Abstract
Recently, jellyfish venom has gained attention as a promising reservoir of pharmacologically active compounds, with potential applications in new drug development. In this investigation, novel peptides, isolated from the hydrolysates of Nemopilema nomurai jellyfish venom (NnV), demonstrate potent inhibitory activities against angiotensin-converting enzyme (ACE). Proteolytic enzymes—specifically, papain and protamex—were utilized for the hydrolysis under optimized enzymatic conditions, determined by assessing the degree of hydrolysis through the ninhydrin test. Comparative analyses revealed that papain treatment exhibited a notably higher degree of NnV hydrolysis compared to protamex treatment. ACE inhibitory activity was quantified using ACE kit-WST, indicating a substantial inhibitory effect of 76.31% for the papain-digested NnV crude hydrolysate, which was validated by captopril as a positive control. The separation of the NnV-hydrolysate using DEAE sepharose weak-anion-exchange chromatography revealed nine peaks under a 0–1 M NaCl stepwise gradient, with peak no. 3 displaying the highest ACE inhibition of 96%. The further purification of peak no. 3 through ODS-C18 column reverse-phase high-performance liquid chromatography resulted in five sub-peaks (3.1, 3.2, 3.3, 3.4, and 3.5), among which 3.2 exhibited the most significant inhibitory activity of 95.74%. The subsequent analysis of the active peak (3.2) using MALDI–TOF/MS identified two peptides with distinct molecular weights of 896.48 and 1227.651. The peptide sequence determined by MS/MS analysis revealed them as IVGRPLANG and IGDEPRHQYL. The docking studies of the two ACE-inhibitory peptides for ACE molecule demonstrated a binding affinity of −51.4 ± 2.5 and −62.3 ± 3.3 using the HADDOCK scoring function.
Keywords:
Neophilia nomurai; papain enzyme hydrolysate; chromatography; angiotensin-converting enzyme (ACE) inhibitor; peptide identification Key Contribution:
The isolation of potent ACE-inhibitory peptides from Nemopilema nomurai jellyfish venom indicates its potential for drug development. The utilization of proteolytic enzymes for hydrolysis, alongside thorough chromatographic purification, led to the identification of highly active peptide fractions, demonstrating promising inhibitory effects against ACE.
1. Introduction
Animal venoms are the intricate blends of various pharmacologically active elements, encompassing proteins, peptides, and enzymes with specific biological functions related to as-yet-unidentified substances [1,2]. Based on a report in 2020 [3], there are several drugs that have been identified from animal toxins, such as captopril, enalapril (ACE inhibitors; Jararaca pit viper; US drug administration 2020), exenatide (type 2 diabetes mellitus; Gila monster lizard; US drug administration 2020), Tirofiban (acute coronary syndrome; saw-scaled viper; US drug administration 2020), etc.
Hypertension (BP) refers to significant risk factors that contribute to cardiovascular disease (CVD) [4]. It is a worldwide health issue, affecting over 1.3 billion people with elevated blood pressure across the globe [5]. Aiming for one of the global targets on non-communicable diseases (NCDs) set by the World Health Assembly in 2013, there is a goal to reduce the prevalence of elevated blood pressure by 25% by 2025, as compared to its level in 2010. According to self-reported data from a survey on hypertension prevalence involving 533,306 adults, eradicating hypertension in women would reduce population mortality by approximately 7.3% compared with 0.1% for hyperlipidemia, 4.1% for diabetes, 4.4% for cigarette smoking, and 1.7% for obesity. Conversely, in men, eradicating hypertension would decrease population mortality by approximately 3.8%, compared to 2.0% for hyperlipidemia, 1.7% for diabetes, 5.1% for cigarette smoking, and 2.6% for obesity [6]. Elevated blood pressure is characterized by a systolic blood pressure (SBP) of ≥140 mmHg or diastolic blood pressure (DBP) of ≥90 mmHg [7,8]. The first-line treatments for hypertension consist of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), loop and thiazide diuretics, and dihydropyridine calcium channel blockers (CCBs) [9]. Among these, ACE inhibitors constitute a significant class of drugs. ACE inhibitors are prescribed for uncomplicated hypertension and are also recommended for conditions such as hypertension with concurrent CAD (including post-myocardial infarction), chronic kidney disease (CKD), type 2 diabetes, heart failure with reduced ejection fraction, or atrial fibrillation [10].
Jellyfish have thrived in areas where numerous other species have faced decline. The adaptations of these species, which have been around for 500 million years, are so effective that they now significantly influence human activities. Nematocyte, a unique type of cell found in jellyfish, possesses stinging capabilities and is utilized for defense, prey capture, and locomotion. These specialized cells consist of a thread-like aperture that resembles an anchor, serving as a reservoir for jellyfish venom. Jellyfish venom causes swelling, which leads to a rapid heart rate, difficulty in respiration, severe-to-mild back pain, and brain hemorrhages [11]. On the other hand, jellyfish venom also possesses many pharmacological activities in addition to its toxic effects. For example, Cyanea capillata—anti-arrhythmic activity [12]; Chiropsalmus quadrigatus—anti-hypertensive activity [13]; Rhopilema esculentum—immunomodulatory activity [14]; Chrysaora quinquecirrha [15]—anti-inflammatory, anti-microbial, and analgesic activity; Pelagia noctiluca—anti-inflammatory activity [16]; and Carybdea marsupialis—anti-microbial activity [17].
Nemopilema nomurai, a rhizostome jellyfish within the phylum Cnidaria, holds the distinction of being one of the world’s largest jellyfish, featuring a bell diameter of 2 m and a body weight reaching up to 200 kg [18]. In recent times, there has been a proliferation of jellyfish species, including N. nomurai, in the coastal waters of East Asia [19]. NnV harbors diverse pharmacological characteristics such as anti-inflammatory and anti-cancer properties [20,21,22,23]. Previously, it has been reported that whole Nemopilema nomurai jellyfish proteolytic hydrolysate shows ACE-inhibitory activity [24]. Therefore, in this study, we specifically aim for the identification of ACE-inhibitory peptides from Nemopilema nomurai jellyfish venom (NnV) using papain hydrolysate.
