Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2
Abstract
:1. Introduction
2. Results
2.1. Effects of AZA-2 on the Viability and Apoptosis of Caco-2 Cells
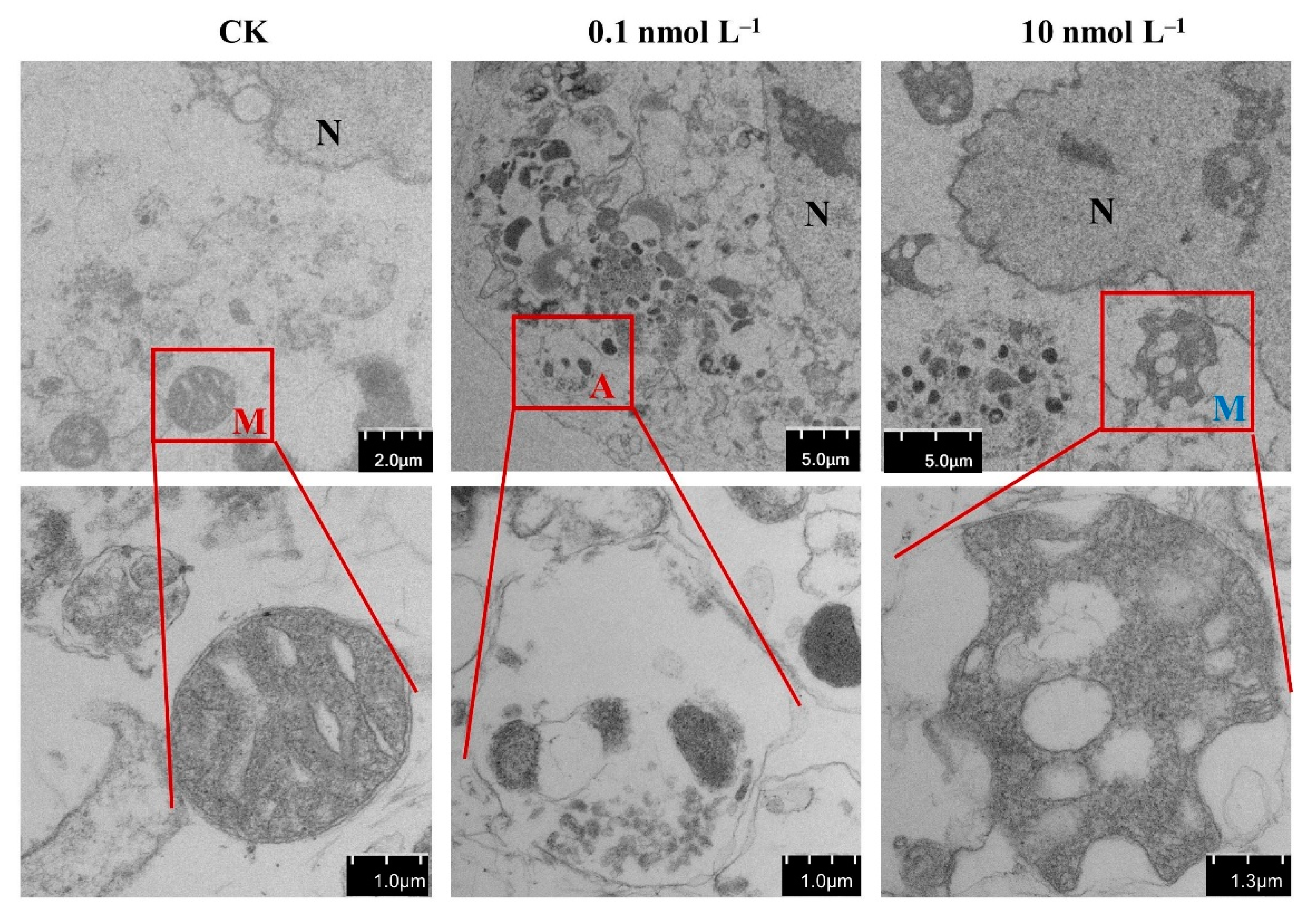
2.2. Effect of AZA-2 on the Mitochondria of Caco-2 Cells
2.3. Oxidative Stress on Caco-2 Cells by AZA-2 Exposure
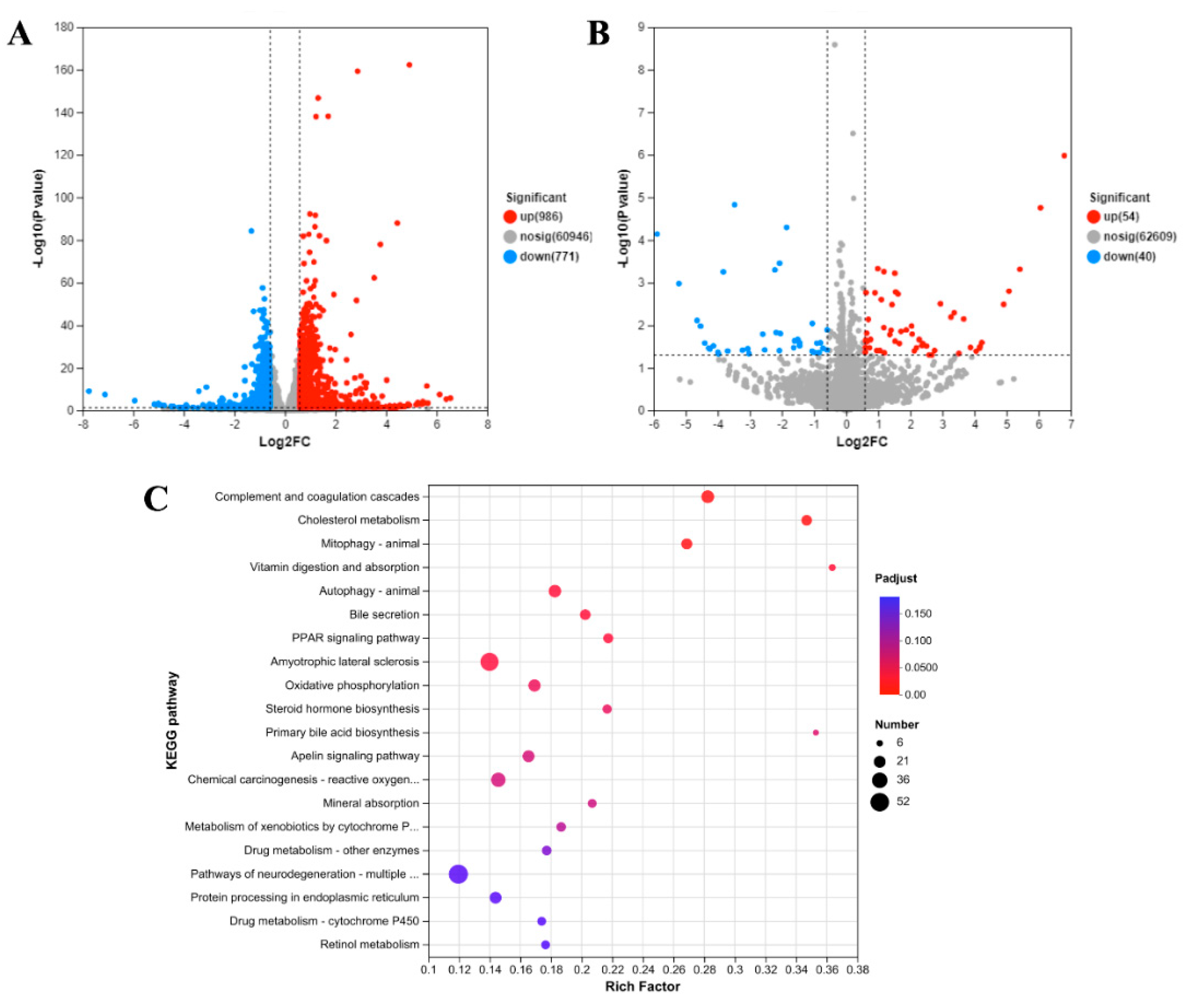
2.4. Transcriptional Response to AZA-2 Exposure
3. Discussion
3.1. Cytotoxicity of AZA-2 on Caco-2 Cells
3.2. Autophagy of Caco-2 Cells Promoted by AZA-2
3.3. Effects of AZA-2 on the Lipid Metabolism of Caco-2 Cells
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Materials
5.2. Cell Culture
5.3. Cell Viability Assay
5.4. Acridine Orange/Propidium Iodide Staining
5.5. Oxidative Stress Test
5.6. Observation by Transmission Electron Microscopy (TEM)
5.7. RNA Extraction, cDNA Library Construction and RNA-Sequencing Analysis
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO/PI | acridine orange/propidium iodide |
| ATF6 | activating transcription factor 6 |
| ATG | autophagy-related protein |
| AZA | azaspiracid |
| cAMP | cyclic adenosine monophosphate |
| cNDA | complementary deoxyribonucleic acid |
| COX | cyclooxygenase |
| C9orf72 | chromosome 9 open reading frame 72 |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DEGs | differential expression genes |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | dimethyl sulfoxide |
| EC50 | median effective concentration |
| ER | endoplasmic reticulum |
| FIP200 | focal adhesion kinase family-interacting protein |
| GPx | glutathione peroxidase |
| IRE1 | Inositol-requiring enzyme 1 |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LC3 | light chains 3 |
| MDA | malondialdehyde |
| NBR1 | neighbor of breast cancer gene 1 |
| NDP52 | nuclear dot protein 52 |
| NF-κB | nuclear factor kappa-B |
| NRU | neutral red |
| OPTN | optineurin |
| PBS | phosphate buffer solution |
| PCR | polymerase chain reaction |
| PE | phosphatidylethanolamine |
| PERK | protein kinase R-like endoplasmic reticulum kinase |
| PINK1 | phosphatase and tensin homolog-induced putative kinase 1 |
| PPAR | peroxisome proliferator-activated receptor |
| RNA-seq | ribonucleic acid sequences |
| ROS | reactive oxygen species |
| RQN | RNA quality number |
| SLC23A1 | solute carrier family 23 member 1 |
| SMCR8 | Smith–Magenis syndrome chromosomal region candidate gene 8 |
| SOD | superoxide dismutase |
| SQSTM1 | sequestosome-1 |
| ULK1 | unc-51-like kinases 1 |
References
- EFSA (European Food Safety Authority). Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission on marine biotoxins in shellfish azaspiracids group. EFSA J. 2008, 723, 1–52. [Google Scholar]
