Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing
Abstract
1. Introduction
2. Results
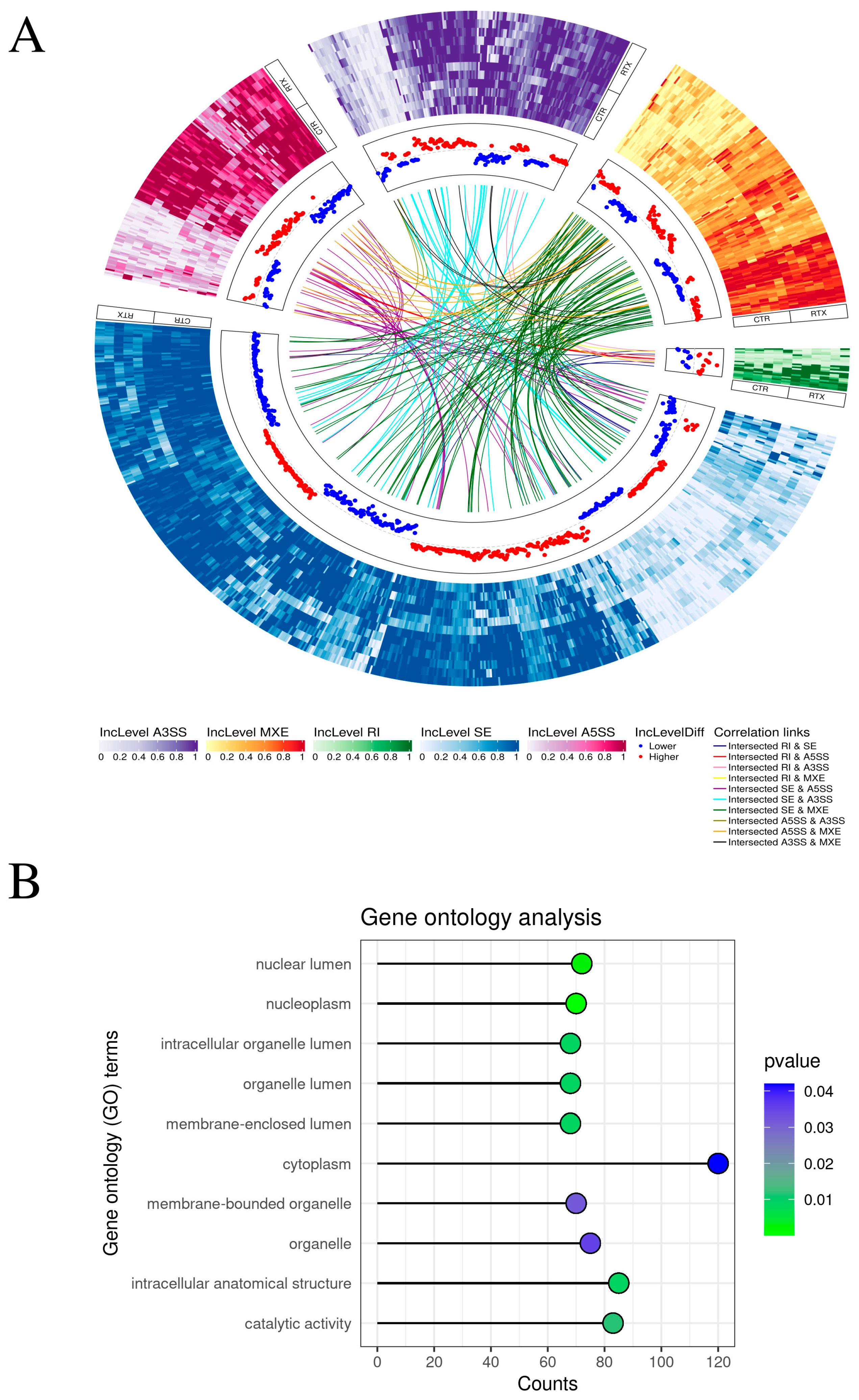
2.1. Transcriptomic Signatures of Differentially Expressed Genes (DEGs) and Functional Annotations
2.2. Alternative Splicing
2.3. Differential Single Nucleotide Variants
2.4. Multi-Level Transcriptomic Modification
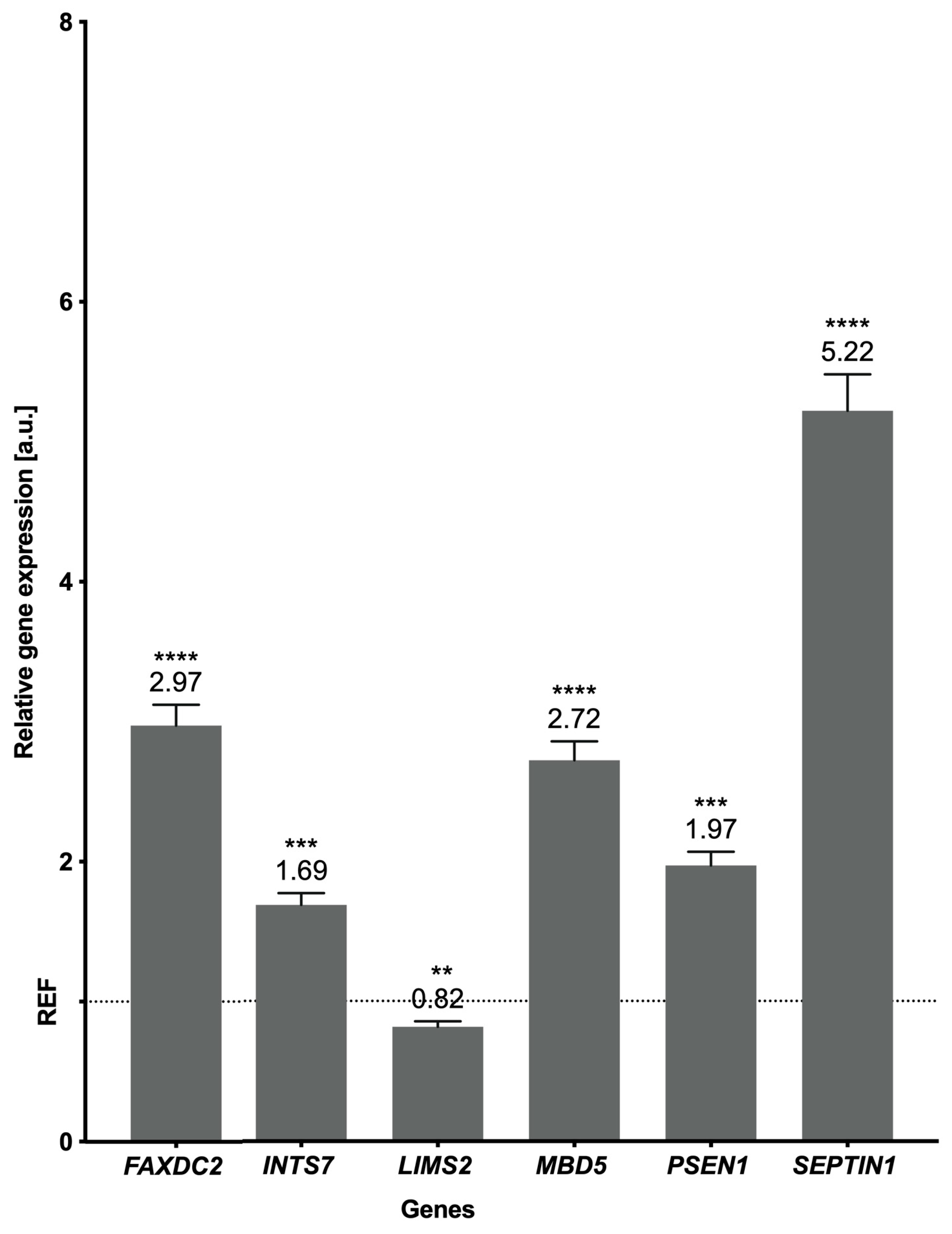
2.5. Validation of the Results
2.6. The Distribution and Relative Frequency of 5-HT-Immunoreactive Nerve Fibers
3. Discussion
3.1. Transcriptomic Changes Following RTX Administration Suggest Altered Expression of Genes Related to the Affected Sensory Fibers and Nerve Degeneration Onset
3.2. Potential Anti-Cancer Mechanisms of Intravesical RTX Administration
3.3. An Immunomodulating Effect Exerted by RTX in the Treated UBT
3.3.1. TRPV1 and Its Role in Immune System Modulation
3.3.2. Immune-Related Gene Expression Changes Following RTX Treatment
3.3.3. Chemokine Signaling and Immune Regulation
3.3.4. Other Key Immune-Related Genes Affected by RTX
3.4. Differential Expression of Genes Involved in Synaptic Plasticity and Neuromodulation
4. Conclusions
5. Materials and Methods
5.1. Laboratory Animals
5.2. RNA Extraction, Library Construction, and Sequencing
5.3. In Silico Profiling of Urinary Bladder Trigone (UBT) Transcriptome Affected by RTX
5.4. Differential Expression Profiles and Functional Annotations
5.5. Differential Alternative Splicing Events Analysis
5.6. Differential Single Nucleotide Variants Analysis
5.7. Functional Annotations
5.8. Real-Time PCR
5.9. Sectioning of the Urinary Bladder Trigone Samples and Immunohistochemical Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrés-Godayol, P.; Jerez-Roig, J.; Minobes-Molina, E.; Yildirim, M.; Molas-Tuneu, M.; Escribà-Salvans, A.; Rierola-Fochs, S.; Romero-Mas, M.; Torres-Moreno, M.; Coll-Planas, L.; et al. Urinary Incontinence and Its Association with Physical and Psycho-Cognitive Factors: A Cross-Sectional Study in Older People Living in Nursing Homes. Int. J. Environ. Res. Public Health 2022, 19, 1500. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.A.; Green, A.L. The Central Autonomic Network and Regulation of Bladder Function. Front. Neurosci. 2019, 13, 535. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Yoshimura, N. Anatomy and Physiology of the Lower Urinary Tract. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 61–108. [Google Scholar] [PubMed]
