Selective Modulation of Osteoclast Function by Bothrops moojeni Venom and Its Fractions: Implications for Therapeutic Targeting in Bone Diseases
Abstract
1. Introduction
2. Results
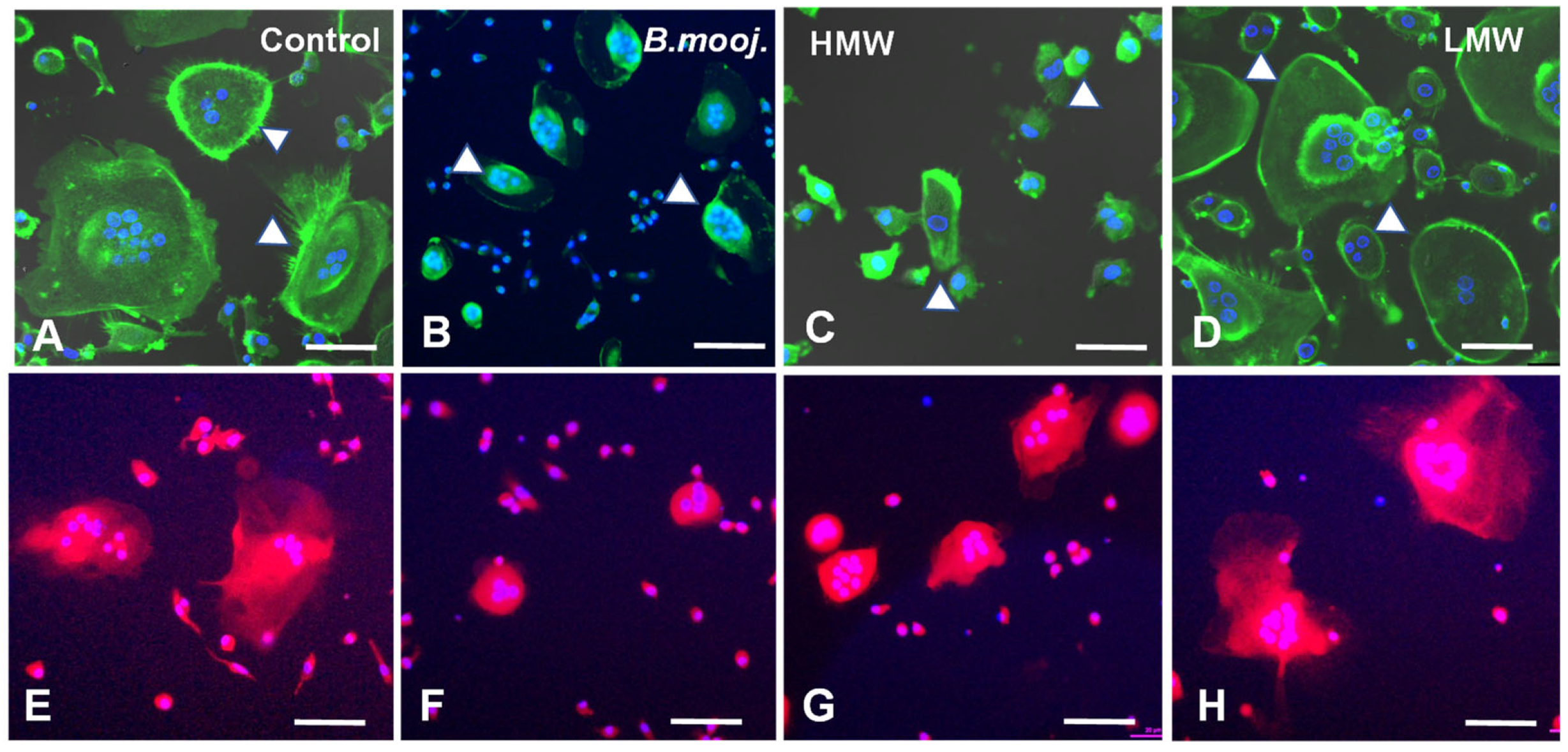
2.1. Effect of B. moojeni Crude Venom and Its Fractions on Osteoclast Morphology and Reabsorption Function: F-Actin Ring Integrity and Mitochondrial Distribution
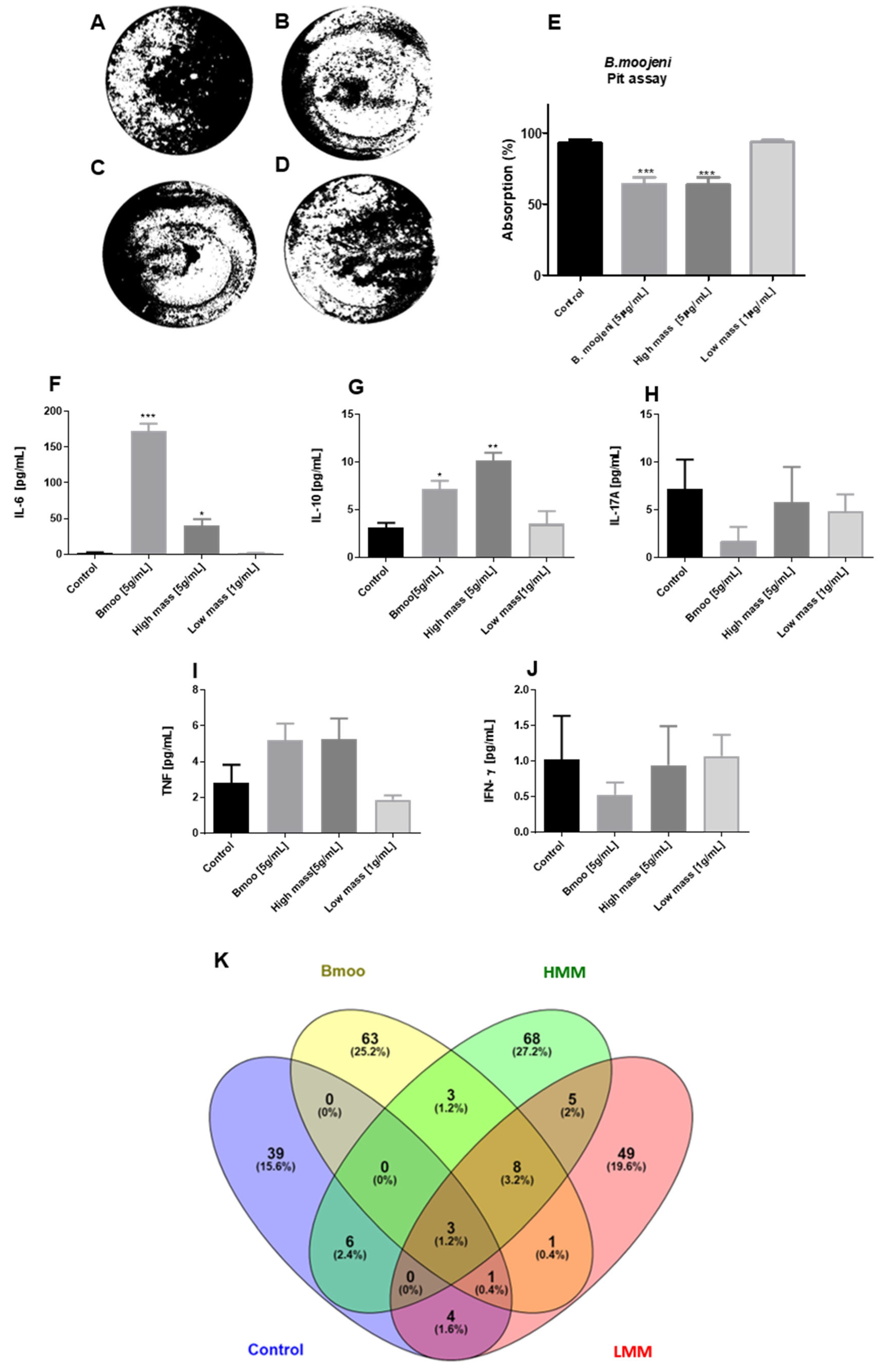
2.2. Impact of B. moojeni Venom and Its Fractions on OC Absorptive Function: Inhibition of Bone Resorption and Associated Cellular Mechanisms
2.3. Distinct Cytokine Modulation in Osteoclastogenesis by B. moojeni Venom and Its Fractions: Insights into Pro-Inflammatory and Regulatory Responses
2.4. Impact of B. moojeni Venom and Its Fractions on Osteoclast Secretome: Cellular and Molecular Pathways of Disruption and Adaptation
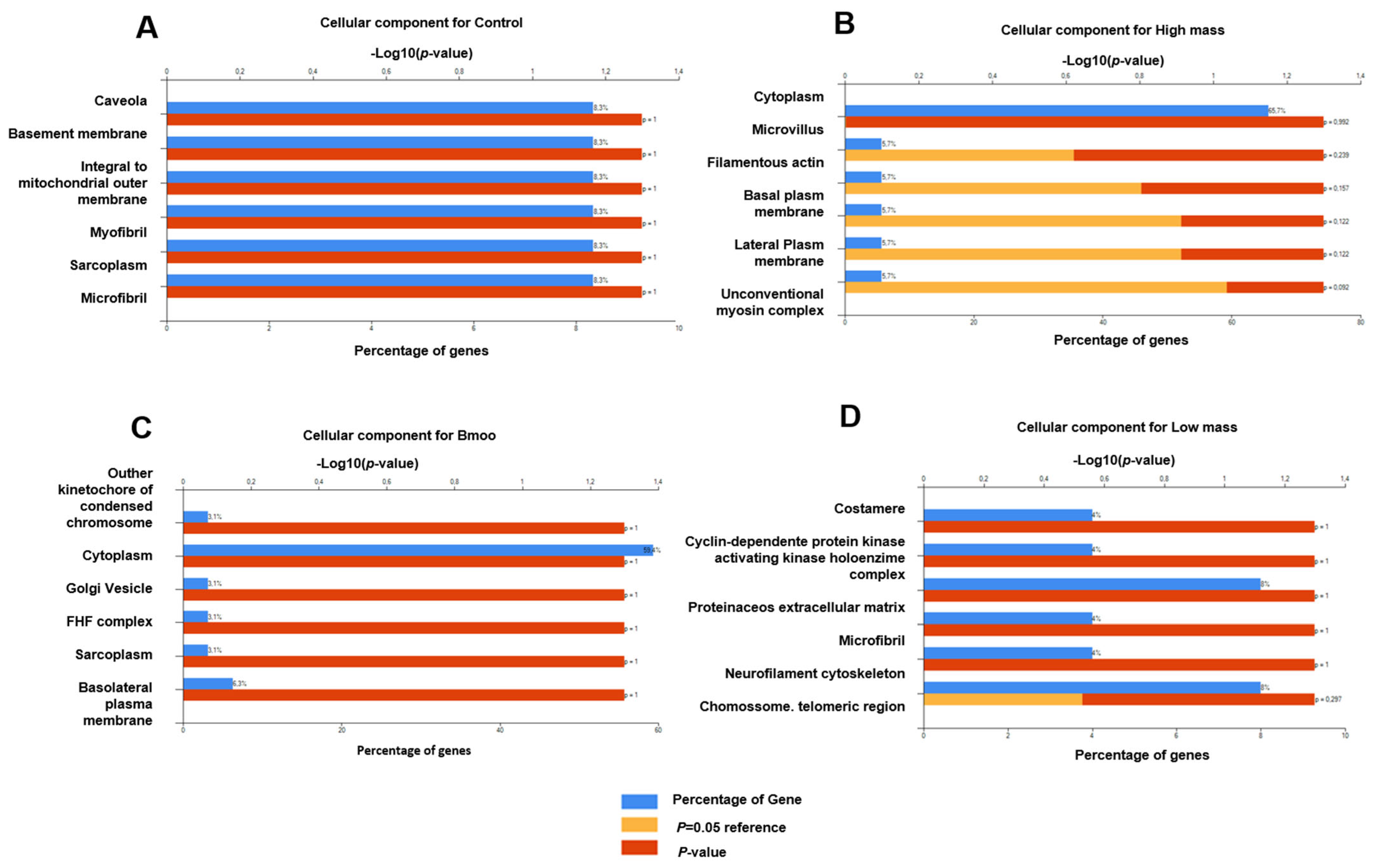
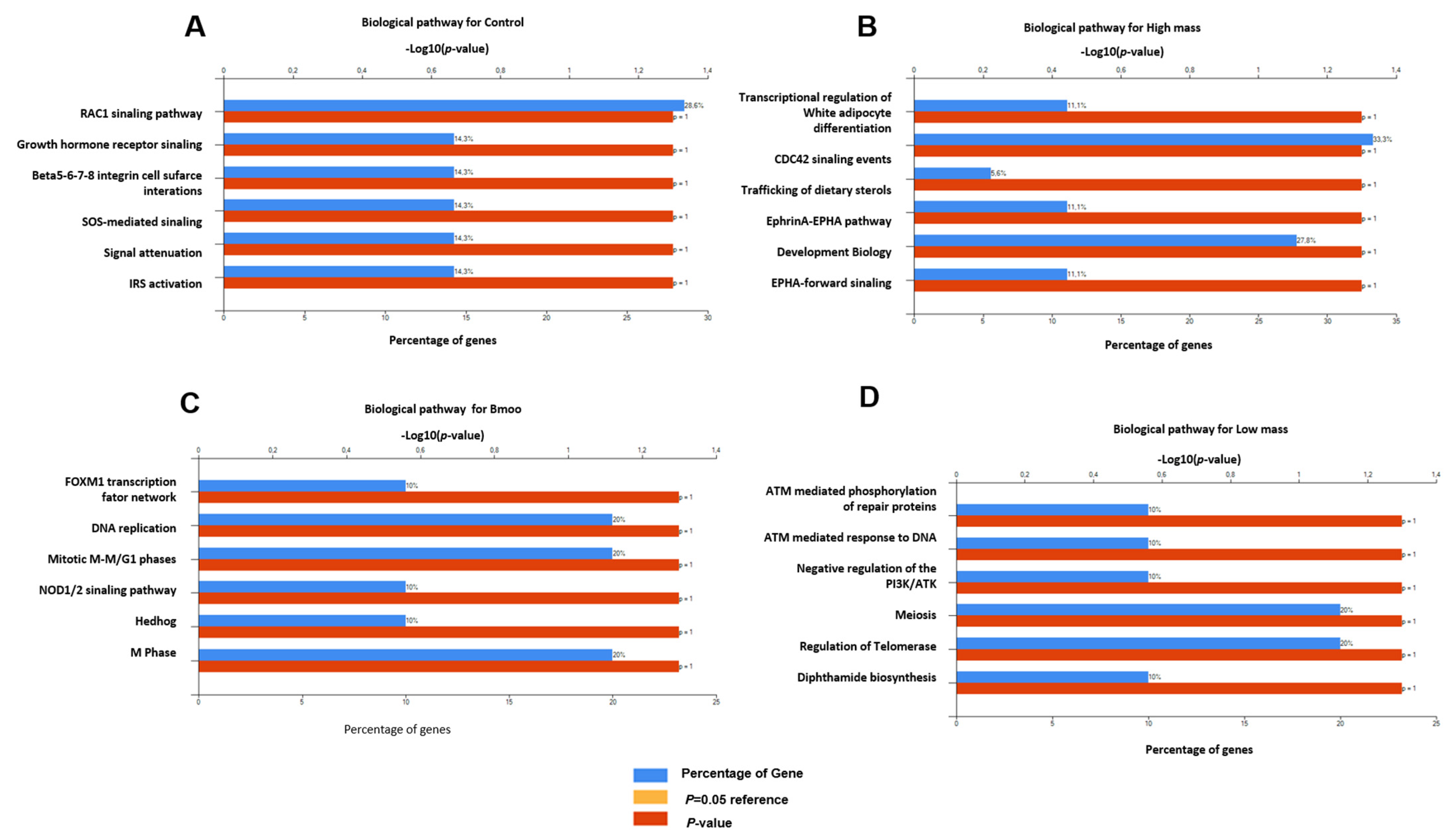
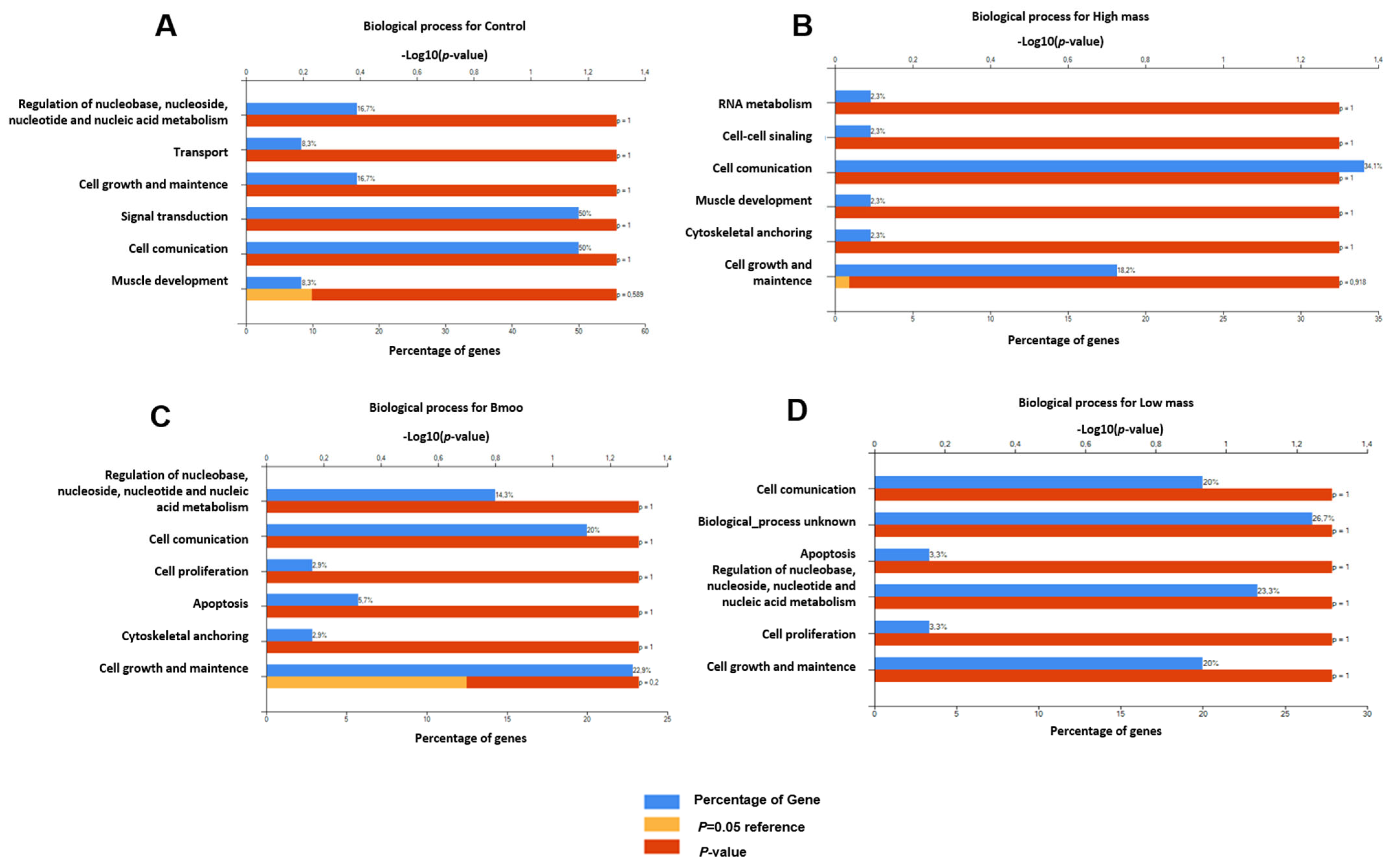
2.5. Gene Expression Profiles in Osteoclast Differentiation: Insights into Immune and Osteogenic Interactions in Mixed Peripheral Blood Cell Cultures
2.6. Molecular Insights into Osteoclast Modulation by B. moojeni Venom and Its Fractions: Differential Expression and Functional Implications of Key Regulatory Genes