2. Results
2.1. Optimization of Enzymatic Condition upon NnV Using the Degree of Hydrolysis
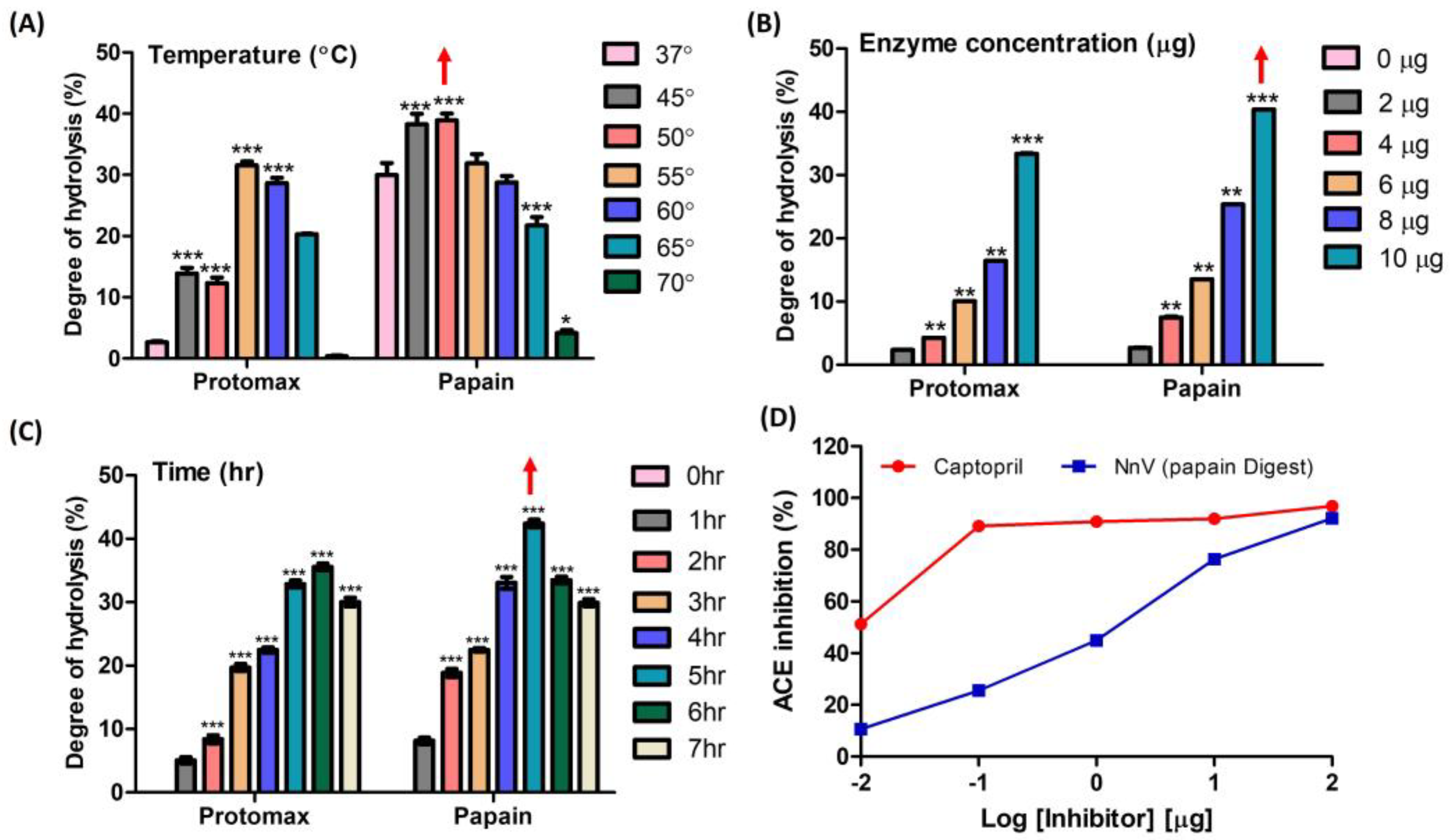
Temperature, reaction time, and concentration play pivotal roles in the enzymatic hydrolysis of NnV. From ninhydrin colorimetry assay, the optimal conditions for papain and protomex were determined as follows: a temperature range of 45–50 °C (papain) and 55–60 °C (protomex), a concentration of 10 µg, and a reaction time of 5–6 h. These conditions yielded the highest degrees of hydrolysis, with values of 31.54%, 33.35% and 35.57% for protomex, and 38.8%, 40.37% and 42.4% for papain (Figure 1A–C). In Figure 1D, the wide range of hydrolysate concentrations were tested for their ACE-inhibitory activities to determine the optimum concentration for further study. For this, 100, 10, 1, 0.1, and 0.01 µg/20 µL of NnV–papain hydrolysate were examined to assess ACE-inhibitory activity. Among these, 10 µg/20 µL was chosen for further study, which shows a concentration-dependent inhibition in ACE assay (76.31% of maximum inhibitory effect) without reaching saturation range. Therefore, we considered a 10 µg/20 µL concentration of the hydrolysate as optimum for further investigation. The positive control, captopril, exhibited a 91.92% inhibition under the same conditions (Figure 1D).
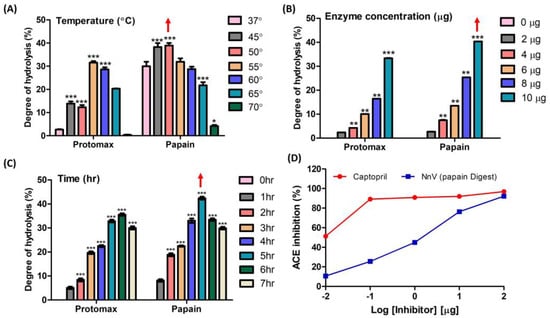
Figure 1.
Degree of enzyme hydrolysis upon NnV based on the Ninhydrin test and preliminary ACE inhibition. (A) Effect of different temperatures of enzyme incubation upon NnV; (B) Effect of enzyme concentrations on NnV; (C) Effect of reaction time of enzymes upon NnV; (D) Log-inhibitory curve of the percent ACE inhibition of papain-hydrolyzed NnV (positive control: captopril). Red arrow denotes highest hydrolysis condition. Results are expressed as mean ± standard deviation (SD), with significance denoted by * p < 0.05, ** p < 0.01, and *** p < 0.001.
2.2. Purification of ACE-Inhibitory Peptides Guided by In Vitro Assay
2.2.1. Ion-Exchange Chromatography
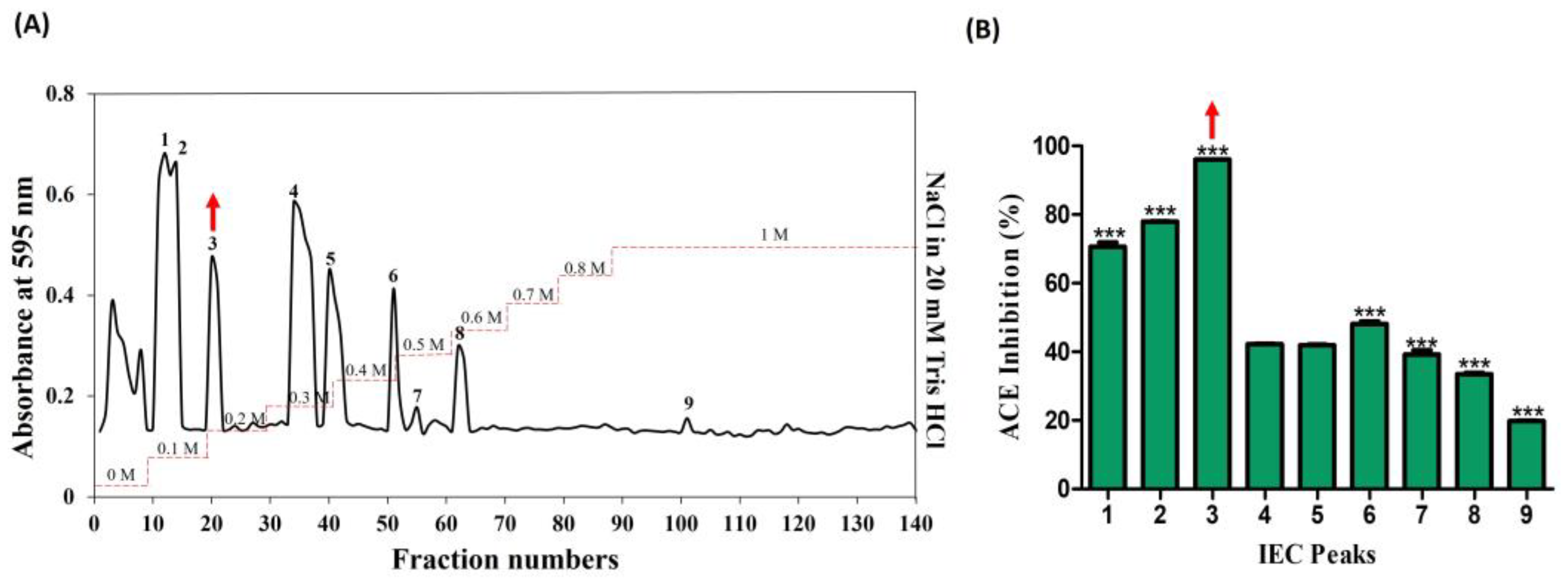
The venom from jellyfish, subjected to papain digestion under optimal conditions (50 °C; 5 h; 10 µg), was freeze-dried and reconstituted in a 20 mM Tris HCl solution (equilibrium buffer) for subsequent sequential chromatography. In the preliminary stage, ion-exchange chromatography revealed the presence of nine peaks, predominantly eluting within the range of 0–1 M NaCl in 20 mM Tris HCl (Figure 2A). The peaks were collected and lyophilized, and protein concentration was determined by protein assay. All the peaks were normalized and their ACE-inhibitory (%) activities corresponding to these nine peaks were determined as follows: 70.64, 77.69, 96.0, 42.21, 41.87, 48.14, 39.21, 33.48, and 19.75. Notably, among these, peak no. 3 demonstrated the highest activity, at 96% (Figure 2B).
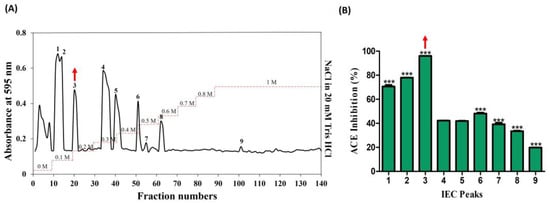
Figure 2.