- Novara, G.; Galfano, A.; Ficarra, V.; Artibani, W. Anticholinergic Drugs in Patients with Bladder Outlet Obstruction and Lower Urinary Tract Symptoms: A Systematic Review. Eur. Urol. 2006, 50, 675–683. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.-E. Muscarinic Receptor Antagonists for Overactive Bladder. BJU Int. 2007, 100, 987–1006. [Google Scholar] [CrossRef]
- Kanai, A.; Andersson, K.-E. Bladder Afferent Signaling: Recent Findings. J. Urol. 2010, 183, 1288–1295. [Google Scholar] [CrossRef]
- Winter, J.; Dray, A.; Wood, J.N.; Yeats, J.C.; Bevan, S. Cellular Mechanism of Action of Resiniferatoxin: A Potent Sensory Neuron Excitotoxin. Brain Res. 1990, 520, 131–140. [Google Scholar] [CrossRef]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of Transient Receptor Potential Vanilloid 1 (TRPV1) by Resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef]
- Acs, G.; Biro, T.; Acs, P.; Modarres, S.; Blumberg, P.M. Differential Activation and Desensitization of Sensory Neurons by Resiniferatoxin. J. Neurosci. 1997, 17, 5622–5628. [Google Scholar] [CrossRef]
- Brown, D. Resiniferatoxin: The Evolution of the “Molecular Scalpel” for Chronic Pain Relief. Pharmaceuticals 2016, 9, 47. [Google Scholar] [CrossRef]
- Cruz, F. Mechanisms Involved in New Therapies for Overactive Bladder. Urology 2004, 63, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.T.; Scheau, A.E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895–7943. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.E.; Kovács, K.J.; Honda, C.N.; Nunez, M.G.; Larson, A.A. Resiniferatoxin (RTX) Causes a Uniquely Protracted Musculoskeletal Hyperalgesia in Mice by Activation of TRPV1 Receptors. J. Pain 2013, 14, 1629–1641. [Google Scholar] [CrossRef]
- Shi, B.; Li, X.; Chen, J.; Su, B.; Li, X.; Yang, S.; Guan, Z.; Wang, R. Resiniferatoxin for Treatment of Lifelong Premature Ejaculation: A Preliminary Study. Int. J. Urol. 2014, 21, 923–926. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Paukszto, Ł.; Wiszpolska, M.; Łopieńska-Biernat, E.; Bossowska, A.; Majewski, M.K.; Majewska, M. Molecular Influence of Resiniferatoxin on the Urinary Bladder Wall Based on Differential Gene Expression Profiling. Cells 2023, 12, 462. [Google Scholar] [CrossRef]