3. Discussion
4. Conclusions
5. Methodology
5.1. hPBMCs and OC Differentiation Protocol
5.2. F-Actin
5.3. Mitotracker
5.3.1. Pit Assay
5.3.2. Cytometric Bead Array (CBA)
5.4. Establishment of Non-Lethal Concentrations of Bothrops moojeni Venom and Its Fractions for Osteoclast Precursor Viability Assays
5.4.1. Experimental Setup and Data Generation
5.4.2. Sample Preparation
5.4.3. Chromatography
5.4.4. Mass Spectrometry Analysis
5.4.5. Protein Identification
5.4.6. Functional Enrichment and Pathway Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Amélio, F.; Vigerelli, H.; Kerkis, I. Bothrops moojeni venom and its components—An overview. J. Venom. Res. 2021, 11, 26. [Google Scholar]
- Oliveira, A.L.; Viegas, M.F.; Da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Frihling, B.E.F.; Boleti, A.P.d.A.; de Oliveira, C.F.R.; Sanches, S.C.; Cardoso, P.H.d.O.; Verbisck, N.; Macedo, M.L.R.; Rita, P.H.S.; Carvalho, C.M.E.; Migliolo, L. Purification, Characterization and Evaluation of the Antitumoral Activity of a Phospholipase A2 from the Snake Bothrops moojeni. Pharmaceuticals 2022, 15, 724. [Google Scholar] [CrossRef]