- Luo, Z.; Krock, B.; Mertens, K.N.; Price, A.M.; Turner, R.E.; Rabalais, N.N.; Gu, H. Morphology, molecular phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the Gulf of Mexico. Harmful Algae 2016, 55, 56–65. [Google Scholar] [CrossRef]
- Tillmann, U.; Edvardsen, B.; Krock, B.; Smith, K.F.; Paterson, R.F.; Voss, D. Diversity, distribution, and azaspiracids of Amphidomataceae (Dinophyceae) along the Norwegian coast. Harmful Algae 2018, 80, 15–34. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Krock, B.; Witt, M.; Tillmann, U. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae 2013, 21–22, 64–75. [Google Scholar] [CrossRef]
- Wietkamp, S.; Krock, B.; Gu, H.; Voss, D.; Klemm, K.; Tillmann, U. Occurrence and distribution of Amphidomataceae (Dinophyceae) in Danish coastal waters of the North Sea, the Limfjord and the Kattegat/Belt area. Harmful Algae 2019, 88, 101637. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Tillmann, U.; Tebben, T.; Trefault, N.; Gu, H. Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphidomataceae, (Dinophyceae). Harmful Algae 2019, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qiu, J.; Xie, T.; McCarron, P.; Li, A. Accumulation and transformation of azaspiracids in scallops (Chlamys farreri) and mussels (Mytilus galloprovincialis) fed with Azadinium poporum, and response of antioxidant enzymes. Toxicon 2018, 143, 20–28. [Google Scholar] [CrossRef]
- Aboualaalaa, H.; Leblad, B.R.; Hervé, F.; Hormat-Allah, A.; Baudy, L.; Ennaskhi, I.; Hammi, I.; Ibghi, M.; Elmortaji, H.; Abadie, E.; et al. Development of harmful algal blooms species responsible for lipophilic and amnesic shellfish poisoning intoxications in southwestern Mediterranean coastal waters. Toxicon 2022, 219, 106916. [Google Scholar] [CrossRef]
- Li, J.; Ruan, Y.; Mak, Y.L.; Zhang, X.; Lam, J.C.; Leung, K.M.; Lam, P.K. Occurrence and trophodynamics of marine lipophilic phycotoxins in a subtropical marine food web. Environ. Sci. Technol. 2021, 55, 8829–8838. [Google Scholar] [CrossRef]
- Talić, S.; Škobić, D.; Dedić, A.; Nazlić, N.; Ujević, I.; Ivanković, A.; Pavela-Vrančić, M. The occurrence of lipophilic toxins in shellfish from the Middle Adriatic Sea. Toxicon 2020, 186, 19–25. [Google Scholar] [CrossRef]
- Nicolas, J.; Hoogenboom, R.L.A.P.; Hendriksen, P.J.M.; Bodero, M.; Bovee, T.F.H.; Rietjens, I.M.C.M.; Gerssen, A. Marine biotoxins and associated outbreaks following seafood consumption: Prevention and surveillance in the 21st century. Glob. Food Secur. 2017, 15, 11–21. [Google Scholar] [CrossRef]
