Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity
Abstract
1. Introduction
2. Results
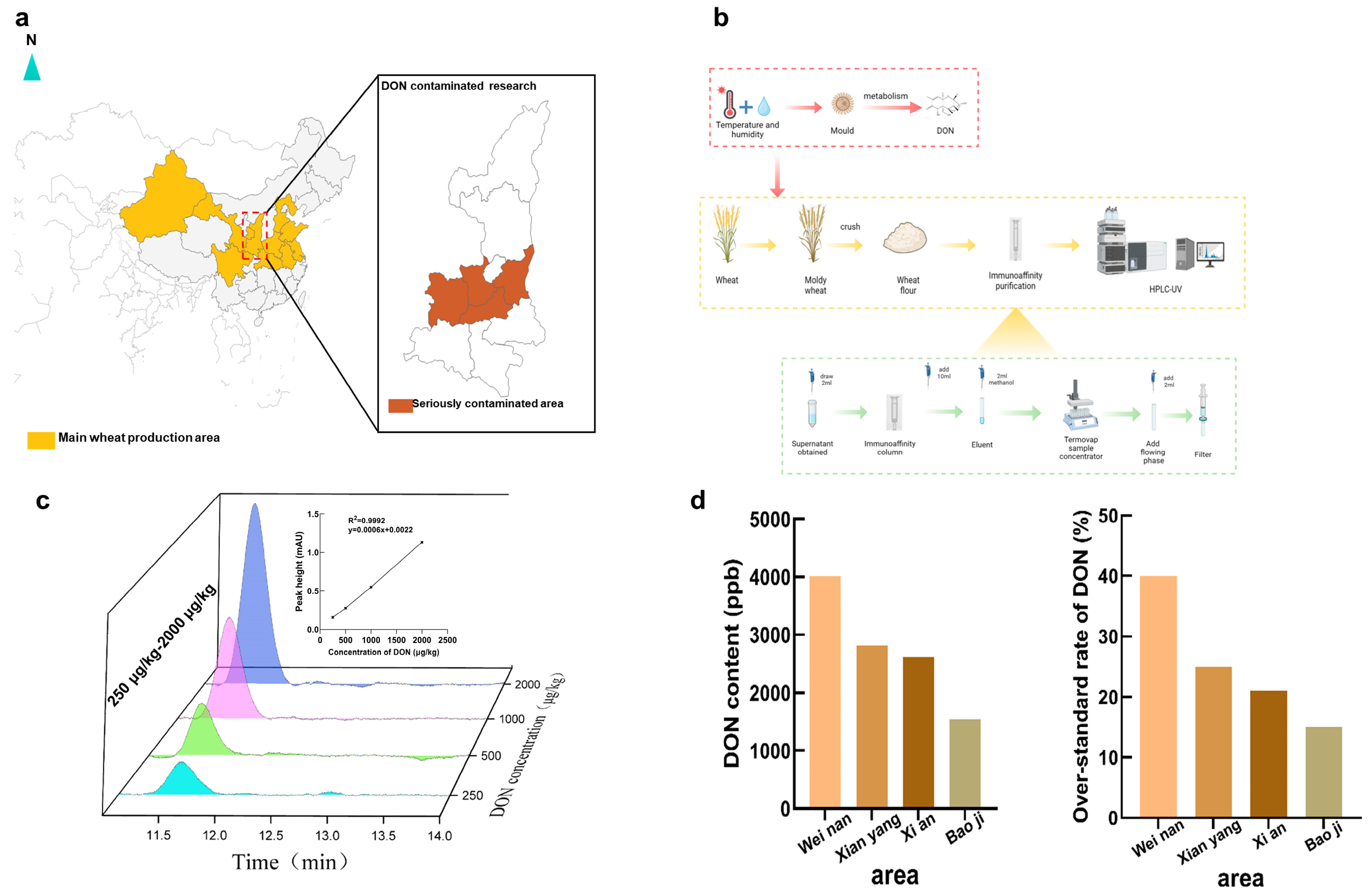
2.1. DON Contamination at Harvest in Shaanxi
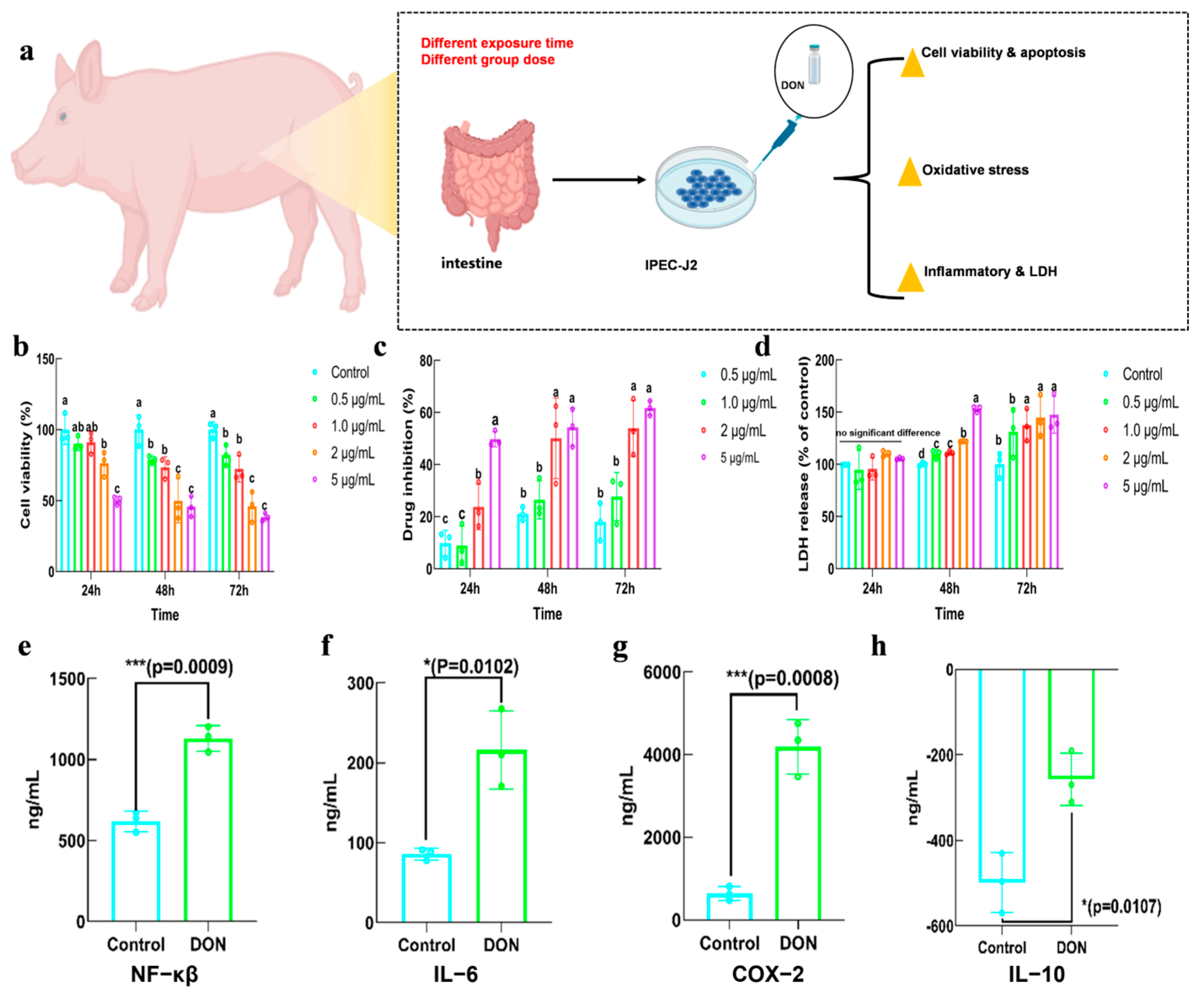
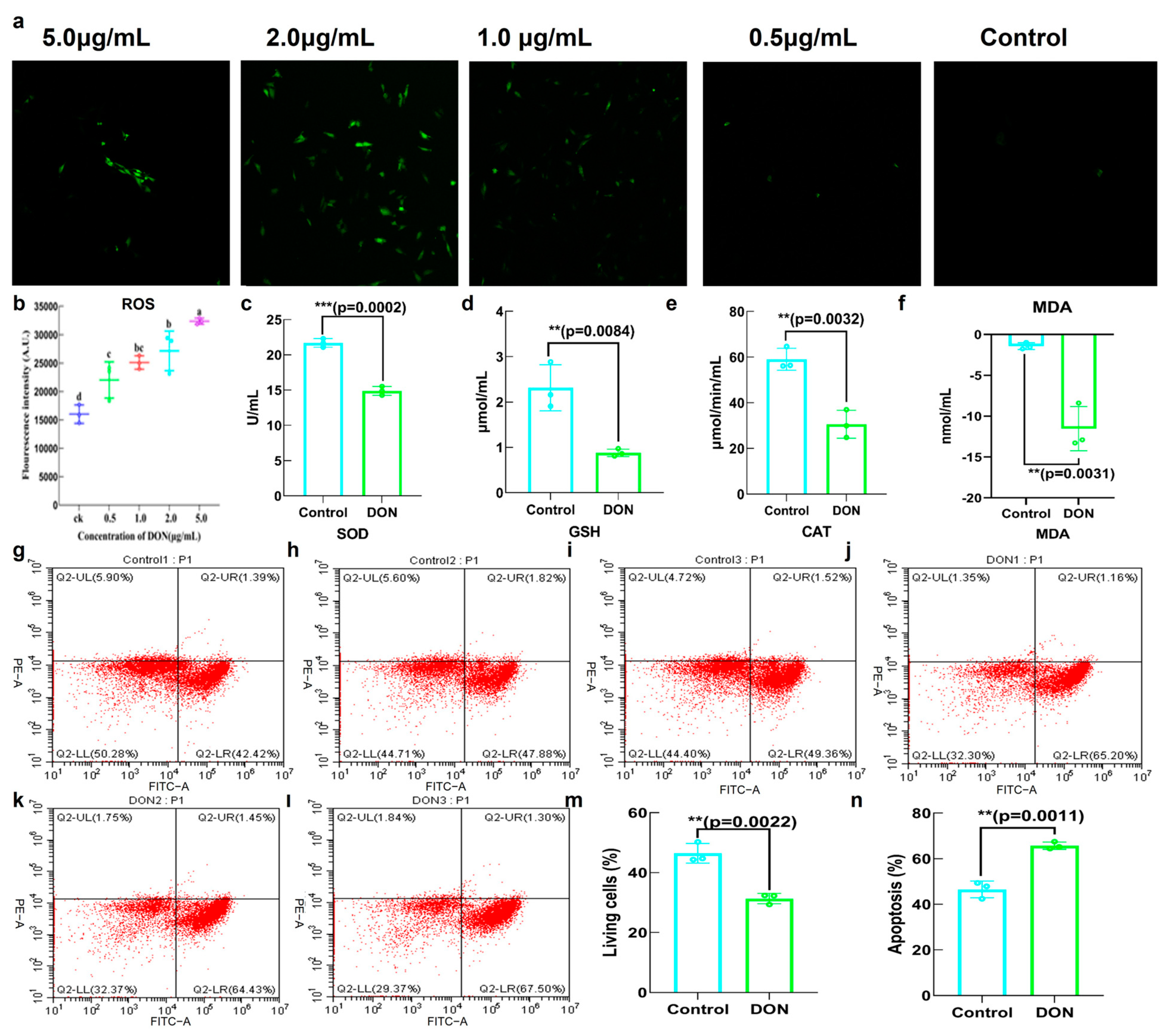
2.2. Toxicity of DON in IPEC−J2
2.3. The Effects of DON on General Physical Indicators in Mice
2.4. The Effects of DON on Intestinal Metabolic Pathways and Gene Expression
2.5. The Effects of DON on Gut Microbiota
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Wheat Sample Collection and DON Detection
5.3. In Vitro Cytotoxicity of DON
5.3.1. The Effect of DON on Cell Viability and Drug Inhibition Rate
5.3.2. The Effect of DON on Lactate Dehydrogenase (LDH) Release
5.3.3. The Effect of DON on Cell Apoptosis
5.3.4. The Effect of DON on Inflammatory Factor Expression
5.3.5. The Effect of DON on Antioxidant Capacity of IPEC−J2
5.4. In Vivo Cytotoxicity of DON
5.4.1. Animals Feed and Observation
5.4.2. RNA Sequencing Analysis
5.4.3. Gut Microbiota Analysis
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, S.; Terciolo, C.; Neves, M.; Puel, S.; Naylies, C.; Lippi, Y.; Pinton, P.; Oswald, I.P. Comparative sensitivity of proliferative and differentiated intestinal epithelial cells to the food contaminant, deoxynivalenol. Environ. Pollut. 2021, 277, 116818. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, L.; Xiao, J.; Zhou, X.; Nüssler, A.K.; Liu, L.; Liu, J.; Yang, W. Review of mechanisms of deoxynivalenol-induced anorexia: The role of gut microbiota. J. Appl. Toxicol. 2017, 37, 1021–1029. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Xu, S.; Meng, Z.; Li, D.; Zhou, X.; Zhang, R.; Shi, S.; Hao, L.; Liu, L.; et al. Deoxynivalenol Exposure Induced Colon Damage in Mice Independent of the Gut Microbiota. Mol. Nutr. Food Res. 2023, 67, e2300317. [Google Scholar] [CrossRef]
- Song, X.; Qiao, L.; Chang, J.; Dou, X.; Zhang, X.; Pi, S.; Xu, C. Dietary supplementation with selenium nanoparticles-enriched Lactobacillus casei ATCC 393 alleviates intestinal barrier dysfunction of mice exposed to deoxynivalenol by regulating endoplasmic reticulum stress and gut microbiota. Ecotoxicol. Environ. Saf. 2022, 248, 114276. [Google Scholar] [CrossRef]