- Silva, N.B.; Dias, E.H.V.; Costa, J.D.O.; Mamede, C.C.N. Bothrops Moojeni Snake Venom: A Source of Potential Therapeutic Agents Against Hemostatic Disorders. Int. J. Cardiovasc. Sci. 2024, 37, e20220075. [Google Scholar] [CrossRef]
- Silva, M.C.; Sales-Campos, H.; Oliveira, C.J.F.; Silva, T.L.; França, F.B.F.; Oliveira, F.; Mineo, J.R. Treatment with a Zinc Metalloprotease Purified from Bothrops moojeni Snake Venom (BmooMP-Alpha-I) Reduces the Inflammation in an Experimental Model of Dextran Sulfate Sodium-Induced Colitis. Mediat. Inflamm. 2019, 2019, 519. [Google Scholar] [CrossRef]
- Almeida, G.d.O.; Cintra, A.C.O.; Silva, T.A.; de Oliveira, I.S.; Correia, L.I.V.; Torquato, R.J.S.; Junior, R.S.F.; Arantes, E.C.; Sampaio, S.V. Moojecin: The first disintegrin from Bothrops moojeni venom and its antitumor activity in acute myeloid leukemia. Int. J. Biol. Macromol. 2024, 279, 135066. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Uehara, S.; Udagawa, N.; Mukai, H.; Ishihara, A.; Maeda, K.; Yamashita, T.; Murakami, K.; Nishita, M.; Nakamura, T.; Kato, S.; et al. Protein kinase N3 promotes bone resorption by osteoclasts in response to Wnt5a-Ror2 signaling. Sci. Signal. 2017, 10, eaan0023. [Google Scholar] [CrossRef]