- Viana, R.; Batourina, E.; Huang, H.; Dressler, G.R.; Kobayashi, A.; Behringer, R.R.; Shapiro, E.; Hensle, T.; Lambert, S.; Mendelsohn, C. The Development of the Bladder Trigone, the Center of the Anti-Reflux Mechanism. Development 2007, 134, 3763–3769. [Google Scholar] [CrossRef]
- Sánchez Freire, V.; Burkhard, F.C.; Schmitz, A.; Kessler, T.M.; Monastyrskaya, K. Structural Differences between the Bladder Dome and Trigone Revealed by MRNA Expression Analysis of Cold-Cut Biopsies. BJU Int. 2011, 108, E126–E135. [Google Scholar] [CrossRef]
- Fry, C.H.; Meng, E.; Young, J.S. The Physiological Function of Lower Urinary Tract Smooth Muscle. Auton. Neurosci. 2010, 154, 3–13. [Google Scholar] [CrossRef]
- Juszczak, K.; Ziomber, A.; Wyczolkowski, M.; Thor, P.J. Urodynamic Effects of the Bladder C-Fiber Afferent Activity Modulation in Chronic Model of Overactive Bladder in Rats. J. Physiol. Pharmacol. 2009, 60, 85–91. [Google Scholar]
- Dobberfuhl, A.D.; van Uem, S.; Versi, E. Trigone as a Diagnostic and Therapeutic Target for Bladder-Centric Interstitial Cystitis/Bladder Pain Syndrome. Int. Urogynecol. J. 2021, 32, 3105–3111. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The Neural Control of Micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M. The Development of Swine Models in Drug Discovery and Development. Future Med. Chem. 2012, 4, 1771–1772. [Google Scholar] [CrossRef] [PubMed]
- Schook, L.B.; Collares, T.V.; Darfour-Oduro, K.A.; De, A.K.; Rund, L.A.; Schachtschneider, K.M.; Seixas, F.K. Unraveling the Swine Genome: Implications for Human Health. Annu. Rev. Anim. Biosci. 2015, 3, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Smith, A.C. Swine in the Laboratory; Swindle, M.M., Smith, A.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466553521. [Google Scholar]
- Shen, J.D.; Chen, S.J.; Chen, H.Y.; Chiu, K.Y.; Chen, Y.H.; Chen, W.C. Review of Animal Models to Study Urinary Bladder Function. Biology 2021, 10, 1316. [Google Scholar] [CrossRef]
- Hall, P.A.; Jung, K.; Hillan, K.J.; Russell, S.H. Expression Profiling the Human Septin Gene Family. J. Pathol. 2005, 206, 269–278. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, Y.; Wang, M.; Suraneni, P.K.; Li, D.; Crispino, J.D.; Fan, J.; Huang, Z. Novel Function of Faxdc2 in Megakaryopoiesis. Blood Cancer J. 2016, 6, e478. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, S.; Zhang, Q.; Yang, X.; Yuan, W.; Wang, Y.; Li, Y.; Zhu, P.; Wu, X.; Jiang, Z.; et al. FAXDC2 Inhibits the Proliferation and Invasion of Human Liver Cancer HepG2 Cells. Exp. Ther. Med. 2023, 27, 27. [Google Scholar] [CrossRef]
