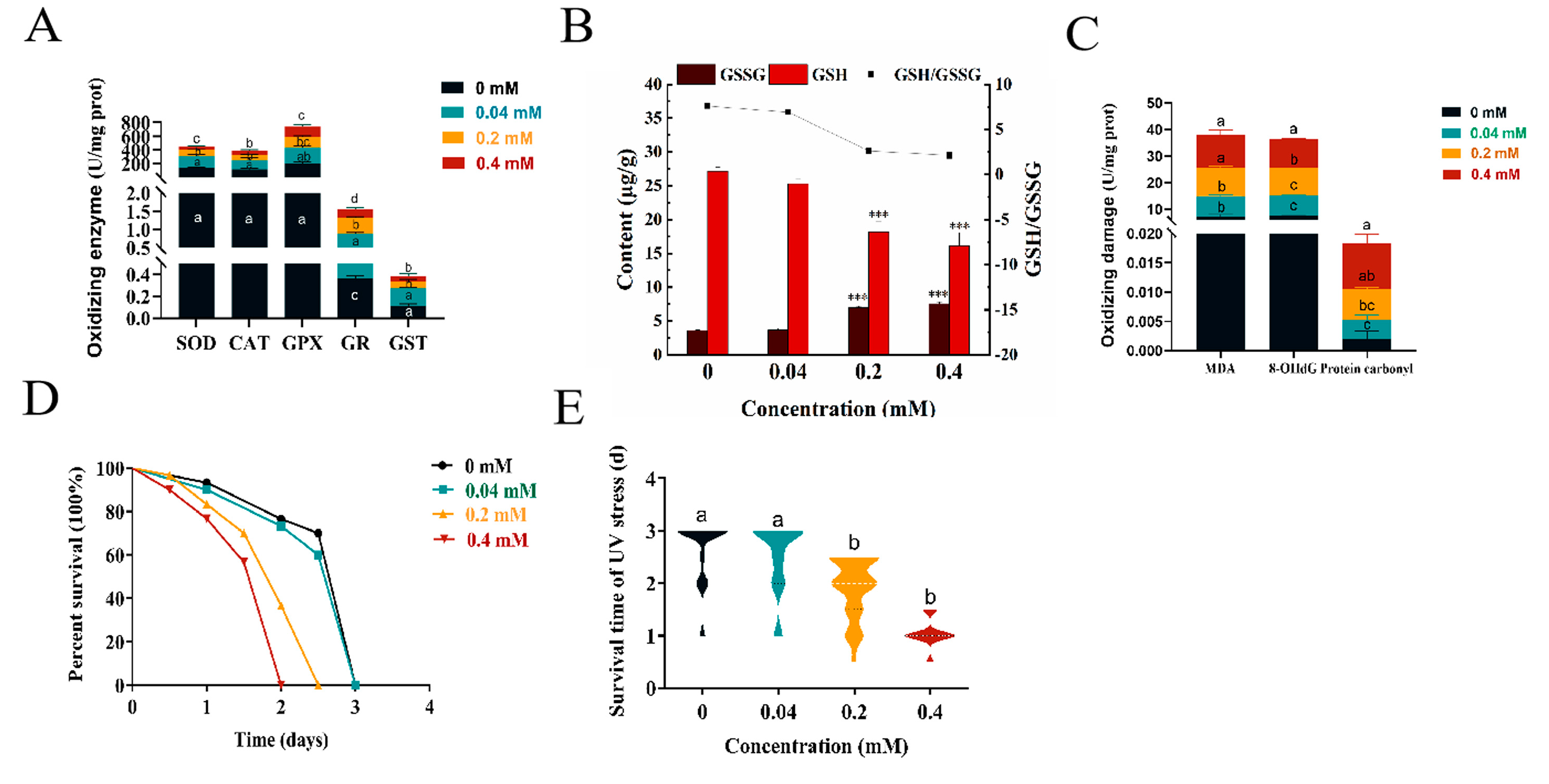
2.4. Effect of Arecoline on Oxidative Stress C. elegans
2.5. Effect of Arecoline on the Internal Antioxidant Enzyme System of C. elegans
2.6. Oxidative Damage by Arecoline on C. elegans
2.7. Effect of Arecoline on the Mitochondria of C. elegans
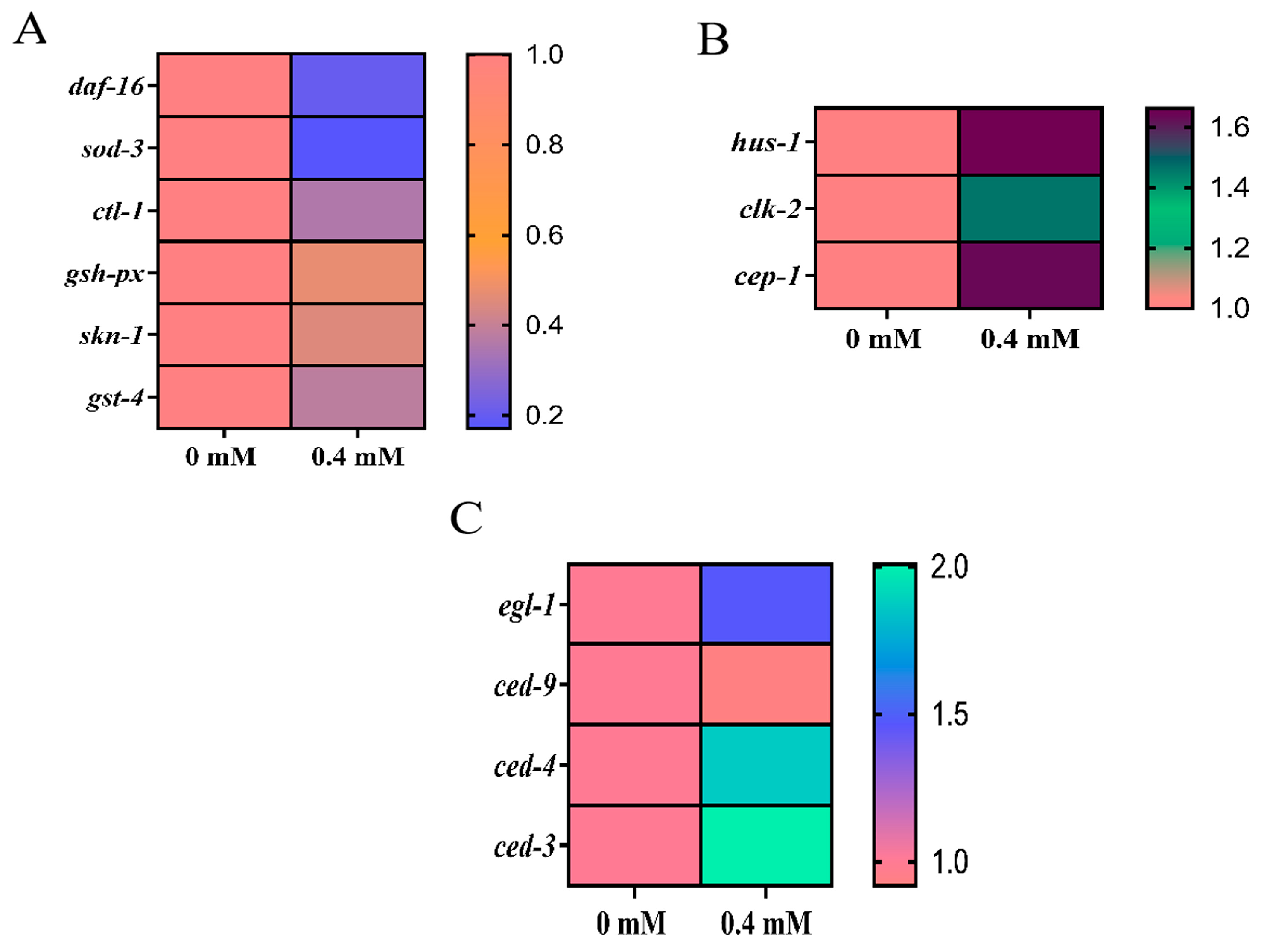
2.8. Detection of Oxidative Stress Genes by Arecoline in C. elegans
2.9. DNA Damage Response by ROS Regulation Involved in the Induction of Apoptosis in C. elegans Cells
2.10. Results of Pharmacological Mitigation of Arecoline Toxicity to C. elegans
2.10.1. Reduction of Arecoline-Induced ROS Production Levels Using Five Antioxidants
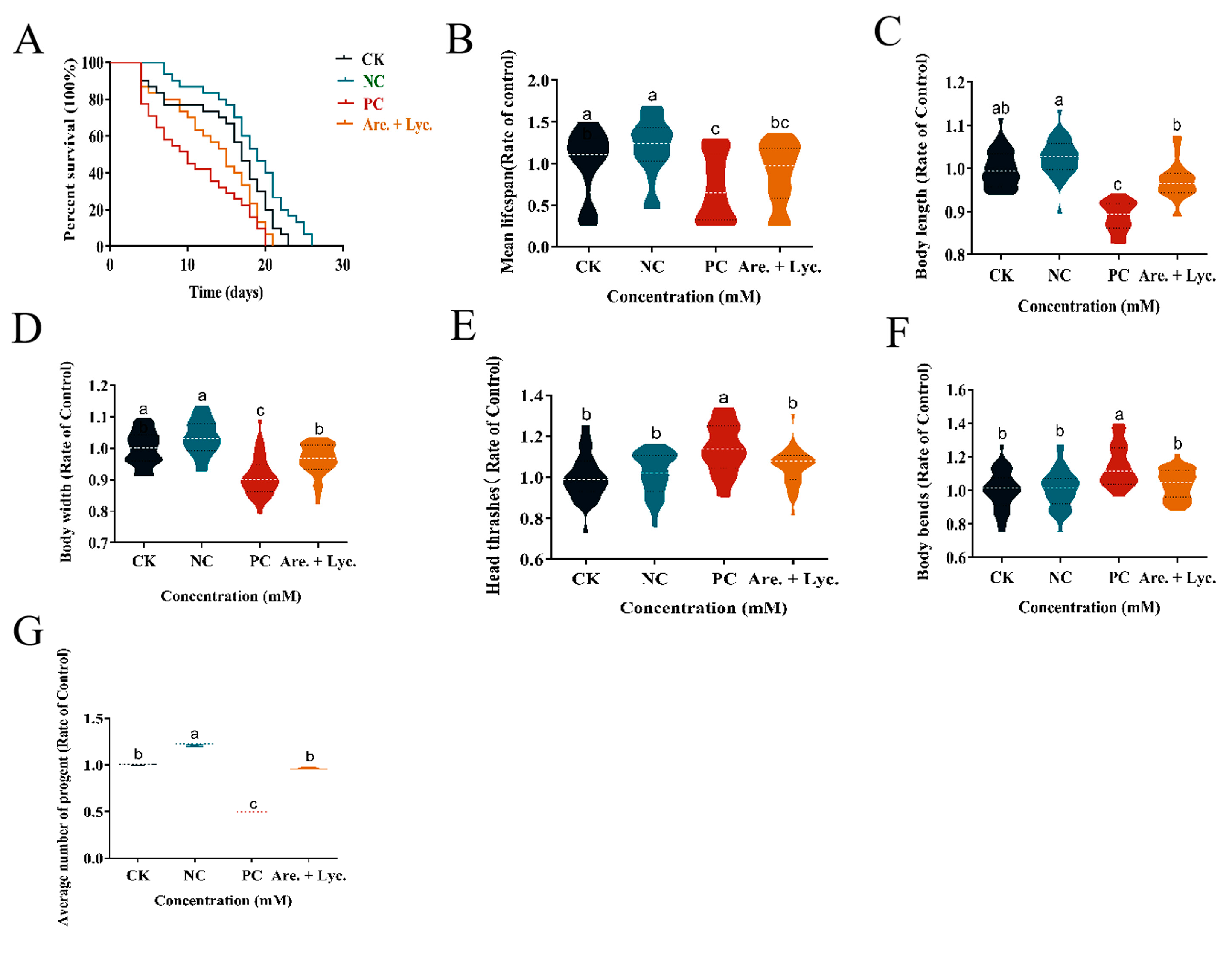
2.10.2. Longevity Experiment of Arecoline + Lycopene on C. elegans
2.10.3. Effect of Arecoline + Lycopene on Body Length and Body Width of C. elegans
2.10.4. Effect of Arecoline + Lycopene on Head Oscillation and Body Bending of C. elegans
2.10.5. Effect of Arecoline + Lycopene on Egg Production of C. elegans
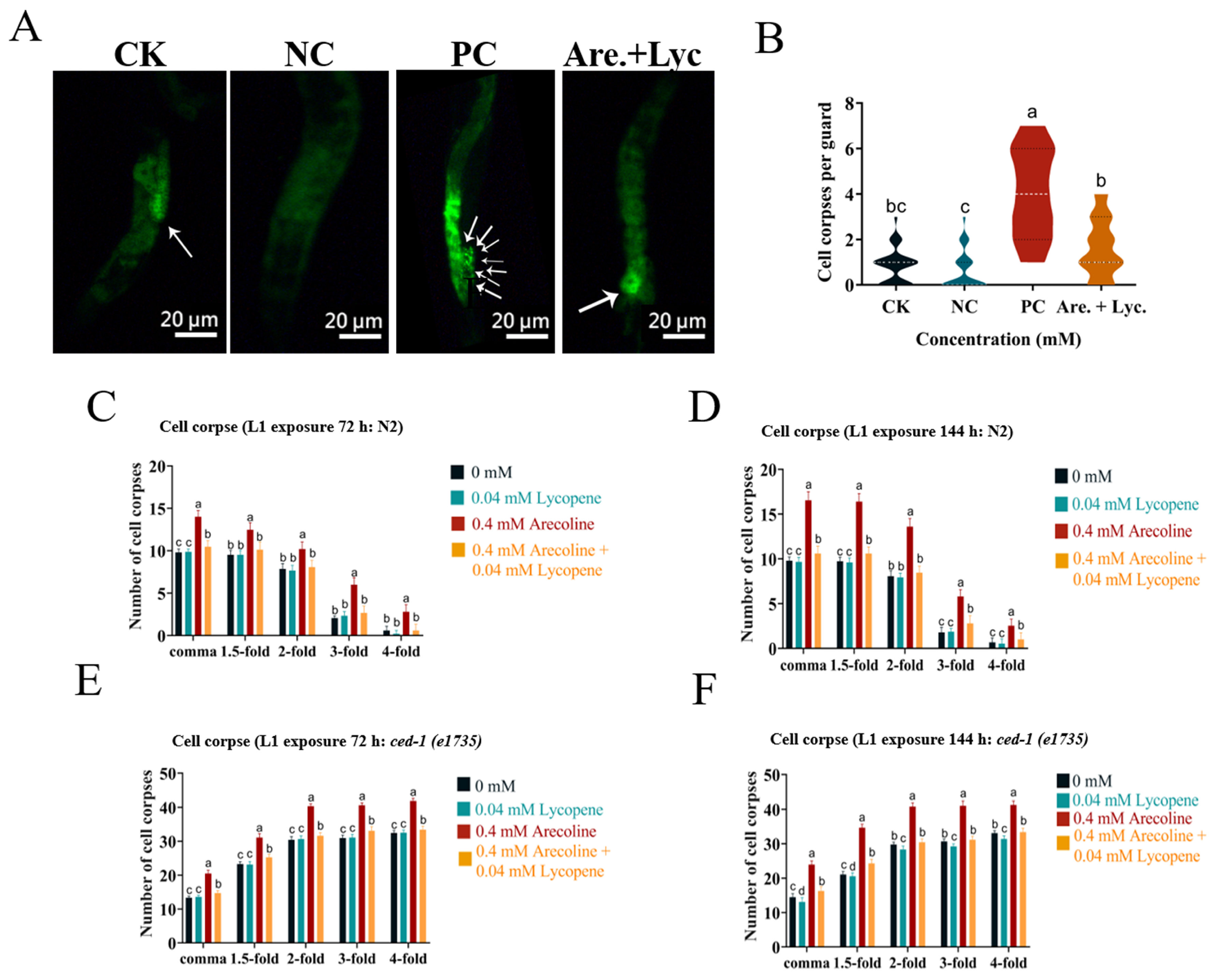
2.10.6. Effect of Arecoline + Lycopene on the Apoptosis Level of C. elegans
3. Discussion
3.1. Effect of Arecoline on the Developmental Toxicity of C. elegans
3.2. Effect of Arecoline on the Neurotoxicity of C. elegans
3.3. Effect of Arecoline on Reproductive Toxicity of C. elegans
3.4. Induction of Oxidative Stress and Apoptosis by Arecoline in C. elegans
3.5. Toxic Reduction Effect
4. Conclusions
5. Materials and Methods
5.1. C. elegans Maintenance
5.2. Arecoline Exposure Assay
5.3. Antioxidant Detoxification Exposure Assay
5.4. Lifespan Assay
5.5. Body Length and Body Width
5.6. Head Thrashing and Body Bending
5.7. Determination of the Content of Muscarinic Acetylcholine Receptors
5.8. Brood Size Measurement
5.9. Detection of Arecoline-Induced Oxidative Stress