- Kim, H.; Takegahara, N.; Choi, Y. IgSF11-mediated phosphorylation of pyruvate kinase M2 regulates osteoclast differentiation and prevents pathological bone loss. Bone Res. 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Takegahara, N.; Kim, H.; Choi, Y. Unraveling the intricacies of osteoclast differentiation and maturation: Insight into novel therapeutic strategies for bone-destructive diseases. Exp. Mol. Med. 2024, 56, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Vuoti, E.; Lehenkari, P.; Tuukkanen, J.; Glumoff, V.; Kylmäoja, E. Osteoclastogenesis of human peripheral blood, bone marrow, and cord blood monocytes. Sci. Rep. 2023, 13, 3763. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.; Yao, Z.; Xing, L. Osteoclasts Have Multiple Roles in Bone in Addition to Bone Resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Kim, A.S.; Mulholland, B.S.; Rauner, M. New Insights Into Osteoclast Biology. JBMR Plus 2021, 5, e10539. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- D’amélio, F.; Vigerelli, H.; Prieto-Da-Silva, Á.R.d.B.; Frare, E.O.; Batista, I.d.F.C.; Pimenta, D.C.; Kerkis, I. Bothrops moojeni Venom and Its Components Strongly Affect Osteoclasts’ Maturation and Protein Patterns. Toxins 2021, 13, 459. [Google Scholar] [CrossRef]
- Novack, D.V.; Faccio, R. Osteoclast motility: Putting the brakes on bone resorption. Ageing Res. Rev. 2011, 10, 54–61. [Google Scholar] [CrossRef]