- Federico, A.; Rienzo, M.; Abbondanza, C.; Costa, V.; Ciccodicola, A.; Casamassimi, A. Pan-Cancer Mutational and Transcriptional Analysis of the Integrator Complex. Int. J. Mol. Sci. 2017, 18, 936. [Google Scholar] [CrossRef]
- Ham, S.; Kim, T.K.; Ryu, J.; Kim, Y.S.; Tang, Y.P.; Im, H.I. Comprehensive MicroRNAome Analysis of the Relationship between Alzheimer Disease and Cancer in PSEN Double-Knockout Mice. Int. Neurourol. J. 2018, 22, 237–245. [Google Scholar] [CrossRef]
- Su, C.; Cai, X.; Xu, T.; Wu, Y.; Wang, L.; Chen, P.; Su, C. LIMS2 Is Downregulated in Osteosarcoma and Inhibits Cell Growth and Migration. J. Oncol. 2022, 4811260. [Google Scholar] [CrossRef]
- Avelino, A.; Cruz, F. TRPV1 (Vanilloid Receptor) in the Urinary Tract: Expression, Function and Clinical Applications. Naunyn Schmiedeberg’s Arch. Pharmacol. 2006, 373, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Olah, Z.; Szabo, T.; Karai, L.; Hough, C.; Fields, R.D.; Caudle, R.M.; Blumberg, P.M.; Iadarola, M.J. Ligand-Induced Dynamic Membrane Changes and Cell Deletion Conferred by Vanilloid Receptor 1. J. Biol. Chem. 2001, 276, 11021–11030. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, F. Peptide Immunoreactivity and Ultrastructure of Rat Urinary Bladder Nerve Fibers after Topical Desensitization by Capsaicin or Resiniferatoxin. Auton. Neurosci. 2000, 86, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Gordon, J.; Escobar, A.; Mitchell, K.; Trenholme, H.N.; Grimes, J.A.; Jiménez-Andrade, J.M.; Nahama, A.; Cisternas, A. Safety and Efficacy of Intravesical Instillation of Resiniferatoxin in Healthy Cats: A Preliminary Study. Front. Vet. Sci. 2023, 9, 922305. [Google Scholar] [CrossRef]
- Apostolidis, A.; Brady, C.M.; Yiangou, Y.; Davis, J.; Fowler, C.J.; Anand, P. Capsaicin Receptor TRPV1 in Urothelium of Neurogenic Human Bladders and Effect of Intravesical Resiniferatoxin. Urology 2005, 65, 400–405. [Google Scholar] [CrossRef]
- McEwan, J.R.; Benjamin, N.; Larkin, S.; Fuller, R.W.; Dollery, C.T.; MacIntyre, I. Vasodilatation by Calcitonin Gene-Related Peptide and by Substance P: A Comparison of Their Effects on Resistance and Capacitance Vessels of Human Forearms. Circulation 1988, 77, 1072–1080. [Google Scholar] [CrossRef]
- Ossipov, M.H. Growth Factors and Neuropathic Pain. Curr. Pain Headache Rep. 2011, 15, 185–192. [Google Scholar] [CrossRef]