5.10. Detection of Oxidative Damage Index
5.11. Measurement of Mitochondrial Membrane Potential
5.12. Gonad Apoptosis and Cell Corpse Assay
5.13. qRT-PCR Analysis
5.14. Statistical Analysis of Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, A.L. Metabolism of the areca alkaloids—Toxic and psychoactive constituents of the areca (betel) nut. Drug Met. Rev. 2022, 54, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Orlan, E.; Duncan, K.; Thomas, H.; Ndumele, A.; Ilbawi, A.; Parascandola, M. Areca nut and betel quid control interventions: Halting the epidemic. Subst. Use Misuse 2020, 55, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.G.; Ramos, D.L.; Dinis-Oliveira, R.J. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: An updated review. Arch. Toxicol. 2021, 95, 375–393. [Google Scholar] [CrossRef]
- Gao, S.-L.; Tang, Y.-Y.; Jiang, J.-M.; Zou, W.; Zhang, P.; Tang, X.-Q. Improvement of autophagic flux mediates the protection of hydrogen sulfide against arecoline-elicited neurotoxicity in PC12 cells. Cell Cycle 2022, 21, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Guan, P.; Huang, C.; Huang, S.; Zhou, P.; Zhang, C. Arecoline induces an excitatory response in ventral tegmental area dopaminergic neurons in anesthetized rats. Front. Pharmacol. 2022, 13, 872212. [Google Scholar] [CrossRef] [PubMed]
- Volgin, A.D.; Bashirzade, A.; Amstislavskaya, T.G.; Yakovlev, O.A.; Demin, K.A.; Ho, Y.-J.; Wang, D.; Shevyrin, V.A.; Yan, D.; Tang, Z.; et al. Dark classics in chemical neuroscience: Arecoline. ACS Chemi. Neurosci. 2019, 10, 2176–2185. [Google Scholar] [CrossRef]