- Faeste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and metabolomic changes from chronic dietary exposure to low-level deoxynivalenol reveal impact on mouse well-being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhang, R.-Q.; Zhai, Q.-Y.; Liu, J.-C.; Li, N.; Liu, W.-X.; Li, L.; Shen, W. Metagenomic analysis of gut microbiota alteration in a mouse model exposed to mycotoxin deoxynivalenol. Toxicol. Appl. Pharmacol. 2019, 372, 47–56. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, C.; Ye, J.L.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z.Y. Protection of Porcine Intestinal-Epithelial Cells from Deoxynivalenol-Induced Damage by Resveratrol via the Nrf2 Signaling Pathway. J. Agric. Food Chem. 2018, 67, 1726–1735. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, X.; Zong, Y.; Hua, X.; Xing, F.; Wang, Y.; Wang, F.; Liu, Y. Deoxynivalenol in wheat from the Northwestern region in China. Food Addit. Contam. Part B 2018, 11, 281–285. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shen, J.; Lin, Z.; Chen, Z.; Zhou, M.; Ma, X. PXR Activation Relieves Deoxynivalenol-Induced Liver Oxidative Stress Via Malat1 LncRNA m6A Demethylation. Adv. Sci. 2024, 11, e2308742. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, Z.; Li, L.; Chen, X.; Zou, T.; Chen, J.; You, J. Selenomethionine Supplementation Mitigates Liver Dysfunction, Oxidative Injury and Apoptosis through Enhancing Antioxidant Capacity and Inhibiting JNK MAPK Pathway in Piglets Fed Deoxynivalenol-Contaminated Diets. Antioxidants 2024, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jin, J.; Huangfu, B.; He, X.; Zhang, B.; Li, X.; Xu, W.; Xing, F. Phlorizin Alleviates Inflammation Caused by Deoxynivalenol by Regulating the Gut Microbiome and Inhibiting the TLR4/MyD88/NF-κB Signaling Pathway in Mice. ACS Food Sci. Technol. 2024, 4, 333–343. [Google Scholar] [CrossRef]
- Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Sun, X. The disorder metabolic profiling in kidney and spleen of mice induced by mycotoxins deoxynivalenol through gas chromatography mass spectrometry. Chemosphere 2017, 180, 267–274. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, C.; Ju, Z.; Zheng, S.; Wen, Z.; Xu, S.; Cheng, Z.; Ma, Z. The mitochondrial membrane protein FgLetm1 regulates mitochondrial integrity, production of endogenous reactive oxygen species and mycotoxin biosynthesis in Fusarium graminearum. Mol. Plant Pathol. 2018, 19, 1595–1611. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Y.; Wu, Z.; Wang, H.; Chen, Z.; Wang, J.; Bao, W. Protective effects of melatonin on deoxynivalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Food Chem. Toxicol. 2023, 177, 113803. [Google Scholar] [CrossRef]
- Li, E.; Horn, N.; Ajuwon, K.M. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch. Toxicol. 2021, 95, 2065–2079. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhang, B.; Wu, K.; Yang, A.; Li, C.; Zhang, J.; Zhang, C.; Rajput, S.A.; Zhang, N.; et al. Deoxynivalenol Impairs Porcine Intestinal Host Defense Peptide Expression in Weaned Piglets and IPEC-J2 Cells. Toxins 2018, 10, 541. [Google Scholar] [CrossRef]
- Wang, P.; Huang, L.; Yang, W.; Liu, Q.; Li, F.; Wang, C. Deoxynivalenol Induces Inflammation in the Small Intestine of Weaned Rabbits by Activating Mitogen-Activated Protein Kinase Signaling. Front. Veter-Sci. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Wang, J.; Wang, H.; Wu, S.; Bao, W. Analysis of the roles of the Notch1 signalling pathway in modulating deoxynivalenol cytotoxicity. Ecotoxicol. Environ. Saf. 2022, 246, 114183. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, C.; Ren, X.; Tai, B.; Xing, F. Metagenome Analysis Identifies Microbial Shifts upon Deoxynivalenol Exposure and Post-Exposure Recovery in the Mouse Gut. Toxins 2023, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Lucke, A.; Böhm, J.; Zebeli, Q.; Metzler-Zebeli, B.U. Dietary Deoxynivalenol Contamination and Oral Lipopolysaccharide Challenge Alters the Cecal Microbiota of Broiler Chickens. Front. Microbiol. 