- Yeo, I.J.; Park, M.H.; Son, D.J.; Kim, J.Y.; Nam, K.T.; Hyun, B.K.; Kim, S.Y.; Jung, M.H.; Song, M.J.; Chun, H.O.; et al. PRDX6 Inhibits Neurogenesis through Downregulation of WDFY1-Mediated TLR4 Signal. Mol. Neurobiol. 2019, 56, 3132–3144. [Google Scholar] [CrossRef]
- Hodge, J.C.; Mitchell, E.; Pillalamarri, V.; Toler, T.L.; Bartel, F.; Kearney, H.M.; Zou, Y.S.; Tan, W.H.; Hanscom, C.; Kirmani, S.; et al. Disruption of MBD5 Contributes to a Spectrum of Psychopathology and Neurodevelopmental Abnormalities. Mol. Psychiatry 2014, 19, 368–379. [Google Scholar] [CrossRef]
- Jobe, E.M.; Zhao, X. DNA Methylation and Adult Neurogenesis. Brain Plast. 2016, 3, 5–26. [Google Scholar] [CrossRef]
- Zou, Z.; Li, Z.; Sun, W.; Gao, W.; Liu, B.; Liu, J.; Guo, Y. Establishment of Prognostic Model of Bladder Cancer Based on Apoptosis-Related Genes, in Which P4HB Promotes BLCA Progression. BMC Urol. 2023, 23, 167. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, R.; Chen, Y.; Huang, X.; Li, H.; Liang, P.; Chen, S. Dihydropyrimidinase Like 2 Promotes Bladder Cancer Progression via Pyruvate Kinase M2-Induced Aerobic Glycolysis and Epithelial–Mesenchymal Transition. Front. Cell Dev. Biol. 2021, 9, 641432. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Resiniferatoxin: Nature’s Precision Medicine to Silence TRPV1-Positive Afferents. Int. J. Mol. Sci. 2023, 24, 15042. [Google Scholar] [CrossRef] [PubMed]
- Farfariello, V.; Liberati, S.; Morelli, M.B.; Tomassoni, D.; Santoni, M.; Nabissi, M.; Giannantoni, A.; Santoni, G.; Amantini, C. Resiniferatoxin Induces Death of Bladder Cancer Cells Associated with Mitochondrial Dysfunction and Reduces Tumor Growth in a Xenograft Mouse Model. Chem. Biol. Interact. 2014, 224, 128–135. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Li, F.; Liu, C.S.; Wu, P.; Ling, A.S.; Pan, Q.; Li, X.N. CCT4 Suppression Inhibits Tumor Growth in Hepatocellular Carcinoma by Interacting with Cdc20. Chin. Med. J. 2021, 134, 2721–2729. [Google Scholar] [CrossRef]
- Simon, R.; Atefy, R.; Wagner, U.; Forster, T.; Fijan, A.; Bruderer, J.; Wilber, K.; Mihatsch, M.J.; Gasser, T.; Sauter, G. HER-2 and TOP2A Coamplification in Urinary Bladder Cancer. Int. J. Cancer 2003, 107, 764–772. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, A.; Dai, L.; Yu, X.; Zhang, Z.; Xiong, Q.; Yang, J.; Liu, F.; Xu, J.; Xue, Y.; et al. Prognostic Value of TOP2A in Bladder Urothelial Carcinoma and Potential Molecular Mechanisms. BMC Cancer 2019, 19, 604. [Google Scholar] [CrossRef]