- Serikuly, N.; Alpyshov, E.T.; Wang, D.; Wang, J.; Yang, L.; Hu, G.; Yan, D.; Demin, K.A.; Kolesnikova, T.O.; Galstyan, D.; et al. Effects of acute and chronic arecoline in adult zebrafish: Anxiolytic-like activity, elevated brain monoamines and the potential role of microglia. Prog. Neuro-Psychoph. 2021, 104, 109977. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-T.; Young, G.-C.; Lee, Y.-C.; Chang, Y.-F. A preliminary report on the toxicity of arecoline on early pregnancy in mice. Food Chem. Toxicol. 2011, 49, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-D.; Zang, C.-j.; Yin, S.; Shen, W.; Sun, Q.-Y.; Zhao, M. Metformin protects against mouse oocyte apoptosis defects induced by arecoline. Cell Prolif. 2020, 53, e12809. [Google Scholar] [CrossRef]
- Cox, S.; Vickers, E.R.; Ghu, S.; Zoellner, H. Salivary arecoline levels during areca nut chewing in human volunteers. J. Oral Pathol. Med. 2010, 39, 465–469. [Google Scholar] [CrossRef]
- Lee, H.H.; Chen, L.Y.; Wang, H.L.; Chen, B.H. Quantification of salivary arecoline, arecaidine and N-methylnipecotic acid levels in volunteers by liquid chromatography-tandem mass spectrometry. J. Analyt. Toxicol. 2015, 39, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, D.; Puranik, R.S.; Vanaki, S.S.; Puranik, S.R. Study of salivary arecoline in areca nut chewers. J. Oral Maxillofac. Pathol. 2018, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Floyd, J.; Haroon, S.; Ying, M.; Entezari, A.A.; Jaeger, C.; Vermulst, M.; Gidalevitz, T. Unexpected cell type-dependent effects of autophagy on polyglutamine aggregation revealed by natural genetic variation in C. elegans. BMC Biol. 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Redl, M.; Shayegan, A.; Rollinger, J.M. Application of 3Rs in Caenorhabditis elegans Research for the Identification of Health-Promoting Natural Products. Planta Med. 2024, 90, 576–587. [Google Scholar] [CrossRef]