2018, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhong, H.; Li, T.; Wang, Z.; Chen, X.; Zou, T.; You, J.; Chen, J. Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation. Antioxidants 2024, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 497. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, L.; Yuan, L.; Li, J.; Wang, X. The characteristics of patulin degradation by probiotic yeast—Pichia guilliermondii S15-8. Food Control. 2021, 133, 108627. [Google Scholar] [CrossRef]
- Van Houten, B.; Woshner, V.; Santos, J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair 2005, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, J.; Li, F.; Hu, C.-A.A.; Liao, P.; Tan, K.; Tan, B.; Xiong, X.; Liu, G.; Li, T.; et al. Autophagy protects intestinal epithelial Cells against Deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free. Radic. Biol. Med. 2015, 89, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Pomothy, J.M.; Szabó, O.; Czimmermann, Á.E.; Babiczky, Á.; Jerzsele, Á.; Pászti-Gere, E. Investigation of the inflammatory and oxidative stress-inducing effects of deoxynivalenol and T-2 toxin exposure in non-tumorigenic human intestinal cell model. Toxicon 2021, 200, 78–86. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, S.; Meng, X.; Lin, X.; Duan, N.; Zhang, Y.; Yuan, W.; Wu, S.; Wang, Z. Deoxynivalenol photocatalytic detoxification products alleviate intestinal barrier damage and gut flora disorder in BLAB/c mice. Food Chem. Toxicol. 2021, 156, 112510. [Google Scholar] [CrossRef]
- Sun, L.-H.; Lei, M.-Y.; Zhang, N.-Y.; Zhao, L.; Krumm, C.S.; Qi, D.-S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, M.; Hong, X.; Fu, Y.; Chen, Y.; Wu, H.; Sun, Y.; Wang, X.; Zhou, E.; Wang, J.; et al. Quercetin Alleviates Deoxynivalenol-Induced Intestinal Damage by Suppressing Inflammation and Ferroptosis in Mice. J. Agric. Food Chem. 2023, 71, 10761–10772. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Yin, H.-H.; Zhu, J.