New Report of Cyanobacteria and Cyanotoxins in El Pañe Reservoir: A Threat for Water Quality in High-Andean Sources from PERU
Abstract
:1. Introduction
2. Results
2.1. Morphological Analysis
2.2. Molecular Analysis of Cyanobacteria Genes
2.2.1. Molecular Analysis of Cyanobacteria 16S rRNA Genes
2.2.2. Molecular Analysis of Cyanotoxin-Encoding Genes
2.2.3. Molecular Analysis of Microcystin-Encoding Genes from Microcystis sp.
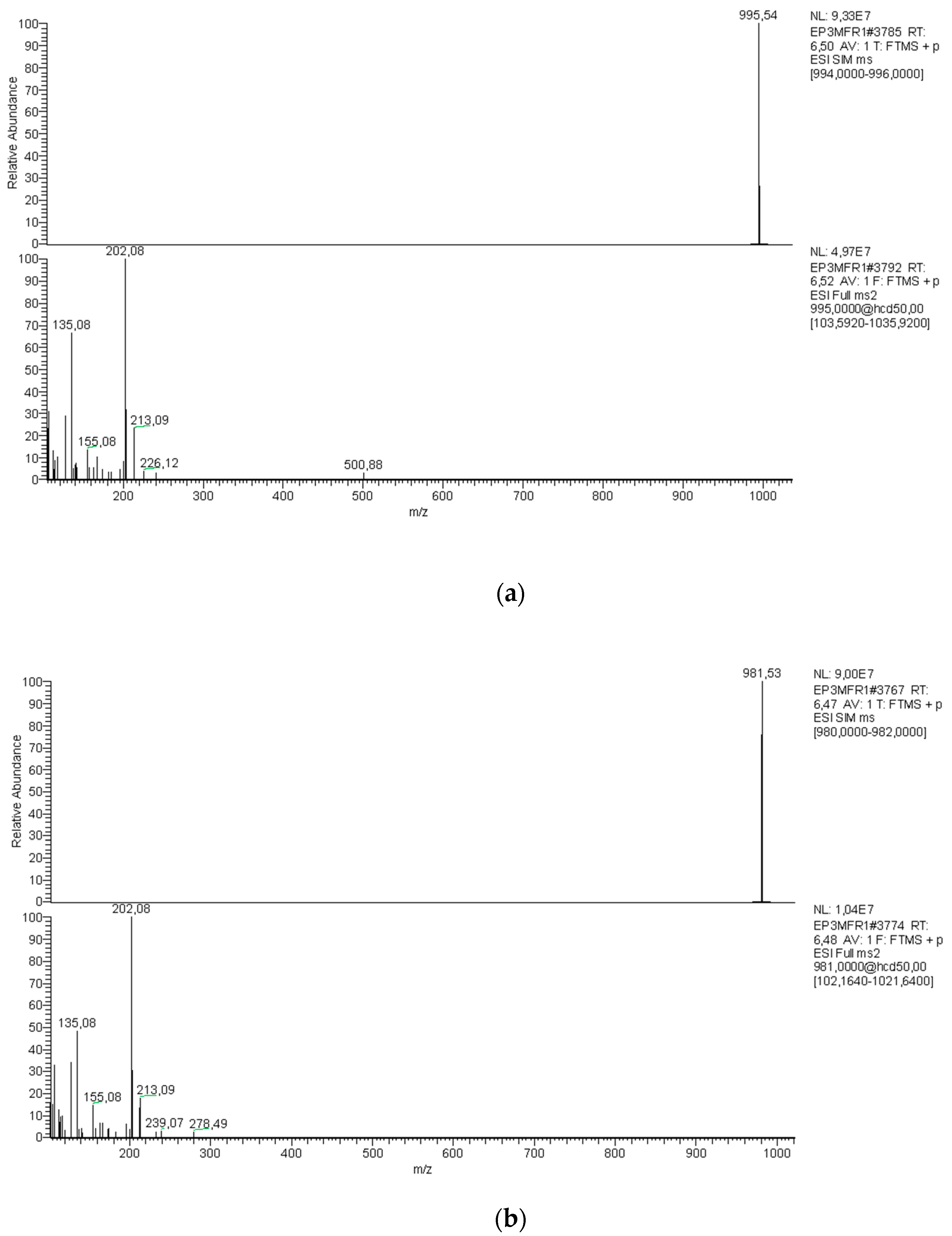