- Chowdhury, M.I.; Sana, T.; Panneerselvan, L.; Dharmarajan, R.; Megharaj, M. Acute toxicity and transgenerational effects of perfluorobutane sulfonate on Caenorhabditis elegans. Environ. Toxicol. Chem. 2021, 40, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, C.; Jiang, K.; Fang, Y.; Kong, C.; Fu, J.; Liu, Y. A preliminary study on the neurotoxic mechanism of harmine in Caenorhabditis elegans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 245, 109038. [Google Scholar] [CrossRef]
- Van Gilst, M.R.; Hadjivassiliou, H.; Yamamoto, K.R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. USA 2005, 102, 13496–13501. [Google Scholar] [CrossRef]
- Raiders, S.; Black, E.C.; Bae, A.; MacFarlane, S.; Klein, M.; Shaham, S.; Singhvi, A. Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior. eLife 2021, 10, e63532. [Google Scholar] [CrossRef]
- Izquierdo, P.G.; O’Connor, V.; Green, A.C.; Holden-Dye, L.; Tattersall, J.E.H. C. elegans pharyngeal pumping provides a whole organism bio-assay to investigate anti-cholinesterase intoxication and antidotes. NeuroToxicology 2021, 82, 50–62. [Google Scholar] [CrossRef]
- Silva, M.H. Effects of low-dose chlorpyrifos on neurobehavior and potential mechanisms: A review of studies in rodents, zebrafish, and Caenorhabditis elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Athar, F.; Templeman, N.M. C. elegans as a model organism to study female reproductive health. Comp. Biochem. Physiol. Part A 2022, 266, 111152. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, S.; Qian, Z.; Pereira, R.F.; Lee, J.; Doherty, J.J.; Zhang, Z.; Peng, Y.; Clark, J.M.; Timme-Laragy, A.R.; et al. Perfluorooctanesulfonic acid (PFOS) and perfluorobutanesulfonic acid (PFBS) impaired reproduction and altered offspring physiological functions in Caenorhabditis elegans. Food Chem. Toxicol. 2020, 145, 111695. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vizuete, A.; Veal, E.A. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 2017, 11, 708–714. [Google Scholar] [CrossRef]