Purification of ACE-inhibitory fractions using DEAE sepharose anion-exchange chromatography. (A) Chromatogram of fractions that were isolated from hydrolyzed crude NnV. (B) ACE-inhibitory activity of separated fractions. The red arrows denote the active peak. Results are expressed as mean ± standard deviation (SD), with significance denoted by *** p < 0.001. The numbers 1–9 in (A) denote isolated peaks.
2.2.2. Reverse-Phase High-Pressure Liquid Chromatography
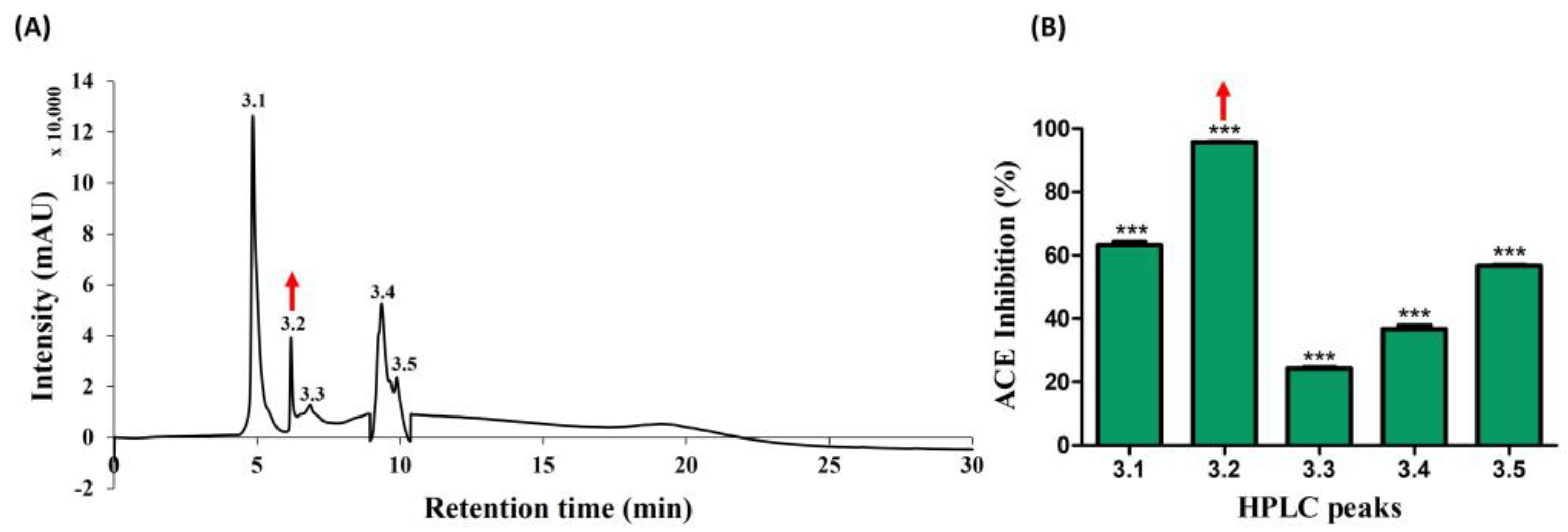
Subsequently, the active candidate (peak no. 3) underwent further purification through reverse-phase high-pressure liquid chromatography (RP-HPLC), employing an ODS-C18 column. Five distinct peaks (3.1, 3.2, 3.3, 3.4, and 3.5) were successfully isolated, as illustrated in Figure 3A, with retention times falling within the range of 5–10 min. Later, peaks were collected using a fraction collector, acetonitrile was removed using a decompression concentrator, and the ACE-inhibitory activities (%) were assessed based on protein concentration. The respective activities of the five peaks ranged from 20% to 96%, with values determined as follows: 65.19, 95.74, 24.57, 37.87, and 56.50 (shown in Figure 3B). A noteworthy observation was exhibited in peak no. 3.2 with 95.74%, mirroring the previously identified active peak. As a result, this potent candidate was chosen for further analysis using MALDI–TOF/MS.
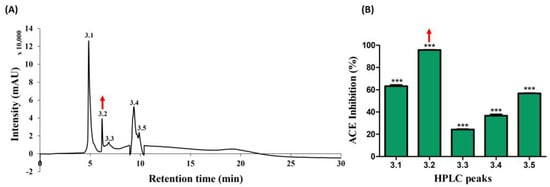
Figure 3.
Further separation of ACE-inhibitory fractions using RP-HPLC. (A) Chromatogram of sub peaks from active peak no. 3 isolated (B) ACE-inhibitory activity. The red arrow marks denote the active peak. Results were expressed as mean ± standard deviation (SD), with significance denoted by *** p < 0.001. The numbers 3.1–3.5 in (A) denotes isolated peaks.
2.3. MALDI–TOF/MS Examination
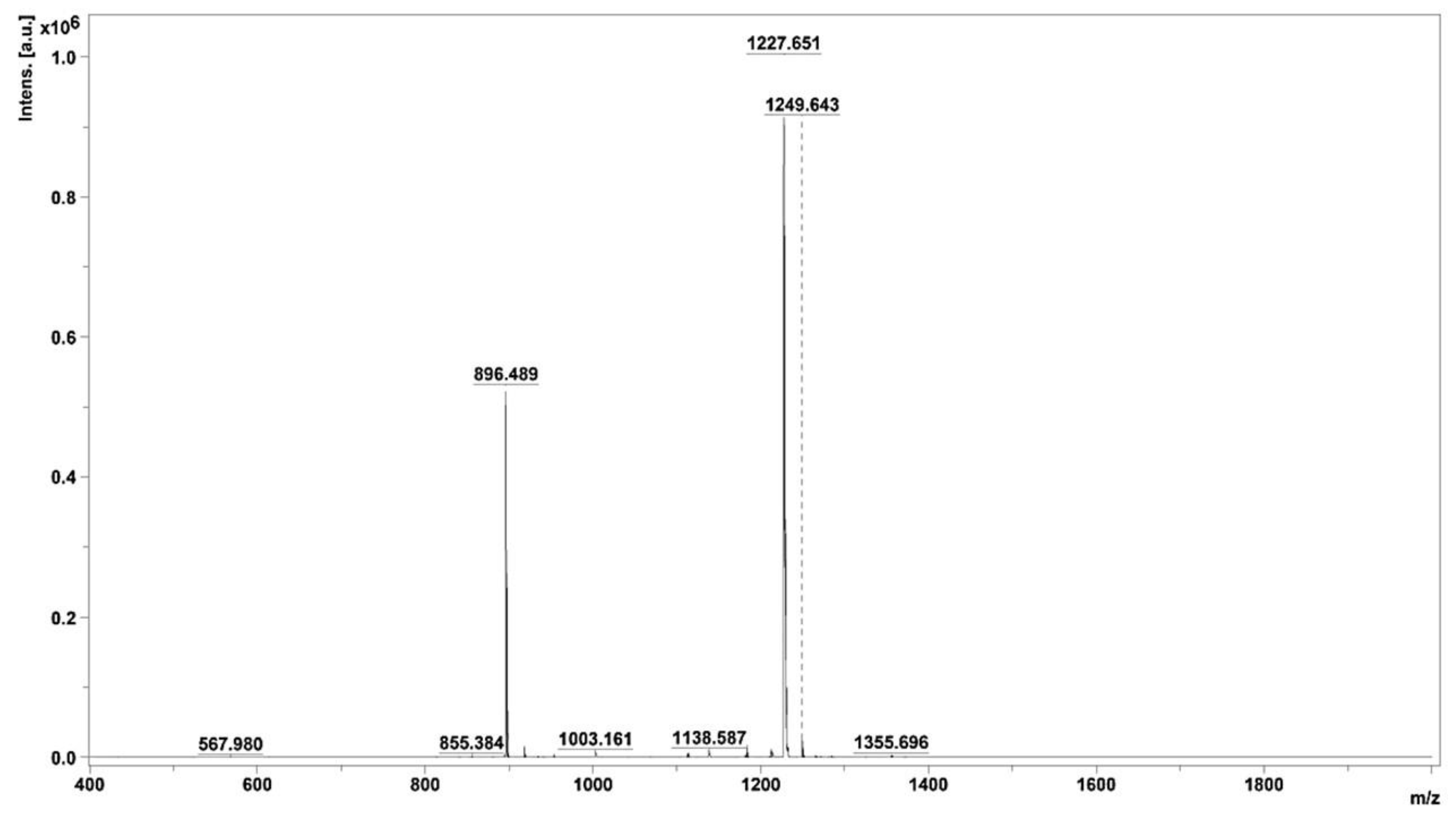
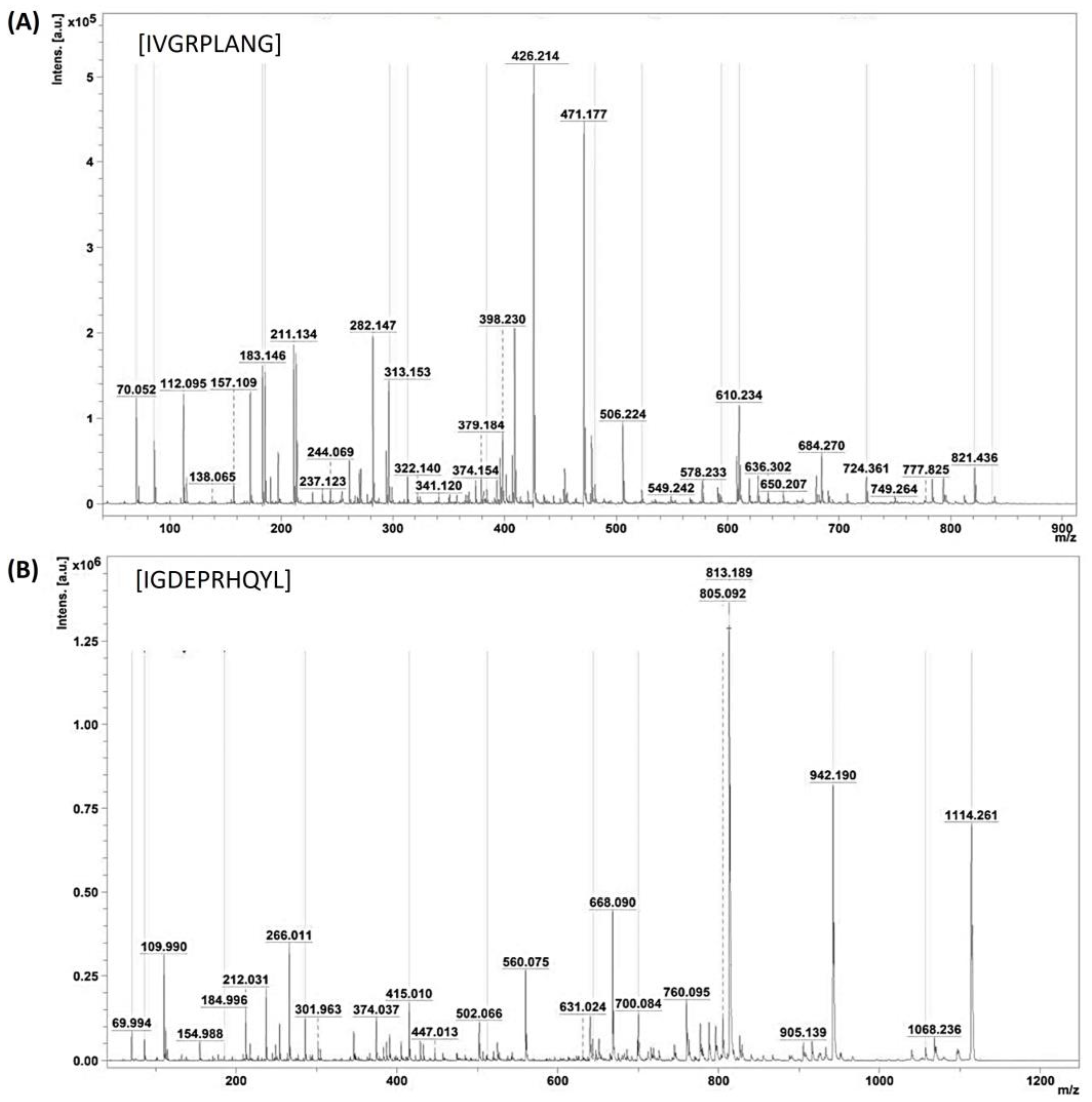
Peak 3.2, displaying noteworthy activity, underwent comprehensive analysis utilizing MALDI–TOF/MS, thereby elucidating the identification of two distinct peptides characterized by varying molecular sizes. The average molecular masses of these identified peptides were meticulously determined, resulting in values of 896.489 and 1227.651, as visually represented in Figure 4. This analytical insight provides crucial information regarding the molecular composition of the peptides residing within active peak 3.2.