- Alfonso, A.; Román, Y.; Vieytes, M.R.; Ofuji, K.; Satake, M.; Yasumoto, T.; Botana, L.M. Azaspiracid-4 inhibits Ca2+ entry by stored operated channels in human T lymphocytes. Biochem. Pharmacol. 2005, 69, 1627–1636. [Google Scholar] [CrossRef]
- Doerr, B.; OHalloran, J.; OBrien, N.; van Pelt, F. Investigation of the genotoxic potential of the marine biotoxins azaspiracid 1–3. Toxicon 2016, 121, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Nicolaou, K.C.; Frederick, M.O.; Vieytes, M.R.; Botana, L.M. Irreversible cytoskeletal disarrangement is independent of caspase activation during in vitro azaspiracid toxicity in human neuroblastoma cells. Biochem. Pharmacol. 2007, 74, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Lepage, K.T.; Frederick, M.O.; Nicolaou, K.C.; Murray, T.F. Involvement of caspase activation in azaspiracid-induced neurotoxicity in neocortical neurons. Toxicol. Sci. 2010, 114, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Hess, P.; Rehmann, N.; Rossini, G.P. Azaspiracid-1 alters the E-cadherin pool in epithelial cells. Toxicol. Sci. 2007, 95, 427–435. [Google Scholar] [CrossRef]
- Twiner, M.J.; Ryan, J.C.; Morey, J.S.; Smith, K.J.; Hammad, S.M.; Van Dolah, F.M.; Hess, P.; Mcmahon, T.; Satake, M.; Yasumoto, T.; et al. Transcriptional profiling and inhibition of cholesterol biosynthesis in human T lymphocyte cells by the marine toxin azaspiracid. Genomics 2008, 91, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Román, Y.; Alfonso, A.; Vieytes, M.R.; Ofuji, K.; Satake, M.; Yasumoto, T.; Botana, L.M. Effects of azaspiracids 2 and 3 on intracellular cAMP, [Ca2+], and pH. Chem. Res. Toxicol. 2004, 17, 1338–1349. [Google Scholar] [CrossRef]
- Twiner, M.J.; El-Ladki, R.; Kilcoyne, J.; Doucette, G.J. Comparative effects of the marine algal toxins azaspiracid-1, -2, and-3 on Jurkat T lymphocyte cells. Chem. Res. Toxicol. 2012, 25, 747–754. [Google Scholar] [CrossRef]
- Pelin, M.; Kilcoyne, J.; Florio, C.; Hess, P.; Tubaro, A.; Sosa, S. Azaspiracids increase mitochondrial dehydrogenases activity in hepatocytes: Involvement of potassium and chloride ions. Mar. Drugs 2019, 17, 276. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, J.; Li, A. Cytotoxicity and intestinal permeability of phycotoxins assessed by the human Caco-2 cell model. Ecotoxicol. Environ. Saf. 2023, 249, 114447. [Google Scholar] [CrossRef]
- Twiner, M.J.; Hanagriff, J.C.; Butler, S.; Madhkoor, A.K.; Doucette, G.J. Induction of apoptosis pathways in several cell lines following exposure to the marine algal toxin azaspiracid. Chem. Res. Toxicol. 2012, 25, 1493–1501. [Google Scholar] [CrossRef]