- Shah, D.; Sah, S.; Nath, S.K. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun. Rev. 2013, 12, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Nasimian, A.; Farzaneh, P.; Tamanoi, F.; Bathaie, S.Z. Cytosolic and mitochondrial ROS production resulted in apoptosis induction in breast cancer cells treated with Crocin: The role of FOXO3a, PTEN and AKT signaling. Biochem. Pharmacol. 2020, 177, 113999. [Google Scholar] [CrossRef] [PubMed]
- Rossbach, L.M.; Oughton, D.H.; Maremonti, E.; Coutris, C.; Brede, D.A. In vivo assessment of silver nanoparticle induced reactive oxygen species reveals tissue specific effects on cellular redox status in the nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 721, 137665. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Song, J.-Y.; Fan, B.; Wang, Y.; Pan, Y.-R.; Che, L.; Sun, Y.-J.; Li, G.-Y. Resveratrol protects photoreceptors by blocking caspase- and PARP-dependent cell death pathways. Free Rad. Biol. Med. 2018, 129, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Zhang, Y.; Zhang, S.; Ahmad, S.; Luo, Y. Synthesis and Photoactivated Toxicity of 2-Thiophenylfuranocoumarin Induce Midgut Damage and Apoptosis in Aedes aegypti Larvae. J. Agr. Food Chem. 2021, 69, 1091–1106. [Google Scholar] [CrossRef]
- Jahedsani, A.; Khezri, S.; Ahangari, M.; Bakhshii, S.; Salimi, A. Apigenin attenuates Aluminum phosphide-induced cytotoxicity via reducing mitochondrial/Lysosomal damages and oxidative stress in rat Cardiomyocytes. Pest. Biochem. Physiol. 2020, 167, 104585. [Google Scholar] [CrossRef]