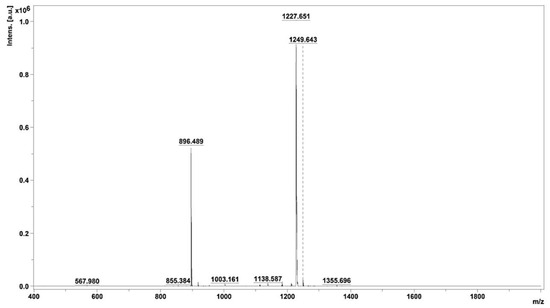
Figure 4.
Mass spectrum of fraction 3.2, obtained using MALDI–TOF/MS.
2.4. ACE-Inhibitory Peptides Sequencing Using MS/MS
Following the identification of the two peptides within active peak 3.2—namely, those with average molecular masses of 896.489 and 1227.651—an in-depth exploration of their amino acid sequences was undertaken. This involved a thorough analysis of the MS/MS ionic patterns, as illustrated in Figure 5A,B, utilizing the advanced capabilities of Brucker Flex analysis version 3.4 software. The intricacies of the peptides’ structural compositions, denoted as IVGRPLANG and IGDEPRHQYL, were thereby elucidated through the intricate patterns discerned during the MS/MS analysis. It is noteworthy that the laser conditions employed for this analytical procedure were meticulously set at 45% laser intensity, with each peptide subjected to 1000 shots for optimal resolution and accuracy.

Figure 5.
Identification of ACE-inhibitory peptide sequence. (A,B) MS/MS peaks of identified peptides IVGRPLANG and IGDEPRHQYL from NnV-hydrolysate.
2.5. Peptide Structure Prediction and Protein–Ligand Interactions through a Computational Approach
AlphaFold generates five peptide structures for a single model. In this, the top-ranked structure was selected based on both the pLDDT and pTM score. The three-dimensional structures of two peptides were modeled using the AlphaFold2 Colab notebook, which is depicted in Figures S1 and S2. The pTM metric, ranging from 0 to 1, serves to assess peptide structure predictions by providing a 3D error measurement. Additionally, the per-residue confidence score is determined through the predicted local distance difference test (pLDDT) score, which spans from 0 to 100. Scores exceeding 90 indicate a high confidence level, while scores below 50 indicate a low confidence level. For the IGDEPRHQYL peptide structure, the pLDDT and pTM scores of the top-ranked model were 72.9 and 0.0461, respectively. Similarly, for the IVGRPLANG peptide structure, the corresponding scores for the top-ranked model were 73.8 and 0.04, respectively. The analysis of the pLDDT results indicates a low confidence in the linker region, suggesting potential flexibility in this region. Detailed information on pLDDT and pTM score are illustrated in Table S1.