-C. Increased gut absorptive capacity in rats with severe head injury after feeding with probiotics. Nutrition 2011, 27, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, J.; Wang, P.; Liu, C.; Liu, M.; Zhou, T.; Yin, Q.; Yan, G. Glycyrrhizic Acid and Compound Probiotics Supplementation Alters the Intestinal Transcriptome and Microbiome of Weaned Piglets Exposed to Deoxynivalenol. Toxins 2022, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shang, M.; Jiang, Y.; Qian, J.; Duan, B.; Yang, Y. The complete chloroplast genome of Rumex hastatus D. DON and its phylogenetic analysis. Mitochondrial DNA Part B 2020, 5, 1681–1682. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, Y.; Zuo, M.; Ding, S.; Li, J.; Feng, S.; Xiao, Y.; Tao, S. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ. Int. 2024, 185, 108525. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. NPJ Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Hsu, P.-I.; Pan, C.-Y.; Kao, J.Y.; Tsay, F.-W.; Peng, N.-J.; Kao, S.-S.; Wang, H.-M.; Tsai, T.-J.; Wu, D.-C.; Chen, C.-L.; et al. Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter 2018, 23, e12498. [Google Scholar] [CrossRef]
- Wang, N.; Meng, F.; Ma, S.; Fu, L. Species-level gut microbiota analysis in ovariectomized osteoporotic rats by Shallow shotgun sequencing. Gene 2022, 817, 146205. [Google Scholar] [CrossRef]
- Huang, W.; Chen, H.; He, Q.; Xie, W.; Peng, Z.; Ma, Q.; Huang, Q.; Chen, Z.; Liu, Y. Nobiletin protects against ferroptosis to alleviate sepsis-associated acute liver injury by modulating the gut microbiota. Food Funct. 2023, 14, 7692–7704. [Google Scholar] [CrossRef]
- Miri, S.; Hassan, H.; Esmail, G.A.; Njoku, E.N.; Chiba, M.; Yousuf, B.; Ahmed, T.A.E.; Hincke, M.; Mottawea, W.; Hammami, R. A Two Bacteriocinogenic Ligilactobacillus Strain Association Inhibits Growth, Adhesion, and Invasion of Salmonella in a Simulated Chicken Gut Environment. Probiotics Antimicrob. Proteins 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kyoung, H.; Park, K.I.; Oh, S.; Song, M.; Kim, Y. Postbiotic heat-killed lactobacilli modulates on body weight associated with gut microbiota in a pig model. AMB Express 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ping, L.; Xie, Q.; Liu, D.; Zhao, L.; Evivie, S.E.; Qin, G.; Zhang, X.; Zhao, W.; Aschalew, N.; et al. Lactobacillus plantarum KLDS1.0386 with antioxidant capacity ameliorates the lipopolysaccharide-induced acute liver injury in mice by NF-κB and Nrf2 pathway. Food Bioscience 2022, 47, 101589. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Han, J.; Li, C.; Tang, J.; Wang, X.; Ma, Y.; Chen, Y.; Xiao, D.; Guo, X. Tibetan highland barley fiber improves obesity and regulates gut microbiota in high-fat diet-fed mice. Food Biosci. 2023, 53, 102620. [Google Scholar] [CrossRef]
- Chang, T.-T.; Chen, J.-W. Direct CCL4 Inhibition Modulates Gut Microbiota, Reduces Circulating Trimethylamine N-Oxide, and Improves Glucose and Lipid Metabolism in High-Fat-Diet-Induced Diabetes Mellitus. J. Inflamm. Res. 2021, 14, 6237–6250. [Google Scholar] [CrossRef]
- Gu, S.; Xie, Q.; Chen, C.; Liu, C.; Xue, W. Gut Microbial Signatures Associated with Peanut Allergy in a BALB/c Mouse Model. Foods 2022, 11, 1395. [Google Scholar] [CrossRef]
- Alam, A.; Leoni, G.; Quiros, M.; Wu, H.; Desai, C.; Nishio, H.; Jones, R.M.; Nusrat, A.; Neish, A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol. 2016, 1, 15021. [Google Scholar] [CrossRef]
- Xu, H.; Wu, B.; Guo, L.; Chen, J.; Lin, N.; Qin, L.; Xie, J. Preparation of deoxynivalenol and mask deoxynivalenol. Toxicon 2019, 158, S65–S66. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, M.; Li, K.; Zhang, C.; Tian, H.; Luo, Y. Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity. Toxins 2024, 16, 353. https://doi.org/10.3390/toxins16080353
Wang Y, Zhang M, Li K, Zhang C, Tian H, Luo Y. Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity. Toxins. 2024; 16(8):353. https://doi.org/10.3390/toxins16080353
Chicago/Turabian StyleWang, Yebo, Minjie Zhang, Ke Li, Chune Zhang, Honglei Tian, and Ying Luo. 2024. "Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity" Toxins 16, no. 8: 353. https://doi.org/10.3390/toxins16080353
APA StyleWang, Y., Zhang, M., Li, K., Zhang, C., Tian, H., & Luo, Y. (2024). Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity. Toxins, 16(8), 353. https://doi.org/10.3390/toxins16080353