- Kuzmic, M.; Galas, S.; Lecomte-Pradines, C.; Dubois, C.; Dubourg, N.; Frelon, S. Interplay between ionizing radiation effects and aging in C. elegans. Free Rad. Biol. Med. 2019, 134, 657–665. [Google Scholar] [CrossRef]
- Omari Shekaftik, S.; Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Cri. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Zhang, J.; Wang, L.; Zhao, X.; Luo, Y. Xanthotoxin induced photoactivated toxicity, oxidative stress and cellular apoptosis in Caenorhabditis elegans under ultraviolet A. Comp. Biochem. Physiol. Part C 2022, 251, 109217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fu, K.; Xiang, K.-p.; Wang, L.-y.; Zhang, Y.-f.; Luo, Y.-p. Comparison of the chronic and multigenerational toxicity of racemic glufosinate and l-glufosinate to Caenorhabditis elegans at environmental concentrations. Chemosphere 2023, 316, 137863. [Google Scholar] [CrossRef] [PubMed]
- Qi, X. The Mechanism of ABT-737-Induced Apoptosis of Gonadal Cells in Caenorhabditis elegans. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2021. [Google Scholar]
- Cheng, W.; Yang, X.; Xue, H.; Huang, D.; Cai, M.; Huang, F.; Zheng, L.; Yu, Z.; Zhang, J. Reproductive toxicity of furfural acetone in meloidogyne incognita and Caenorhabditis elegans. Cells 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.G.; Amorim, A.d.G.N.; dos Santos, R.C.; Souza, J.M.T.; de Souza, L.K.M.; Araújo, T.d.S.L.; Nicolau, L.A.D.; de Lima Carvalho, L.; de Aquino, P.E.A.; da Silva Martins, C.; et al. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Food Res. Inter. 2017, 99, 959–968. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, L.; He, S.; Ren, H.-L.; Zhou, N.; Hu, Z.-M. Lycopene alleviates disc degeneration under oxidative stress through the Nrf2 signaling pathway. Mol. Cellul. Prob. 2020, 51, 101559. [Google Scholar] [CrossRef]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Wen, W. Lycopene Increases the Proportion of Slow-Twitch Muscle Fiber by Ampk Signaling to Improve Muscle Anti-Fatigue Ability. Ph.D. Thesis, Sichuan Agricultural University, Yaan, China, 2022. [Google Scholar]