2.3. Chemical Analysis of Cyanotoxins
2.3.1. Cyanotoxins Analysis
2.3.2. Microcystin Quantification
3. Discussion
4. Conclusions
5. Materials and Methods
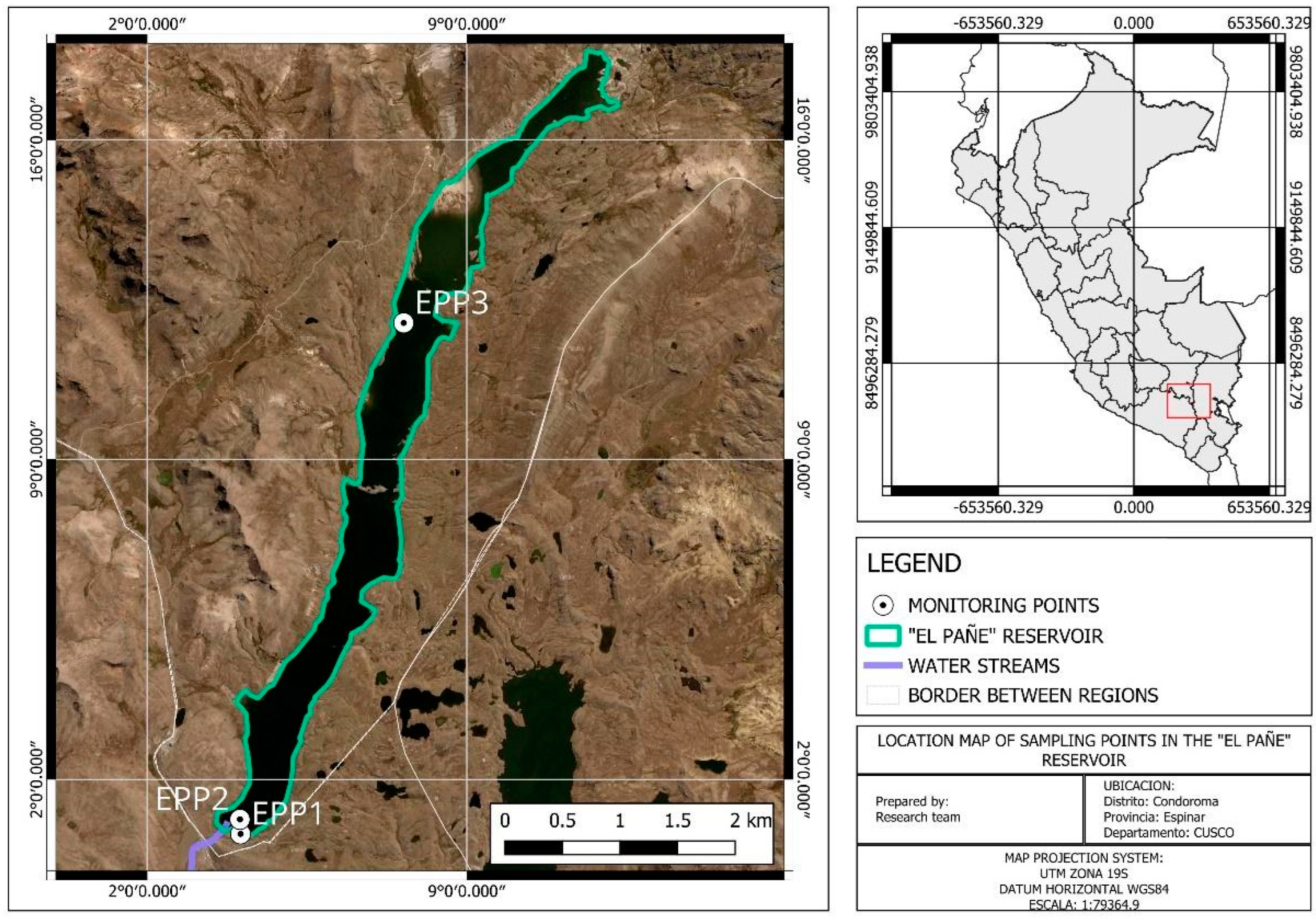
5.1. Sampling
5.2. Field and Laboratory Analyses
5.2.1. Microscopy Observation
5.2.2. Molecular Analysis
Molecular Analysis of Cyanobacteria
Molecular Analysis of Cyanotoxins-Encoding Genes
Molecular Analysis of Microcystin-Encoding Genes from Microcystis sp.