- Dobson, P.F.; Dennis, E.P.; Hipps, D.; Reeve, A.; Laude, A.; Bradshaw, C.; Stamp, C.; Smith, A.; Deehan, D.J.; Turnbull, D.M.; et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci. Rep. 2020, 10, 11643. [Google Scholar] [CrossRef]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, L.; Chen, Y.; Zhu, C.; Chen, F.; Li, A. Research progress on the hedgehog signalling pathway in regulating bone formation and homeostasis. Cell Prolif. 2022, 55, e13162. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 Inhibits Bone Resorption: A Potential Therapeutic Strategy in Periodontitis and Other Bone Loss Diseases. BioMed Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef]
- Zhang, F.; Nakanishi, G.; Kurebayashi, S.; Yoshino, K.; Perantoni, A.; Kim, Y.S.; Jetten, A.M. Characterization of Glis2, a Novel Gene Encoding a Gli-related, Krüppel-like Transcription Factor with Transactivation and Repressor Functions. J. Biol. Chem. 2002, 277, 10139–10149. [Google Scholar] [CrossRef]
- Lv, W.-T.; Du, D.-H.; Gao, R.-J.; Yu, C.-W.; Jia, Y.; Jia, Z.-F.; Wang, C.-J. Regulation of Hedgehog signaling Offers A Novel Perspective for Bone Homeostasis Disorder Treatment. Int. J. Mol. Sci. 2019, 20, 3981. [Google Scholar] [CrossRef]