- Zhu, J.; Li, Y.; Shan, X. Lycopene for the treatment of chronic prostatitis/chronic pelvic pain syndrome: Effect and mechanism based on network pharmacology and molecular docking. Natl. J. Androl. 2022, 28, 792–799. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. DAF-16: FOXO in the context of C. elegans. Curr. Top. Dev. Biol. 2018, 127, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; He, S.; Li, G.; Zhu, H. Damage mechanism of arecoline on HUVECs triggered by oxidative stress-mediated endothelial-to-mesenchymal transition. Anti-Tumor Pharm 2022, 12, 488–495. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, L.; Qiu, Z.; Lee, D.Y.; Settle, S.J.; Que Hee, S.; Telesca, D.; Yang, X.; Allard, P. Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLOS Genetics 2016, 12, e1006223. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Brooks, D.R.; Shepherd, S.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria extend lifespan in C. elegans. Aging 2023, 15, 11764–11781. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Chen, W.; Jia, R.; Ye, X.; Li, Y.; Liu, Y.; Jiang, Y.; Zheng, X. Tetrastigma hemsleyanum leaves extract against acrylamide-induced toxicity in HepG2 cells and Caenorhabditis elegans. J. Hazard. Mater. 2020, 393, 122364. [Google Scholar] [CrossRef]
- Luan, Y.K. The Effect of Reducing BCCIP Expression on Stress Resistance of Caenorhabditis elegans. Master’s Thesis, Jilin University, Changchun, China, 2021. [Google Scholar]