5.2.3. Chemical Analysis of Cyanotoxins
Extraction
LC-MS/MS Analysis
Microcystins Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorador, C.; Vila, I.; Imhoff, J.F.; Witzel, K.P. Cyanobacterial Diversity in Salar de Huasco, a High Altitude Saline Wetland in Northern Chile: An Example of Geographical Dispersion? FEMS Microbiol. Ecol. 2008, 64, 419–432. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, I.; Motta, C.; Forastier, M.; Polla, W.; Otaño, S.; Meichtry, N.; Devercelli, M.; Lombardo, R. Ecological Meta-Analysis of Bloom-Forming Planktonic Cyanobacteria in Argentina. Harmful Algae 2019, 83, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Hawkes, K.; Calvaruso, R.; Reyes-Prieto, A.; Lawrence, J. Seasonality and Distribution of Cyanobacteria and Microcystin Toxin Genes in an Oligotrophic Lake of Atlantic Canada. J. Phycol. 2021, 57, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.; Vasconcelos, V.; Antunes, A. Cyanobacterial Blooms: Current Knowledge and New Perspectives. Earth 2022, 3, 127–135. [Google Scholar] [CrossRef]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic Classification of Cyanoprokaryotes (Cyanobacterial Genera) 2014, Using a Polyphasic Approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Ramos, V.; Moreira, C.; Mankiewicz-Boczek, J.; Vasconcelos, V. Application of Molecular Tools in Monitoring Cyanobacteria and Their Potential Toxin Production. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria; Kurmayer, R., Sivonen, K., Wilmotte, A., Salmaso, N., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 310–313. [Google Scholar]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global Geographical and Historical Overview of Cyanotoxin Distribution and Cyanobacterial Poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water; A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 15–162. [Google Scholar]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing Organisms, Occurrence, Toxicity, Mechanism of Action and Human Health Toxicological Risk Evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Arman, T.; Clarke, J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W.; Collart, L.P.; Mueller, R.S.; Halsey, K.H.; Bildfell, R.J.; Schreder, P.; Sobhakumari, A.; Ferry, R. Anabaena/Dolichospermum as the Source of Lethal Microcystin Levels Responsible for a Large Cattle Toxicosis Event. Toxicon X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Piontek, M.; Czyżewska, W.; Mazur-Marzec, H. Effects of Harmful Cyanobacteria on Drinking Water Source Quality and Ecosystems. Toxins 2023, 15, 703. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Vignale, F.A.; Kurth, D.; Lencina, A.I.; Poiré, D.G.; Chihuailaf, E.; Muñoz-Herrera, N.C.; Novoa, F.; Contreras, M.; Turjanski, A.G.; Farías, M.E. Geobiology of Andean Microbial Ecosystems Discovered in Salar de Atacama, Chile. Front. Microbiol. 2021, 12, 762076. [Google Scholar] [CrossRef] [PubMed]
- Coayla-P, P.; Cheneaux-D, A.A.; Moreno-S, C.V.; Cruz-R, C.E.; Colque-R, E.W.; Damborenea, C. Littoral Macrobenthic Communities and Water Quality in El Pañe Reservoir, Arequipa, Peru. Neotrop. Biodivers. 2022, 8, 99–107. [Google Scholar] [CrossRef]