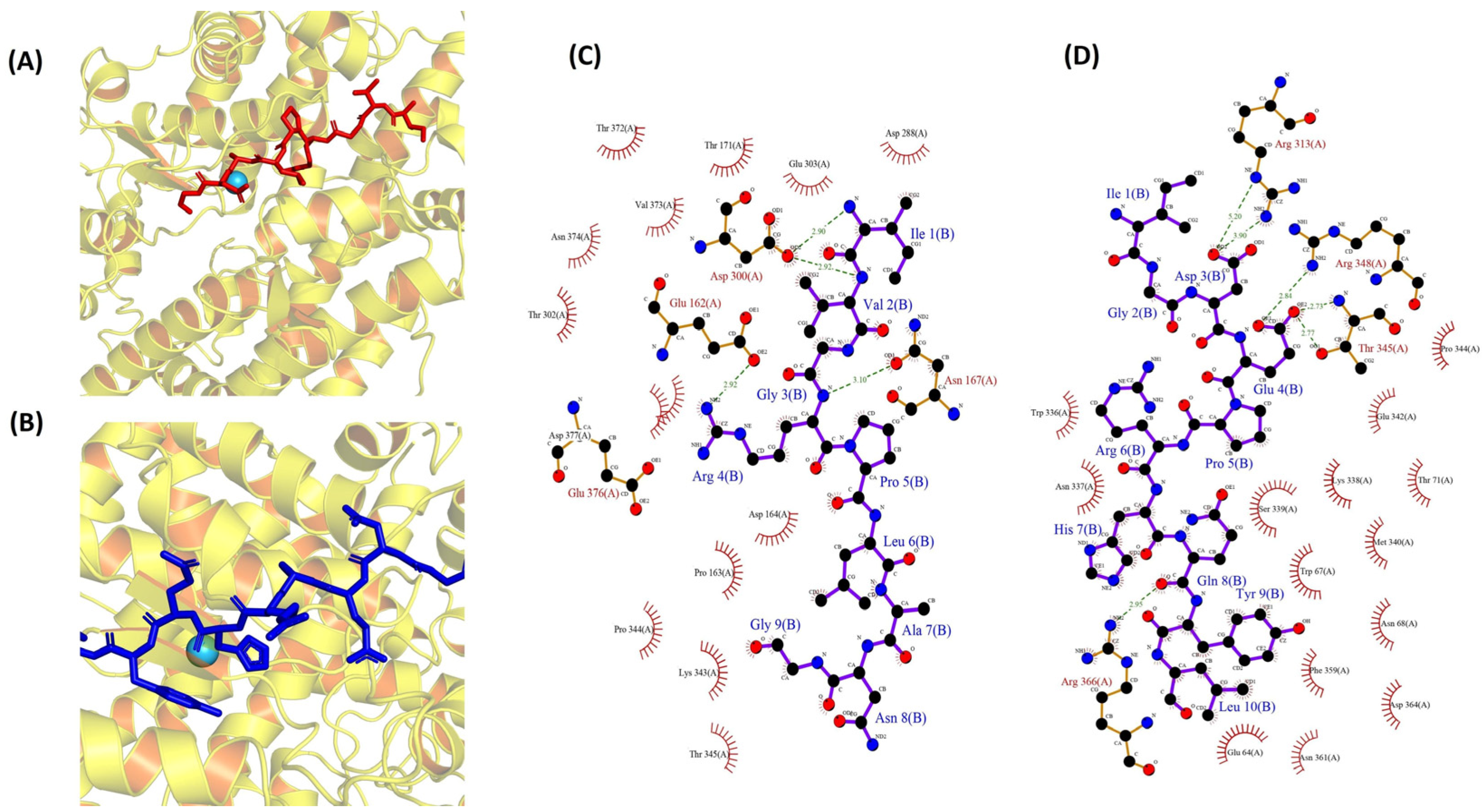
To investigate the molecular interactions between two peptides and ACE, a docking simulation was conducted, utilizing the flexible docking tool within the HADDOCK software (version 2.4). The docking investigation, involving the peptides IVGRPLANG and IGDEPRHQYL, revealed optimal poses (Figure 6A,B) with HADDOCK scores of −51.4 ± 2.5 (cluster 5) and −62.3 ± 3.3 (cluster 7), respectively. Detailed information regarding other interactions, along with their scores, is presented in Tables S2 and S3. In our study, the bonded interactions for IVGRPLANG against the ACE inhibitor involve GLU 162, ASN 167, ASP 300, ASP 300, ARG 4, ARG 4, ILE 1, and VAL 2, highlighted in red. Meanwhile, the non-bonded interactions include GLU 162, PRO 163, ASP 164, ASP 300, ASP 300, LYS 343, PRO 344, and THR 302, denoted by the black color in Figure 6C. The bonded interactions for IGDEPRHQYL against the ACE inhibitor encompass ARG 313, ARG 313, THR 345, THR 345, ARG 348, ARG 366, ASP 3, ASP 3, GLU 4, GLU 4, and GLN 8, highlighted in red. Conversely, the non-bonded interactions consist of GLU 64, TYR 9, LEU 10, TRP 67, LYS 338, SER 339, PRO 344, GLU 4, HIS 7, ARG 366, and MET 340, denoted by the black color in Figure 6D. The pivotal role of the Zn(II) ion in the mechanism of ACE inhibition necessitated the validation of its positioning through ligplot analysis. Following peptide docking, notable alterations in the position, bond lengths, and hydrogen bonds associated with the Zn(II) ion within the ACE protein molecule were observed, as illustrated in Figure S3.
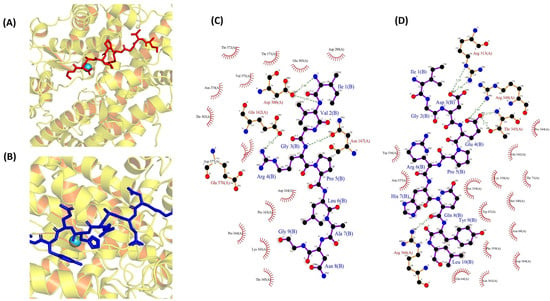
Figure 6.
Molecular docking simulations of ACE (PDB: 1O8A) protein against the isolated peptides. (A,B) Depicting the optimal docking poses at the active site for IVGRPLANG (red) and IGDEPRHQYL (blue). (C,D) The peptides’ (IVGRPLANG and IGDEPRHQYL) interactions with ACE protein residues.
3. Discussion
Numerous studies have been undertaken to investigate the pharmacological characteristics of jellyfish venom [25,26,27,28]. The angiotensin-I-converting enzyme (ACE; EC 3.4.15.1) is considered as one of the essential members in the renin–angiotensin system, playing a crucial physiological role in the regulation of blood pressure [29]. One study has identified that the whole Nemopilema nomurai jellyfish has ACE-inhibitory activity, yet it did not identify the key compound(s) from the jellyfish venom [24]. In addition, the ACE-inhibitory peptides were isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom hydrolysate [13]. In the present study, we have successfully identified two peptides from Nemopilema nomurai jellyfish venom hydrolysate that demonstrate highly significant ACE-inhibitory activity. Therefore, we propose that these isolated peptides have the possibility to be used as candidate molecules for the development of ACE-inhibitor drugs like captopril and enalapril
Various proteases produce polypeptides with distinct compositions and sizes. Enzymatic hydrolysis stands out as the most efficient method for generating bioactive peptides [30,31]. Our results show that the NnV underwent enzyme hydrolysis utilizing two enzymes, protomex and papain. From these, papain was selected as the optimal enzyme based on the degree of hydrolysis. Subsequently, a log-inhibitory analysis was conducted to demonstrate the ACE inhibition of NnV–papain hydrolysate, with captopril utilized as a positive control. The chromatographic purification of proteins is influenced by various factors such as pH, elution volume, gradient characteristics, and ionic strength. The out-standing flow properties of these ion exchangers position them as the primary choice for separating crude protein mixtures during the initial stages of the purification process [32,33]. Peptides with lower molecular weights generally exhibit higher activity compared to those with higher molecular weights, aligning with previous research findings [34,35]. It is postulated that short-chain peptides can adopt a spatial conformation, enabling them to align within the three-dimensional structure of ACE, thereby restricting access for high-molecular-weight peptides [36]. The purification of ACE-inhibitory peptides can be achieved based on their molecular weight, charge, affinity, and polarity [37,38,39]. Various purification methods, including size exclusion chromatography (utilizing Sephadex, Sepharose, Superdex, etc.), ion-exchange chromatography (employing DEAE-cellulose, DE-AE-Sephadex, etc.), and RP-HPLC (utilizing C18, and other columns), are commonly employed, based on the specific characteristics of the peptides. In our study, the enzyme digest NnV-hydrolysate comprised a complex mixture of peptides; it underwent initial separation through ion-exchange chromatography to isolate peptides according to their charges. After the sequential chromatographic separation and ACE-inhibitory evaluation, peak no. 3.2 was identified as a potent candidate, and two peptides were identified using MALDI–TOF/MS. The overall method demonstrated efficient separation, confirming its effectiveness in isolating ACE inhibitors. Hydrophobicity plays a crucial role in facilitating peptide binding to the hydrophobic active site of ACE, consequently enhancing inhibitory activity. Moreover, hydrophobic peptides composed of four to nine amino acids have been observed to passively traverse cell membranes through mechanisms like transcytosis or paracellular diffusion [40,41,42,43]. Thus, applying this hypothesis, the two isolated peptides (IVGRPLANG and IGDEPRHQYL) would have possibly been absorbed, enabling their passage across the intestinal barrier and entry into systemic circulation. The presence of a high concentration of aromatic amino acids and hydrophobic aliphatic amino acids such as isoleucine, leucine, alanine, methionine, and proline are noteworthy, as previous studies [44,45] suggest that these amino acids may enhance ACE-inhibitory activity.