- Ferron, P.J.; Dumazeau, K.; Beaulieu, J.F.; Le Hegarat, L.; Fessard, V. Combined effects of lipophilic phycotoxins (okadaic acid, azapsiracid-1 and yessotoxin) on human intestinal cells models. Toxins 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Tillmann, U.; Witt, M.; Gu, H. Azaspiracid variability of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae 2014, 36, 22–28. [Google Scholar] [CrossRef]
- Klinge, M.W.; Sutter, N.; Mark, E.B.; Haase, A.M.; Borghammer, P.; Schlageter, V.; Lund, S.; Fleischer, J.; Knudsen, K.; Drewes, A.M.; et al. Gastric emptying time and volume of the small intestine as objective markers in patients with symptoms of diabetic enteropathy. J. Neurogastroenterol. Motil. 2021, 27, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Rambla-Alegre, M.; Braga, A.C.; Maulvault, A.L.; Barbosa, V.; Campàs, M.; Reverté, L.; Caixach, J.; Kilcoyne, J.; Costa, P.R.; et al. Bioaccessibility of lipophilic and hydrophilic marine biotoxins in seafood: An in vitro digestion approach. Food Chem. Toxicol. 2019, 129, 153–161. [Google Scholar] [CrossRef]
- Kudin, A.P.; Bimpong-Buta, N.; Vielhaber, S.; Elger, C.E.; Kunz, W.S. Characterization of superoxide-producing sites in isolated brain mitochondria. J. Biol. Chem. 2004, 279, 4127–4135. [Google Scholar] [CrossRef]
- Kadenbach, B.; Huettemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64–76. [Google Scholar] [CrossRef]
- Ong, G.; Logue, S.E. Unfolding the interactions between endoplasmic reticulum stress and oxidative stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 2018, 63, 18–29. [Google Scholar] [CrossRef]
- Liang, W.; Moyzis, A.G.; Lampert, M.A.; Diao, R.Y.; Najor, R.H.; Gustafsson, Å.B. Aging is associated with a decline in Atg9b mediated autophagosome formation and appearance of enlarged mitochondria in the heart. Aging Cell 2020, 19, e13187. [Google Scholar] [CrossRef]
- Themistokleous, C.; Bagnoli, E.; Parulekar, R.; Muqit, M.M.K. Role of autophagy pathway in Parkinsons disease and related genetic neurological disorders. J. Mol. Biol. 2023, 435, 168144. [Google Scholar] [CrossRef]
- Fakih, R.; Sauve, V.; Gehring, K. Structure of the second phosphoubiquitin-binding site in parkin. J. Biol. Chem. 2022, 298, 102114. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.P.; Smith, E.F.; Bauer, C.S.; Moller, A.; Hautbergue, G.M.; Ferraiuolo, L.; Myszczynska, M.A.; Higginbottom, A.; Walsh, M.J.; Whitworth, A.J.; et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. Embo J. 2016, 35, 1656–1676. [Google Scholar] [CrossRef]