- Mamani Larico, A.J.; Zúñiga Medina, S.A. Application of WASP Model for Assessment of Water Quality for Eutrophication Control for a Reservoir in the Peruvian Andes. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2019, 24, 37–47. [Google Scholar] [CrossRef]
- Mamani Larico, A.J.; Rendón Dávila, V.O.; Figueroa Tapia, Á.M.; Quiroz Valdivia, J.; Zúñiga Medina, S.A. Bioenergetic and Water Quality Modeling for Eutrophication Assessment of El Pañe Reservoir, Peru. Ecohydrol. Hydrobiol. 2021, 21, 114–128. [Google Scholar] [CrossRef]
- Salazar-Torres, A.; Robles, D.; Reyes, A.; Santa-Maria, M.C.; Venail, P. Evaluation of Planktonic Cyanobacteria in Peruvian Freshwater Lentic Water Bodies: Prevalence and Regulatory Framework to Aid Policy Making. Environ. Monit. Assess. 2023, 195, 852. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J. Cyanoprokaryota: 3rd Part: Heterocystous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; Volume 19/3, pp. 1–1130. [Google Scholar]
- Paquis, P.; Hengst, M.B.; Florez, J.Z.; Tapia, J.; Molina, V.; Pérez, V.; Pardo-Esté, C. Short-Term Characterisation of Climatic-Environmental Variables and Microbial Community Diversity in a High-Altitude Andean Wetland (Salar de Huasco, Chile). Sci. Total Environ. 2023, 859, 160291. [Google Scholar] [CrossRef] [PubMed]
- Gaysina, L.A.; Saraf, A.; Singh, P. Cyanobacteria in Diverse Habitats. In Cyanobacteria: From Basic Science to Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 9780128146682. [Google Scholar]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A New Wine in an Old Bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Komárková, J.; Montoya, H.; Komárek, J. Cyanobacterial Water Bloom of Limnoraphis Robusta in the Lago Mayor of Lake Titicaca. Can It Develop? Hydrobiologia 2016, 764, 249–258. [Google Scholar] [CrossRef]
- Montoya, H. Cyanobacterial Species, Potentially Forming Water-Blooms in the Lake Titicaca (Peru). Arnaldoa 2014, 21, 381–390. [Google Scholar]
- Stark, G.F.; Martin, R.M.; Smith, L.E.; Wei, B.; Hellweger, F.L.; Bullerjahn, G.S.; McKay, R.M.L.; Boyer, G.L.; Wilhelm, S.W. Microcystin Aids in Cold Temperature Acclimation: Differences between a Toxic Microcystis Wildtype and Non-Toxic Mutant. Harmful Algae 2023, 129, 102531. [Google Scholar] [CrossRef] [PubMed]
- Capelli, C.; Ballot, A.; Cerasino, L.; Papini, A.; Salmaso, N. Biogeography of Bloom-Forming Microcystin Producing and Non-Toxigenic Populations of Dolichospermum Lemmermannii (Cyanobacteria). Harmful Algae 2017, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.; Gomes, C.; Vasconcelos, V.; Antunes, A. Cyanotoxins Occurrence in Portugal: A New Report on Their Recent Multiplication. Toxins 2020, 12, 154. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An Overview of Diversity, Occurrence, Genetics and Toxin Production of Bloom-Forming Dolichospermum (Anabaena) Species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, X.; Wu, Z.; Sun, T.; Tong, Y. Recent Advances in Cyanotoxin Synthesis and Applications: A Comprehensive Review. Microorganisms 2023, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Cirés, S.; de Pedro, Z.M.; Colina, J.Á.; Velásquez-Figueroa, Y.; Carmona-Jiménez, J.; Caro-Borrero, A.; Salazar, A.; Santa María Fuster, M.C.; Contreras, D.; et al. Overview of Toxic Cyanobacteria and Cyanotoxins in Ibero-American Freshwaters: Challenges for Risk Management and Opportunities for Removal by Advanced Technologies. Sci. Total Environ. 2021, 761, 143197. [Google Scholar] [CrossRef] [PubMed]