- Xu, G.; Lu, X.; Huang, T.; Fan, J. ARHGAP24 inhibits cell cycle progression, induces apoptosis and suppresses invasion in renal cell carcinoma. Oncotarget 2016, 7, 51829–51839. [Google Scholar] [CrossRef]
- Balla, B.; Kósa, J.P.; Kiss, J.; Borsy, A.; Podani, J.; Takács, I.; Lazáry, Á.; Nagy, Z.; Bácsi, K.; Speer, G.; et al. Different Gene Expression Patterns in the Bone Tissue of Aging Postmenopausal Osteoporotic and Non-osteoporotic Women. Calcif. Tissue Int. 2008, 82, 12–26. [Google Scholar] [CrossRef]
- Munson, S.; Wang, Y.; Chang, W.; Bikle, D.D. Myosin 1a Regulates Osteoblast Differentiation Independent of Intestinal Calcium Transport. J. Endocr. Soc. 2019, 3, 1993–2011. [Google Scholar] [CrossRef]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018, 19, 3004. [Google Scholar] [CrossRef]
- Cheng, B.-F.; Feng, X.; Gao, Y.-X.; Jian, S.-Q.; Liu, S.-R.; Wang, M.; Xie, Y.-F.; Wang, L.; Feng, Z.-W.; Yang, H.-J. Neural Cell Adhesion Molecule Regulates Osteoblastic Differentiation Through Wnt/β-Catenin and PI3K-Akt Signaling Pathways in MC3T3-E1 Cells. Front. Endocrinol. 2021, 12, 657953. [Google Scholar] [CrossRef] [PubMed]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-β Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef] [PubMed]