- Ye, F.; Le, H.; He, F.; Tu, H.; Peng, D.; Ruan, S. Prognostic Value of an Integrin-Based Signature in Hepatocellular Carcinoma and the Identification of Immunological Role of LIMS2. Dis. Markers 2022, 2022, 7356297. [Google Scholar] [CrossRef]
- Rogaev, E.I.; Sherrington, R.; Wu, C.; Levesque, G.; Liang, Y.; Rogaeva, E.A.; Ikeda, M.; Holman, K.; Lin, C.; Lukiw, W.J.; et al. Analysis of the 5′ Sequence, Genomic Structure, and Alternative Splicing of Thepresenilin-1Gene (PSEN1) Associated with Early Onset Alzheimer Disease. Genomics 1997, 40, 415–424. [Google Scholar] [CrossRef]
- Barrachina, M.; Ferrer, I. DNA Methylation of Alzheimer Disease and Tauopathy-Related Genes in Postmortem Brain. J. Neuropathol. Exp. Neurol. 2009, 68, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, X.; Zhang, J.R.; Li, Y.; Lu, J.; Huang, F.C.; Zheng, C.H.; Xie, J.W.; Wang, J.B.; Huang, C.M. The Expression of Presenilin 1 Enhances Carcinogenesis and Metastasis in Gastric Cancer. Oncotarget 2016, 7, 10650–10662. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. The MiR-193a-3p Regulated PSEN1 Gene Suppresses the Multi-Chemoresistance of Bladder Cancer. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- da Silva, J.M.; dos Santos, T.P.M.; Saraiva, A.M.; Fernandes de Oliveira, A.L.; Garlet, G.P.; Batista, A.C.; de Mesquita, R.A.; Russo, R.C.; da Silva, T.A. Role of Atypical Chemokine Receptor ACKR2 in Experimental Oral Squamous Cell Carcinogenesis. Cytokine 2019, 118, 160–167. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP Channels Contribute Differentially to Skin Inflammation and Persistent Itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- White, F.A.; Wilson, N.M. Chemokines as Pain Mediators and Modulators. Curr. Opin. Anaesthesiol. 2008, 21, 580–585. [Google Scholar] [CrossRef]
- White, F.A.; Sun, J.; Waters, S.M.; Ma, C.; Ren, D.; Ripsch, M.; Steflik, J.; Cortright, D.N.; LaMotte, R.H.; Miller, R.J. Excitatory Monocyte Chemoattractant Protein-1 Signaling Is up-Regulated in Sensory Neurons after Chronic Compression of the Dorsal Root Ganglion. Proc. Natl. Acad. Sci. USA 2005, 102, 14092–14097. [Google Scholar] [CrossRef]
- Dolat, L.; Hu, Q.; Spiliotis, E.T. Septin Functions in Organ System Physiology and Pathology. Biol. Chem. 2014, 395, 123–141. [Google Scholar] [CrossRef]