The two identified peptides’ sequences were modeled and docked against an ACE protein molecule, and values were obtained using the HADDOCK score. Hydrogen bond interactions are indispensable for stabilizing the structure of the enzyme–substrate complex, thereby playing a crucial role in facilitating the ACE-catalyzed reaction. Based on the molecular docking, the strong hydrogen interaction suggests that the peptides would effectively inhibit the ACE protein. Despite the absence of a direct interaction be-tween either IVGRPLANG or IGDEPRHQYL and Zn(II), post-docking observations indicate alterations in bond lengths and hydrogen bond formations from their initial states. This outcome implies the potential of the peptides to coordinate with Zn(II) within ACE, potentially distorting its tetrahedral geometry and influencing ACE-inhibitory activities. This observation aligns with other findings reported by Mirzaei et al. (2018) [46] and Wu et al. (2018) [47]. However, this study serves as the primary investigation into drug identification. In future endeavors, we will synthesize the peptides and employ animal models to validate the efficacy of the peptides for further exploration
4. Conclusions
In summary, we have identified the angiotensin-converting enzyme inhibitors from Nemopilema nomurai jellyfish venom. The major contributions of this study include (i) the papain hydrolysate of NnV showed higher ACE activity; (ii) peak no. 3.2, isolated through sequential chromatography techniques, displayed similar significant ACE activity; (iii) two peptide sequences, IVGRPLANG (896.48) and IGDEPRHQYL (1227.651), have been obtained; (iv) the molecular docking analysis confirms that the binding affinities of the two peptides against ACE molecules are −51.4 ± 2.5 and −62.3 ± 3.3 using the HADDOCK scoring function. Therefore, these findings highlight the pharmacological significance of jellyfish venom-derived compounds for future therapeutic exploration.
5. Materials and Methods
5.1. Jellyfish Collection and Preparation
Samples of Nemopilema nomurai jellyfish were gathered from the Yellow Sea near the Gunsan coast in South Korea. After separating the tentacles, they were promptly placed on ice for further processing. The dissected tentacles underwent cleansing with cold (4 °C) seawater to remove any debris, and the samples were combined with three volumes (v/v) of chilled fresh seawater. Subsequently, the tentacle-free saltwater was collected and centrifuged at 1000× g for 5 min, following 24 h of agitation on a shaker at 4 °C. The resulting nematocyst-rich pellet underwent triple rinsing with fresh seawater. The residual sedimented tentacles underwent additional autolysis overnight at 4 °C, repeating this process for four days, and the nematocyst-rich pellets were once again rinsed with fresh seawater. Ultimately, the nematocyst sample was centrifuged at 500× g for 5 min. The resulting pellet (nematocyst) was then freeze-dried and stored at −20 °C for further investigation [48].
5.2. Venom Extraction and Preparation
The venom was derived and processed from freeze-dried nematocysts with slight modifications [49]. In summary, venom extraction involved the use of 1 g of nematocyst powder, glass beads (approximately 8000 beads; 0.5 mm in diameter), and 1 mL of ice-cold phosphate-buffered saline (PBS, 0.137 M NaCl, 0.0027 M KCl, 0.01 M Na2HPO4, 0.0018 M KH2PO4, pH 7.4) as an extraction buffer. The extraction was carried out through robust vortexing on the mini bead mill at 3000 rpm for 30 s, with this step being repeated more than 10 times at regular intervals while cooling on ice. Subsequently, the venom extract underwent centrifugation for 30 min at 4 °C at 22,000× g. The resulting final supernatant was designated as NnV for this study. Protein concentration in the venom was determined using the Bradford test [50] (Bio-Rad, Hercules, CA, USA). Finally, the venom, adjusted according to its protein concentration, was employed for subsequent research. Later, the NnV was employed for dialysis (Fisher brand dialysis tubing with 15 m roll; 25 mm width; 1.98 mL volume; 20 µm thickness; 15.9 mm dry ᴓ; MWCO 12–14 kD), to remove excessive salt for further study.
5.3. Enzymatic Hydrolysis of NnV Extract and Assessment of the Degree of Hydrolysis
The hydrolysis of NnV was conducted using protomex and papain, following a slightly modified procedure. The enzyme–substrate reactions were performed with a ratio of 1:100. The reaction conditions, for both papain and protomex, were a pH of 6.8, temperature ranging from 37 to 70 °C, reaction time spanning 0 to 6 h, and concentration range of 0 to 10 µg. To stop the reactions, heat treatment was applied at 90 °C for 15 min. The resulting slurries underwent centrifugation at 3000× g for 10 min, and the supernatants were utilized as hydrolysates for subsequent analysis. The Ninhydrin colorimetric method, with slight modifications, was employed to determine the degree of hydrolysis (DH), as described by Huang et al. (2022) [51]. The detection procedure involved taking 0.5 mL of the hydrolysate supernatant post-centrifugation (3000× g for 10 min) and combining it with 0.4 mL of sodium phosphate buffer (pH 8.0, 2 M) and 0.4 mL of ninhydrin (2%). Then, the mixture was placed in a 100 °C water bath for 15 min, followed by dilution with distilled water to a total volume of 50 mL after cooling to room temperature. The solution was then allowed to stand for 15 min, and its absorbance was measured at a wavelength of 570 nm. The following equation was used to calculate the degree of hydrolysis:
DH (%) = (Hsample − Hblank)/(Htotal − Hblank) × 100
In this context, Hblank denotes the absorbance measured with distilled water as the blank. Htotal represents the absorbance recorded following thorough acid hydrolysis, using 6 M HCl at 120 °C for 24 h.
5.4. Assessment of ACE-Inhibitory Activity
The ACE-inhibitory activity was assessed using the ACE kit-WST from Dojindo Laboratories [52], following the assay procedure outlined in the provided technical manual. In summary, a 20-microliter sample solution was introduced to a sample well, as well as to blank 1 and blank 2 wells. Subsequently, 20 microliters of substrate buffer were added to each well. Deionized water was incorporated into the blank 2 well, while 20 mL of enzyme working solution was added to each sample well and blank 1 well. Incubation was carried out at 37 °C for 1 h. Following this, 200 mL of the indicator working solution were introduced to each well, followed by an additional 10 min incubation at room temperature before measuring absorbance at 450 nm using a microplate reader.
Here, Ablank1 represents the absorbance of the control (without ACE inhibition), Ablank2 corresponds to the absorbance of the reagent alone, and Asample denotes the absorbance of the sample.
ACE inhibition (%) = (Ablank1 − Asample)/(Ablank1 − Ablank2) × 100
5.5. Purification of ACE-Inhibitory Peptides by Sequential Chromatography
The NnV–papain hydrolysate was subjected to ion-exchange chromatography with minor modification, as described by Prakash et al. (2020) [53]. In brief, NnV-hydrolysate was injected into the DEAE Sepharose fast-flow column (1 × 30 cm) (Cytiva), where 20 mM Tris HCl was used as an equilibrium buffer (at pH 6.8). NaCl (0–1 M) in 20 mM Tris HCl buffer was used as an elution buffer at a 0.6 mL/min flow rate by the discontinuous gradient method, and nine peaks were obtained. Each peak’s protein concentration was evaluated using the Bradford Assay. Subsequently, the combined peaks underwent testing for ACE-inhibitory activity, and the most active peak was chosen for further analysis. Peak no. 3 was then subjected to separation using RP-HPLC [54]. The sample was dissolved in 2 mL chromatography water and injected into the ODS-C18 (10 × 250 mm, particle size 5 µm) column. The parameters for elution were 1 mL/min flow, gradient of 0.1% TFA in water (solution A), and gradient in acetonitrile (solution B) as follows: B% as 5, 10, 15, 55, 30, and 5 over time for 0, 5, 10, 20, 25, and 30 min, respectively. UV detection was measured at a wavelength of 214 nm. Then, the peaks were collected and assessed for their ACE-inhibitory activity; potent peaks (peak no. 3.2) were chosen and subjected to MALDI–TOF/MS analysis.