- Kuiperij, H.B.; Van Pel, M.; De Rooij, K.E.; Hoeben, R.C.; Fibbe, W.E. Serpina1 (α1-AT) is synthesized in the osteoblastic stem cell niche. Exp. Hematol. 2009, 37, 641–647. [Google Scholar] [CrossRef]
- Chang, E.-J.; Ha, J.; Oerlemans, F.; Lee, Y.J.; Lee, S.W.; Ryu, J.; Kim, H.J.; Lee, Y.; Kim, H.-M.; Choi, J.-Y.; et al. Brain-type creatine kinase has a crucial role in osteoclast-mediated bone resorption. Nat. Med. 2008, 14, 966–972. [Google Scholar] [CrossRef]
- Deng, P.; Chen, Q.M.; Hong, C.; Wang, C.Y. Histone methyltransferases and demethylases: Regulators in balancing osteogenic and adipogenic differentiation of mesenchymal stem cells. Int. J. Oral. Sci. 2015, 7, 197–204. [Google Scholar] [CrossRef]
- Sugatani, T.; Alvarez, U.; Hruska, K.A. PTEN Regulates RANKL- and Osteopontin-stimulated Signal Transduction during Osteoclast Differentiation and Cell Motility. J. Biol. Chem. 2003, 278, 5001–5008. [Google Scholar] [CrossRef]
- Thouverey, C.; Caverzasio, J. Focus on the p38 MAPK Signaling Pathway in Bone Development and Maintenance. 10 June 2015 BoneKEy Rep. Available online: http://www.portico.org/Portico/article?article=pgk2ph9s9dt (accessed on 12 September 2024).