- Murphy, D.L.; Fox, M.A.; Timpano, K.R.; Moya, P.R.; Ren-Patterson, R.; Andrews, A.M.; Holmes, A.; Lesch, K.P.; Wendland, J.R. How the Serotonin Story Is Being Rewritten by New Gene-Based Discoveries Principally Related to SLC6A4, the Serotonin Transporter Gene, Which Functions to Influence All Cellular Serotonin Systems. Neuropharmacology 2008, 55, 932–960. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Skowrońska, A.; Majewska, M.; Majewski, M.; Majewski, M. The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Sann, H.; Jancsó, G.; Ambrus, A.; Pierau, F.K. Capsaicin Treatment Induces Selective Sensory Degeneration and Increased Sympathetic Innervation in the Rat Ureter. Neuroscience 1995, 67, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Ramage, A.G. The Role of Central 5-Hydroxytryptamine (5-HT, Serotonin) Receptors in the Control of Micturition. Br. J. Pharmacol. 2006, 147, S120–S131. [Google Scholar] [CrossRef] [PubMed]
- Klarskov, P.; Hørby-Petersen, J. Influence of Serotonin on Lower Urinary Tract Smooth Muscle in Vitro. Br. J. Urol. 1986, 58, 507–513. [Google Scholar] [CrossRef]
- Recio, P.; Barahona, M.V.; Orensanz, L.M.; Bustamante, S.; Martínez, A.C.; Benedito, S.; García-Sacristán, A.; Prieto, D.; Hernández, M. 5-hydroxytryptamine Induced Relaxation in the Pig Urinary Bladder Neck. Br. J. Pharmacol. 2009, 157, 271–280. [Google Scholar] [CrossRef]
- Castleden, C.M.; George, C.F.; Renwick, A.G.; Asher, M.J. Imipramine--a Possible Alternative to Current Therapy for Urinary Incontinence in the Elderly. J. Urol. 1981, 125, 318–320. [Google Scholar] [CrossRef]
- Redaelli, M.; Ricatti, M.J.; Simonetto, M.; Claus, M.; Ballabio, M.; Caretta, A.; Mucignat-Caretta, C. Serotonin and Noradrenaline Reuptake Inhibitors Improve Micturition Control in Mice. PLoS ONE 2015, 10, e0121883. [Google Scholar] [CrossRef]
- Adler, M.; Sweeney, R.E.; Hamilton, T.A.; Lockridge, O.; Duysen, E.G.; Purcell, A.L.; Deshpande, S.S. Role of Acetylcholinesterase on the Structure and Function of Cholinergic Synapses: Insights Gained from Studies on Knockout Mice. Cell. Mol. Neurobiol. 2011, 31, 909–920. [Google Scholar] [CrossRef]