5.6. Peptide Identification and Sequencing
The peptides and their sequences were identified through MALDI–TOF/MS at the central facility laboratory in Gyeongsang National University, Republic of Korea. Tests were conducted utilizing Cyano-4-hydroxycinnamic acid (HCCA) as the matrix. The sample (peak no. 3.2), at a volume of of 1 μL, was combined with 1 μL of the matrix, and, subsequently, 2 μL of the resulting mixture was applied onto the target plate. The spectra were obtained using a Bruker auto flex speed MALDI–TOF/MS equipped with a patented smart beam laser, emitting at 355 nm and operating at 200 Hz. Laser-induced fragmentation technology was utilized by initially setting the laser intensity at 30% for the identification of the parent compound, then later adjusted to 45% for fragmentation. The spectra were recorded in reflectron positive ion mode. The de novo sequencing of peptides was determined based on the MS/MS ionic pattern using Bruker Flex analysis version 3.4 [55].
5.7. Peptide Structure Prediction Using AlphaFold
The prediction of the 3D structure for the target proteins was executed within the ColabFold platform, using its interface [56]. The protein sequences inputted for AlphaFold modeling were consistent in both instances. In the ColabFold environment, the MMseqs2 method was specifically chosen to generate Multiple Sequence Alignments (MSAs). During this process, the amber relaxation of the model was deactivated, and the prediction for unpaired MSAs was initiated. AlphaFold, employed for predicting the three-dimensional coordinates of protein residues, utilizes pLDDT (predicted pLDDT-Cα) as a metric, ranging from 0 to 100, to indicate the confidence level associated with each prediction. The pLDDT values, reflecting this confidence, are integrated into the B-factor fields of the PDB files. The extraction of these values was facilitated through the utilization of the Bio Python library, version 1.78 [57].
5.8. Molecular Docking
The crystal structure of human ACE (ACE) complexed with the inhibitor lisinopril (PDB: 1O8A) was sourced from the RCSB Protein Data Bank (https://www.rcsb.org, accessed on 10 June 2024) and employed as the template for subsequent docking studies. In preparation for docking, the removal of all water molecules and lisinopril inhibitor was performed, while retaining the cofactor zinc atoms within the active site of the ACE model. Polar hydrogens were then added to the ACE model. Molecular docking studies were conducted using the HADDOCK software version 2.4. [46], with the best-ranked docking pose of the purified peptides within the ACE active site determined based on scores and binding energy values. Additionally, PDB sum and PYMOL (visualization tool) was utilized to identify hydrogen bonds, as well as hydrophobic interactions between ACE protein and the identified peptides. The impact of peptides on the Zn(II) tetrahedral geometry was elucidated through the examination of docking results using the LigPlot viewer version 2.2. [58,59].
5.9. Statistical Analysis
All the experiments were replicated three times, and the data were subjected to one-way analysis of variance (ANOVA), followed by Dunnett’s test for analysis. Results were expressed as mean ± standard deviation (SD), with significance denoted by * p < 0.05, ** p < 0.01, and *** p < 0.001. Graph Pad prism software was used to conduct all statistical analyses (version 5.1).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins16090410/s1, Figure S1: Prediction of the peptide IVGRPLANG structures by ColabFold; models were ranked based on AlphaFold pTM. Figure S2: Prediction of the peptide IGDEPRHQYL structures by ColabFold; models were ranked based on AlphaFold pTM. Figure S3: The specifics of zinc ion (Zn(II)) coordination with ACE residues are illustrated (A) pre-docking, and post-docking with peptides (B) IVGRPLANG and (C) IGDEPRHQYL. Representation via Ligplot version v.1.4.5 software delineates the formation of hydrogen bonds (indicated by green dotted lines), ligand bonds (represented by blue lines), and non-ligand bonds (depicted by brown lines). Table S1: Peptide structural scoring for the modeled protein using ColabFold and AlphaFold. Table S2: HADDOCK score for IVGRPLANG peptide against ACE protein. Table S3: HADDOCK score for IGDEPRHQYL peptide against ACE protein.
Author Contributions
Conceptualization, E.K.; methodology, R.L.M.P.; software, R.L.M.P., E.K. and D.A.R.; validation, R.L.M.P., D.H.H., C.K. and E.K.; formal analysis, R.L.M.P.; data curation, R.L.M.P., D.H.H. and D.A.R.; writing—original draft preparation, R.L.M.P.; writing—review and editing, R.L.M.P., D.H.H., C.K. and E.K.; supervision, E.K.; project administration, E.K.; funding acquisition, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (RS-2023-00276023 and NRF-2021R1I1A306005711).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
NnV—Nemopilema nomurai jellyfish venom; ACE—Angiotensin-converting enzyme; ACEI—Angiotensin-converting enzyme inhibitors; HADDOCK—High-ambiguity-driven protein–protein docking; DEAE—Diethylaminomethyl; IEC—Ion-exchange chromatography; RP-HPLC—Reverse-phase high-performance liquid chromatography; MALDI–TOF/MS—Matrix-assisted laser desorption ionization–time of flight/mass spectrometry; pTM—Predicted template modeling score; pLDDT—Predicted local distance difference test; ODS-C18—Octadecyl-silica–Cyclocarbon 18; Zn—Zinc; MWCO—Molecular weight cut-off; DH—Degree of hydrolysis; HCCA—Cyano-4-hydroxycinnamic acid; MSA—Multiple sequence alignment; PyMOL—Cross-platform molecular graphic tool; RCSB—Research Collaboratory for Structural Bioinformatics; PDB—Protein data bank; SD—Standard deviation; ANOVA—Analysis of variance.
References
- Tamimi, N.A.; Ellis, P. Drug development: From concept to marketing! Nephron Clin. Pract. 2009, 113, c125–c131. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Porras, J.M. Pharmacology of peptides and proteins in snake venoms. Annu. Rev. Pharmacol. 1968, 8, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.D.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Guzik, T.J.; Nosalski, R. Maffia Immune and inflammatory mechanisms in hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Winkel, M.; Ali, M.K.; Narayan, K.V.; Mehta, N.K. Cardiovascular mortality associated with 5 leading risk factors: National and state preventable fractions estimated from survey data. Ann. Intern. Med. 2015, 163, 245–253. [Google Scholar] [CrossRef]
- Kontis, V.; Mathers, C.D.; Rehm, J.; Stevens, G.A.; Shield, K.D.; Bonita, R.; Riley, L.M.; Poznyak, V.; Beaglehole, R.; Ezzati, M. Contribution of Six Risk Factors to Achieving the 25 × 25 Non-Communicable Disease Mortality Reduction Target: A Modelling Study. Lancet 2014, 384, 427–437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. NCD Global Monitoring Framework; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A. Hypertension. Nat. Rev. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Arai, M.N. Interactions of fish and pelagic coelenterates. Can. J. Zool. 1988, 66, 1913–1927. [Google Scholar] [CrossRef]
- Walker, M.J.A.; Martinez, T.T.; Godin, D.V. Investigations into the cardiotoxicity of a toxin from the nematocysts of the jellyfish, Cyanea capillata. Toxicon 1977, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- So, P.B.T.; Rubio, P.; Lirio, S.; Macabeo, A.P.; Huang, H.Y.; Corpuz, M.J.A.T.; Villaflores, O.B. In vitro angiotensin I converting enzyme inhibition by a peptide isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom hydrolysate. Toxicon 2016, 119, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-M.; Wang, J.-F.; Zha, X.-Q.; Pan, L.-H.; Zhang, H.-L.; Luo, J.-P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, K.; Bragadeeswaran, S. Antimicrobial and immunomodulatory activities of jellyfish (Chrysaora quinquecirrha) venom. In Prospects in Bioscience: Addressing the Issues; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ayed, Y.; Sghaier, R.M.; Laouini, D.; Bacha, H. Evaluation of anti-proliferative and anti-inflammatory activities of Pelagia noctiluca venom in Lipopolysaccharide/Interferon-γ stimulated RAW264.7 macrophages. Biomed. Pharmaco. 2016, 84, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Morales-Landa, J.L.; Zapata-Pérez, O.; Cedillo-Rivera, R.; Segura-Puertas, L.; Simá-Alvarez, R.; Sánchez-Rodríguez, J. Antimicrobial, antiprotozoal, and toxic activities of cnidarian extracts from the Mexican Caribbean Sea. Pharm. Bio. 2007, 45, 37–43. [Google Scholar] [CrossRef]
- Omori, M.; Kitamura, M. Taxonomic Review of Three Japanese Species of Edible Jellyfish (Scyphozoa: Rhizostomeae). Plankt. Biol. Ecol. 2004, 51, 36–51. Available online: http://www.plankton.jp/PBE/issue/vol51_1/vol51_1_036.pdf (accessed on 13 November 2022).