- Triantis, T.M.; Kaloudis, T.; Zervou, S.; Hiskia, A. Solid-Phase Extraction of Microcystins and Nodularin from Drinking Water. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Hoboken, NJ, USA, 2016; pp. 354–357. [Google Scholar]
- Marsin Sanagi, M.; Ling, S.L.; Nasir, Z.; Hermawan, D.; Aini Wan Ibrahim, W.; Abu Naim, A. Comparison of Signal-to-Noise, Blank Determination, and Linear Regression Methods for the Estimation of Detection and Quantification Limits for Volatile Organic Compounds by Gas Chromatography. J. AOAC Int. 2009, 92, 1833–1838. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Del Dot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman4, A.E. RRNA Sequences and Evolutionary Relationships among Toxic and Nontoxic Cyanobacteria of the Genus Microcystis; International Union of Microbiological Societies: Florence, Italy, 1997. [Google Scholar]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR Primers To Amplify 16S RRNA Genes from Cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Doblin, M.A.; Coyne, K.J.; Rinta-Kanto, J.M.; Wilhelm, S.W.; Dobbs, F.C. Dynamics and Short-Term Survival of Toxic Cyanobacteria Species in Ballast Water from NOBOB Vessels Transiting the Great Lakes-Implications for HAB Invasions. Harmful Algae 2007, 6, 519–530. [Google Scholar] [CrossRef]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-Based Identification of Microcystin-Producing Genotypes of Different Cyanobacterial Genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Tillett, D.; Parker, D.L.; Neilan, B.A. Detection of Toxigenicity by a Probe for the Microcystin Synthetase a Gene (McyA) of the Cyanobacterial Genus Microcystis: Comparison of Toxicities with 16S RRNa and Phycocyanin Operon (Phycocyanin Intergenic Spacer) Phylogenies. Appl. Environ. Microbiol. 2001, 67, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Mikalsen, B.; Boison, G.; Skulberg, O.M.; Fastner, J.; Davies, W.; Gabrielsen, T.M.; Rudi, K.; Jakobsen, K.S. Natural Variation in the Microcystin Synthetase Operon McyABC and Impact on Microcystin Production in Microcystis Strains. J. Bacteriol. 2003, 185, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Ouahid, Y.; Pérez-Silva, G.; Del Campo, F.F. Identification of Potentially Toxic Environmental Microcystis by Individual and Multiple PCR Amplification of Specific Microcystin Synthetase Gene Regions. In Proceedings of the Environmental Toxicology. Environ. Toxicol. 2005, 20, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mihali, T.K.; Kellmann, R.; Muenchhoff, J.; Barrow, K.D.; Neilan, B.A. Characterization of the Gene Cluster Responsible for Cylindrospermopsin Biosynthesis. Appl. Environ. Microbiol. 2008, 74, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Ramos, V.; Martins, A.; Sousa, M.; Welker, M.; Antunes, A.; Vasconcelos, V.M. Phylogenetic, Chemical and Morphological Diversity of Cyanobacteria from Portuguese Temperate Estuaries. Mar. Environ. Res. 2012, 73, 7–16. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a Synthetase Gene Cluster of the Cyanobacterium Anabaena Sp. Strain 37 and Molecular Methods to Detect Potential Producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef]