- Costea, R.; Ene, I.; Pavel, R. Pig Sedation and Anesthesia for Medical Research. Animals 2023, 13, 3807. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Park, J.W.; Huang, J.; Dittmar, K.A.; Lu, Z.X.; Zhou, Q.; Carstens, R.P.; Xing, Y. MATS: A Bayesian Framework for Flexible Detection of Differential Alternative Splicing from RNA-Seq Data. Nucleic Acids Res. 2012, 40, e61. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, F.; Eisinger, B.E.; Kelnhofer, L.E.; Jobe, E.M.; Zhao, X. Integrative Single-Cell Transcriptomics Reveals Molecular Networks Defining Neuronal Maturation During Postnatal Neurogenesis. Cereb. Cortex 2017, 27, 2064–2077. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. G:Profiler-Interoperable Web Service for Functional Enrichment Analysis and Gene Identifier Mapping (2023 Update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Timmermans, J.P.; Majewski, M.; Lakomy, M.; Scheuermann, D.W. Immunohistochemical Properties of Nerve Fibres Supplying Accessory Male Genital Glands in the Pig. A Colocalisation Study. Histochem. Cell Biol. 1999, 111, 217–228. [Google Scholar] [CrossRef]



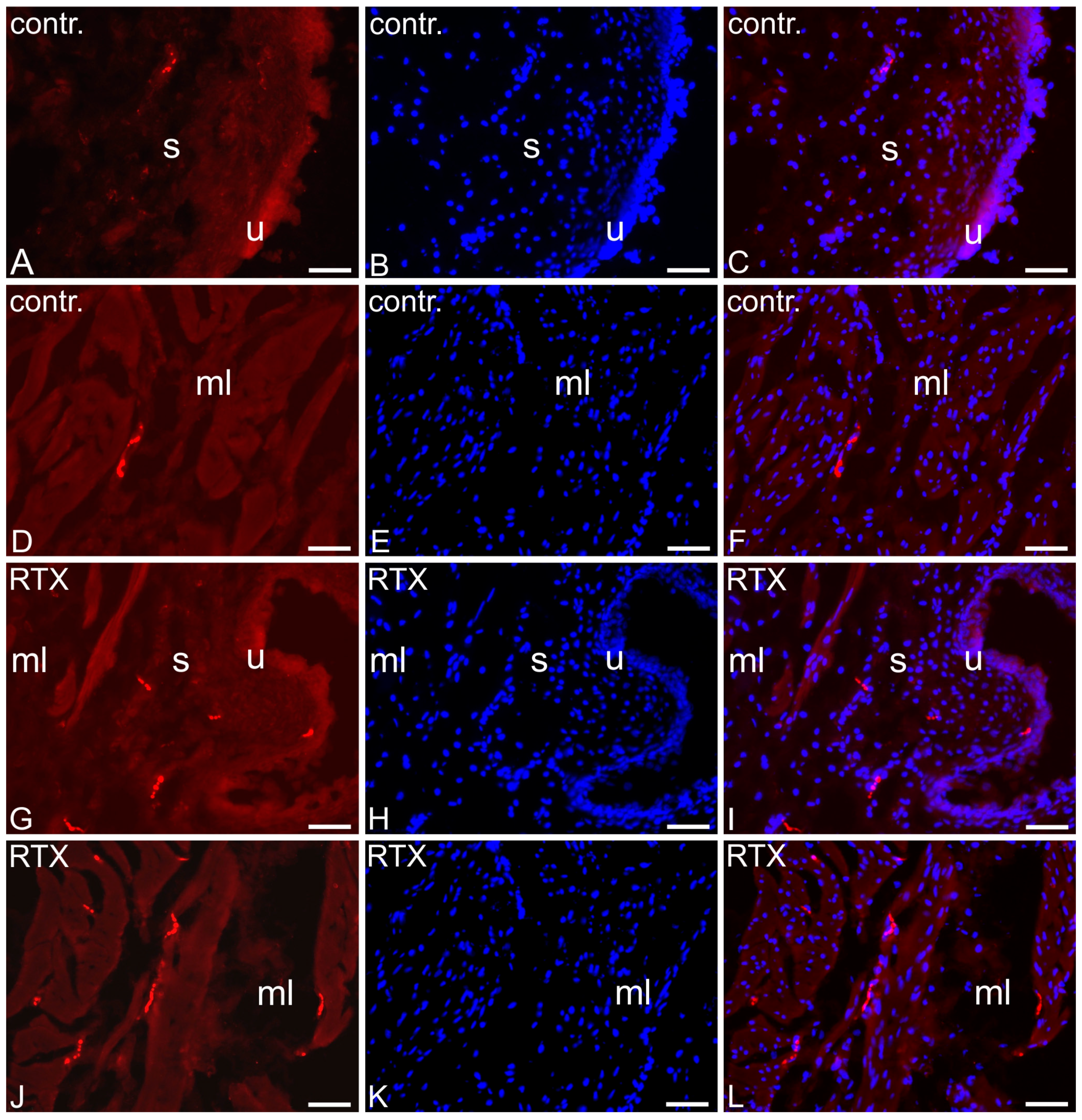
| Part of the Urinary Bladder Trigone | Control Pigs | RTX-Treated Pigs |
|---|---|---|
| Muscle layer | +/− | ++ ↑ |
| Submucosa | +/− | + ↑ |
| Urothelium | +/− | + ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepiarczyk, E.; Maździarz, M.; Paukszto, Ł.; Bossowska, A.; Majewski, M.; Kaleczyc, J.; Łopieńska-Biernat, E.; Jaśkiewicz, Ł.; Skowrońska, A.; Skowroński, M.T.; et al. Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing. Toxins 2025, 17, 127. https://doi.org/10.3390/toxins17030127
Lepiarczyk E, Maździarz M, Paukszto Ł, Bossowska A, Majewski M, Kaleczyc J, Łopieńska-Biernat E, Jaśkiewicz Ł, Skowrońska A, Skowroński MT, et al. Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing. Toxins. 2025; 17(3):127. https://doi.org/10.3390/toxins17030127
Chicago/Turabian StyleLepiarczyk, Ewa, Mateusz Maździarz, Łukasz Paukszto, Agnieszka Bossowska, Mariusz Majewski, Jerzy Kaleczyc, Elżbieta Łopieńska-Biernat, Łukasz Jaśkiewicz, Agnieszka Skowrońska, Mariusz T. Skowroński, and et al. 2025. "Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing" Toxins 17, no. 3: 127. https://doi.org/10.3390/toxins17030127
APA StyleLepiarczyk, E., Maździarz, M., Paukszto, Ł., Bossowska, A., Majewski, M., Kaleczyc, J., Łopieńska-Biernat, E., Jaśkiewicz, Ł., Skowrońska, A., Skowroński, M. T., & Majewska, M. (2025). Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing. Toxins, 17(3), 127. https://doi.org/10.3390/toxins17030127