- Kawahara, M.; Uye, S.I.; Ohtsu, K.; Iizumi, H. Unusual Population Explosion of the Giant Jellyfish Nemopilema Nomurai (Scyphozoa: Rhizostomeae) in East Asian Waters. Mar. Ecol. Prog. Ser. 2006, 307, 161–173. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Hwang, S.J.; Choi, M.-J.; Cho, S.; Lee, H.-J.; Park, Y. Upcycling of jellyfish (Nemopilema nomurai) sea wastes as highly valuable reducing agents for green synthesis of gold nanoparticles and their antitumor and anti-inflammatory activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1127–1136. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.-K.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer effect of Nemopilema nomurai jellyfish venom on HepG2 cells and a tumor xenograft animal model. Evid. Based Complement. Altern. Med. 2017, 2017, 2752716. [Google Scholar] [CrossRef]
- Choudhary, I.; Lee, H.; Pyo, M.J.; Heo, Y.; Chae, J.; Yum, S.S.; Kang, C.; Kim, E. Proteomic investigation to identify anticancer targets of Nemopilema nomurai jellyfish venom in human hepatocarcinoma HepG2 cells. Toxins 2018, 10, 194. [Google Scholar] [CrossRef]
- Lee, H.C.H.H.; Pyo, M.J.; Bae, S.K.; Heo, Y.; Choudhary, I.; Hwang, D.; Yang, H.; Kim, J.; Chae, J. Nemopilema nomurai jellyfish venom exerts an anti-metastatic effect by inhibiting smad-and NF-κB-Mediated epithelial–mesenchymal transition in HepG2 cells. Sci. Rep 2018, 8, 2808. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-D.; Kim, Y.-K.; Lim, C.-W.; Yeun, S.-M.; Lee, M.-H.; Moon, H.-S.; Yoon, N.-Y.; Park, H.-Y.; Lee, D.-S. ACE-inhibitory properties of proteolytic hydrolysates from giant jellyfish Nemopilema nomurai. Fish. Aquat. Sci. 2011, 14, 174–178. [Google Scholar] [CrossRef]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vivo cardiovascular effects of the Irukandji jellyfish (Carukia barnesi) nematocyst venom and a tentacle extract in rats. Toxicol. Lett. 2005, 155, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Noguchi, K.; Matsuzaki, T.; Nakasone, J.; Miyagi, K.; Sakanashi, M. Haemodynamic effects of the crude venom from nematocysts of the box-jellyfish Chiropsalmus quadrigatus (Habu-kurage) in anaesthetized rabbits. Toxicon 2003, 41, 621–631. [Google Scholar] [CrossRef]
- Sakanashi, M.; Matsuzaki, T.; Nakasone, J.; Koyama, T.; Kukita, I. Effects of diltiazem on in vitro cardiovascular actions of crude venom obtained from okinawan box-jellyfish (habu-kurage), Chiropsalmus quadrigatus. Anaesth. Intensiv. Care 2002, 30, 570–577. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Nekliudov, A.D.; Ivankin, A.; Bertudina, A.V. Characteristics and Use of Protein Hydrolysates (Review). Prikl. Biokhimiia Mikrobiol. 2000, 36, 525–534. [Google Scholar]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Lan, Q.; Bassi, A.S.; Zhu, J.X.; Margaritis, A. A modified Langmuir model for the prediction of the effects of ionic strength on the equilibrium characteristics of protein adsorption onto ion exchange/affinity adsorbents. Chem. Eng. J. 2001, 81, 179–186. [Google Scholar] [CrossRef]
- Silveira, S.T.; Quines, L.K.d.M.; Burkert, C.A.V.; Kalil, S.J. Separation of phycocyanin from Spirulina platensis using ion exchange chromatography. Bioprocess Biosyst. Eng. 2008, 31, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Mora, L.; Oseguera-Toledo, M.E.; Aristoy, M.-C.; Ben Amara, I.N.; Toldrá, F. In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 2017, 58, 145–159. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhang, Q.; Ma, Q.; Fu, Y.-H.; Jin, W.-G.; Zhu, B.-W. Affinity purification of angiotensin-converting enzyme inhibitory peptides from Volutharpa ampullacea perryi protein hydrolysate using Zn-SBA-15 immobilized ACE. Eur. Food Res. Technol. 2017, 244, 457–468. [Google Scholar] [CrossRef]
- Tian, L.; Liu, J.; Ma, L.; Zhang, L.; Wang, S.; Yan, E.; Zhu, H. Isolation and purification of antioxidant and ACE-inhibitory peptides from Yak (Bos grunniens) skin. J. Food Process. Preserv. 2017, 41, e13123. [Google Scholar] [CrossRef]
- Mane, S.; Jamdar, S. Purification and identification of Ace-inhibitory peptides from poultry viscera protein hydrolysate. J. Food Biochem. 2017, 41, e12275. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Pan, D.; Cao, J.; Guo, H.; Zhao, B. Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate. Food Chem. 2012, 130, 121–126. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Baraniak, B. Angiotensin I converting enzyme inhibitory peptides obtained after in vitro hydrolysis of pea (Pisum sativum var Bajka) globulins. BioMed. Res. Int. 2014, 2014, 438459. [Google Scholar] [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Nuchprapha, A.; Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K. Karnchanatat Two novel ACE inhibitory peptides isolated from longan seeds: Purification, inhibitory kinetics and mechanisms. RSC Adv. 2020, 10, 12711–12720. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Alaiz, M.; Vioque, J.; Drago, S.R. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbina residual cake. J. Appl. Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Choudhary, I.; Lee, H.; Pyo, M.-J.; Heo, Y.; Bae, S.K.; Kwon, Y.C.; Yoon, W.D.; Kang, C.; Kim, E. Proteomics approach to examine the cardiotoxic effects of Nemopilema nomurai Jellyfish venom. J. Proteom. 2015, 128, 123–131. [Google Scholar] [CrossRef]
- Prakash, R.L.M.; Hwang, D.H.; Hong, I.-H.; Chae, J.; Kang, C.; Kim, E. Danio rerio as an alternative vertebrate model for jellyfish venom study: The toxinological aspects of Nemopilema nomurai venom. Toxicol. Lett. 2020, 335, 91–97. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, C.; Tang, X.; Liu, Z.; Huang, W.; Ye, Y. Enzymes-dependent antioxidant activity of sweet apricot kernel protein hydrolysates. LWT 2022, 154, 112825. [Google Scholar] [CrossRef]
- Kit-WST, D.A. Technical Manual; Dojindo Molecular Technologies: Kumamoto, Japan, 2013. [Google Scholar]
- Prakash, R.L.M.; Hwang, D.H.; Asirvatham, R.D.; Hong, I.H.; Kang, C.; Kim, E. Identification of cardiorespiratory toxic components of Nemopilema nomurai jellyfish venom using sequential chromatography methods. Toxicon 2023, 229, 107126. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y., Jr.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourao, R.H.V.; Chalkidis, H.M.; Valente, R. Functional proteomic analyses of Bothrops atrox venom reveals 635 phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Cheison, S.C.; Brand, J.; Leeb, E.; Kulozik, U. Analysis of the effect of temperature changes combined with different alkaline pH on the β-lactoglobulin trypsin hydrolysis pattern using MALDI-TOF-MS/MS. J. Agric. Food Chem. 2011, 59, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- AlphaFold2.Ipynb—Colaboratory. 2013. Available online: https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb (accessed on 3 March 2024).
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Chalé, F.G.H.; Ruiz, J.C.R.; Fernández, J.J.A.; Ancona, D.A.B.; Campos, M.R.S. ACE inhibitory, hypotensive and antioxidant peptide fractions from Mucuna pruriens proteins. Process Biochem. 2014, 49, 1691–1698. [Google Scholar] [CrossRef]
- Asirvatham, R.D.; Hwang, D.H.; Prakash, R.L.M.; Kang, C.; Kim, E. Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein. Int. J. Mol. Sci. 2023, 24, 8972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).