- Levine, T.P.; Daniels, R.D.; Gatta, A.T.; Wong, L.H.; Hayes, M.J. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics 2013, 29, 499–503. [Google Scholar] [CrossRef]
- Zhang, D.; Iyer, L.M.; He, F.; Aravind, L. Discovery of novel DENN proteins: Implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front. Genet. 2012, 3, 283. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tyndall, E.R.; Bui, V.; Bewley, M.C.; Wang, G.; Hong, X.; Shen, Y.; Flanagan, J.M.; Wang, H.; Tian, F. Multifaceted membrane interactions of human Atg3 promote LC3-phosphatidylethanolamine conjugation during autophagy. Nat. Commun. 2023, 14, 5503. [Google Scholar] [CrossRef]
- Zhang, S.; Yazaki, E.; Sakamoto, H.; Yamamoto, H.; Mizushima, N. Evolutionary diversification of the autophagy-related ubiquitin-like conjugation systems. Autophagy 2022, 18, 2969–2984. [Google Scholar] [CrossRef] [PubMed]
- Sakoh-Nakatogawa, M.; Matoba, K.; Asai, E.; Kirisako, H.; Ishii, J.; Noda, N.N.; Inagaki, F.; Nakatogawa, H.; Ohsumi, Y. Atg12-Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nat. Struct. Mol. Biol. 2013, 20, 433–439. [Google Scholar] [CrossRef]
- Qian, M.; Fang, X.; Wang, X. Autophagy and inflammation. J. Clin. Med. 2017, 6, 24. [Google Scholar] [CrossRef]
- Kaufmann, A.; Beier, V.; Franquelim, H.G.; Wollert, T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell 2014, 156, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B 2005, 827, 65–75. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Subramenium, G.A.; Sabui, S.; Marchant, J.S.; Said, H.M.; Subramanian, V.S. Enterotoxigenic escherichia coli heat labile enterotoxin inhibits intestinal ascorbic acid uptake via a camp-dependent NF-κB-mediated pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G55–G63. [Google Scholar] [CrossRef]
- Kojo, S. Vitamin C: Basic metabolism and its function as an index of oxidative stress. Curr. Med. Chem. 2004, 11, 1041–1064. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Bruene, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Qiu, J.; Zhang, J.; Li, A.; Wang, G. Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2. Toxins 2024, 16, 381. https://doi.org/10.3390/toxins16090381
Zhao L, Qiu J, Zhang J, Li A, Wang G. Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2. Toxins. 2024; 16(9):381. https://doi.org/10.3390/toxins16090381
Chicago/Turabian StyleZhao, Liye, Jiangbing Qiu, Jingrui Zhang, Aifeng Li, and Guixiang Wang. 2024. "Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2" Toxins 16, no. 9: 381. https://doi.org/10.3390/toxins16090381