- Yang, M.; Abudureyimu, M.; Wang, X.; Zhou, Y.; Zhang, Y.; Ren, J. PHB2 ameliorates Doxorubicin-induced cardiomyopathy through interaction with NDUFV2 and restoration of mitochondrial complex I function. Redox Biol. 2023, 65, 102812. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-W.; Yang, P.; Xing, M.-M.; Chen, B.; Xie, X.-H.; Ding, J.-H.; Lu, M.; Liu, Y.; Guo, Y.-W.; Hu, G. Neuroprotective effects of a lead compound from coral via modulation of the orphan nuclear receptor Nurr1. CNS Neur. Therap. 2023, 29, 893–906. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Wang, Z.; Li, L.; Li, Z.; Xiang, M.; Ding, P. Long-term toxicity of lindane through oxidative stress and cell apoptosis in Caenorhabditis elegans. Environ. Pollut. 2021, 272, 116036. [Google Scholar] [CrossRef]
- Kokel, D.; Li, Y.; Qin, J.; Xue, D. The nongenotoxic carcinogens naphthalene and para-dichlorobenzene suppress apoptosis in Caenorhabditis elegans. Nat. Chem. Biol. 2006, 2, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Prot. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Phar. 2023, 14, 1195490. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, K.; Wang, B.; Wang, L.; Zhang, Y.; Li, H.; Luo, Y. Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents. Toxins 2024, 16, 352. https://doi.org/10.3390/toxins16080352
Xiang K, Wang B, Wang L, Zhang Y, Li H, Luo Y. Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents. Toxins. 2024; 16(8):352. https://doi.org/10.3390/toxins16080352
Chicago/Turabian StyleXiang, Kaiping, Bing Wang, Lanying Wang, Yunfei Zhang, Hanzeng Li, and Yanping Luo. 2024. "Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents" Toxins 16, no. 8: 352. https://doi.org/10.3390/toxins16080352
APA StyleXiang, K., Wang, B., Wang, L., Zhang, Y., Li, H., & Luo, Y. (2024). Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents. Toxins, 16(8), 352. https://doi.org/10.3390/toxins16080352