| Code | Cyanobacteria | Microcystis sp. | Dolichospermum sp. |
|---|---|---|---|
| 16S rRNA | 16S rRNA Microcystis sp. | 16S rRNA Dolichospermum sp. | |
| EPP2M | + | − | + |
| EPP3M | + | − | + |
| EPP2N | + | + | + |
| EPP3N | + | − | + |
| Code | Microcystins | Cylindrospermopsins | Anatoxins | Saxitoxins |
|---|---|---|---|---|
| mcyA | cyrJ | AnaC | Sxtl | |
| EPP2M | + | − | − | − |
| EPP3M | + | − | − | − |
| EPP2N | + | − | − | − |
| EPP3N | + | − | − | − |
| Code | Microcystins Microcystis sp. | ||||
|---|---|---|---|---|---|
| mcyA- Microcystis sp. | mcyB- Microcystis sp. | mcyC- Microcystis sp. | mcyD- Microcystis sp. | mcyE- Microcystis sp. | |
| EPP2M | − | − | − | − | − |
| EPP3M | − | − | − | − | − |
| EPP2N | − | + | + | + | + |
| EPP3N | − | − | − | − | − |
| Code | Origin | Month | Results | |||
|---|---|---|---|---|---|---|
| Units | MC-LR Biomass | dmMC-LR 1 Biomass | MC-LR Dissolved | |||
| EPP1M | El Pañe | May | µg/L | 2.17 | - | <LOD |
| EPP2M | El Pañe | May | µg/L | 19.39 | - | <LOD |
| EPP3M | El Pañe | May | µg/L | 25.18 | - | <LOD |
| EPP1N | El Pañe | November | µg/L | - | - | - |
| EPP2N | El Pañe | November | µg/L | 0.46 | 0.11 | 0.14 |
| EPP3N | El Pañe | November | µg/L | 40.60 | 12.3 | <LOD |
| Code | Origin | Coordinates | |
|---|---|---|---|
| GPS Lat. | GPS Long. | ||
| EPP1 | El Pañe | 15°25′07.6″ S | 71°04′01.8″ W |
| EPP2 | El Pañe | 15°25′07.6″ S | 71°04′01.9″ W |
| EPP3 | El Pañe | 15°20′19.6″ S | 71°02′23.8″ W |
| Code | Origin | Month | Analysis | |||
|---|---|---|---|---|---|---|
| Microscopy | Molecular Biology | Chemistry Biomass | Chemistry Water | |||
| EPP1M | El Pañe | May | x | x | x | |
| EPP2M | El Pañe | May | x | x | x | x |
| EPP3M | El Pañe | May | x | x | x | x |
| EPP1N | El Pañe | November | x | |||
| EPP2N | El Pañe | November | x | x | x | x |
| EPP3N | El Pañe | November | x | x | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez Uro, V.H.; Azevedo, J.; Araújo, M.J.; Silva, R.; Bedoya, J.; Paredes, B.; Ranilla, C.; Vasconcelos, V.; Campos, A. New Report of Cyanobacteria and Cyanotoxins in El Pañe Reservoir: A Threat for Water Quality in High-Andean Sources from PERU. Toxins 2024, 16, 378. https://doi.org/10.3390/toxins16090378
Rodriguez Uro VH, Azevedo J, Araújo MJ, Silva R, Bedoya J, Paredes B, Ranilla C, Vasconcelos V, Campos A. New Report of Cyanobacteria and Cyanotoxins in El Pañe Reservoir: A Threat for Water Quality in High-Andean Sources from PERU. Toxins. 2024; 16(9):378. https://doi.org/10.3390/toxins16090378
Chicago/Turabian StyleRodriguez Uro, Victor Hugo, Joana Azevedo, Mário Jorge Araújo, Raquel Silva, Jürgen Bedoya, Betty Paredes, Cesar Ranilla, Vitor Vasconcelos, and Alexandre Campos. 2024. "New Report of Cyanobacteria and Cyanotoxins in El Pañe Reservoir: A Threat for Water Quality in High-Andean Sources from PERU" Toxins 16, no. 9: 378. https://doi.org/10.3390/toxins16090378