- Arthur, A.; Gronthos, S. Eph-Ephrin Signaling Mediates Cross-Talk Within the Bone Microenvironment. Front. Cell Dev. Biol. 2021, 9, 598612. [Google Scholar] [CrossRef]
- Richter, W.F.; Nayak, S.; Iwasa, J.; Taatjes, D.J. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2022, 23, 732–749. [Google Scholar] [CrossRef]
- Urban, W.; Krzystańska, D.; Piekarz, M.; Nazar, J.; Jankowska, A. Osteosarcoma’s genetic landscape painted by genes’ mutations. Acta Biochim. Pol. 2023, 70, 671–678. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Chamcheu, J.C. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef]
- Ji, L.; Xu, S.; Luo, H.; Zeng, F. Insights from DOCK2 in cell function and pathophysiology. Front. Mol. Biosci. 2022, 9, 997659. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhu, Z.; Wu, J.; She, H.; Han, R.; Xu, H.; Qin, Z.H. DRAM1 regulates autophagy and cell proliferation via inhibition of the phosphoinositide 3-kinase-Akt-mTOR-ribosomal protein S6 pathway. Cell Commun. Signal. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Chonat, S.; Risinger, M.; Sakthivel, H.; Niss, O.; Rothman, J.A.; Hsieh, L.; Chou, S.T.; Kwiatkowski, J.L.; Khandros, E.; Gorman, M.F.; et al. The Spectrum of SPTA1-Associated Hereditary Spherocytosis. Front. Physiol. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Pitter, K.L.; Casey, D.L.; Lu, Y.C.; Hannum, M.; Zhang, Z.; Song, X.; Pecorari, I.; McMillan, B.; Ma, J.; Samstein, R.M.; et al. Pathogenic ATM Mutations in Cancer and a Genetic Basis for Radiotherapeutic Efficacy. JNCI J. Natl. Cancer Inst. 2021, 113, 266–273. [Google Scholar] [CrossRef]
- Tseng-Rogenski, S.S.; Munakata, K.; Choi, D.Y.; Martin, P.K.; Mehta, S.; Koi, M.; Zheng, W.; Zhang, Y.; Carethers, J.M. The Human DNA Mismatch Repair Protein MSH3 Contains Nuclear Localization and Export Signals That Enable Nuclear-Cytosolic Shuttling in Response to Inflammation. Mol. Cell. Biol. 2020, 40, e00029-20. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Menendez, A.; Fong, C.; Babey, M.; Tahimic, C.G.; Cheng, Z.; Li, A.; Chang, W.; Bikle, D.D. Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired Endochondral Bone Formation During Fracture Healing. J. Bone Miner. Res. 2015, 30, 1572–1584. [Google Scholar] [CrossRef]
- Ogi, T.; Shinkai, Y.; Tanaka, K.; Ohmori, H. Polκ protects mammalian cells against the lethal and mutagenic effects of benzo[a]pyrene. Proc. Natl. Acad. Sci.USA 2002, 99, 15548–15553. [Google Scholar] [CrossRef]
- Tuntithavornwat, S.; Shea, D.J.; Wong, B.S.; Guardia, T.; Lee, S.J.; Yankaskas, C.L.; Konstantopoulos, K. Giant obscurin regulates migration and metastasis via RhoA-dependent cytoskeletal remodeling in pancreatic cancer. Cancer Lett. 2022, 526, 155–167. [Google Scholar] [CrossRef]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Lian, W.-S.; Wu, R.-W.; Ko, J.-Y.; Chen, Y.-S.; Wang, S.-Y.; Jahr, H.; Wang, F.-S. Inhibition of histone lysine demethylase 6A promotes chondrocytic activity and attenuates osteoarthritis development through repressing H3K27me3 enhancement of Wnt10a. Int. J. Biochem. Cell Biol. 2023, 158, 106394. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.J.R.; Cuesta, R.; Pause, A. Folliculin: A Regulator of Transcription Through AMPK and mTOR Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 667311. [Google Scholar]
- Tong, X.; Zhang, C.; Wang, D.; Song, R.; Ma, Y.; Cao, Y.; Zhao, H.; Bian, J.; Gu, J.; Liu, Z. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020, 53, e12714. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Jin, Z.; Yang, J.; Xu, D.; Zhao, L.; Kiram, A.; Yin, Y.; Zhou, D.; Sun, Z.; Xiao, L.; et al. Muscle-bone cross-talk through the FNIP1-TFEB-IGF2 axis is associated with bone metabolism in human and mouse. Sci. Transl. Med. 2024, 16, eadk9811. [Google Scholar] [CrossRef]
- Touaitahuata, H.; Blangy, A.; Vives, V. Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases 2014, 5, e28119. [Google Scholar] [CrossRef]
- D’Amélio, F.; Vigerelli, H.; Prieto-da-Silva, Á.R.B.; Osório Frare, E.; Batista, I.F.C.; Pimenta, D.C.; Kerkis, I. Bothrops moojeni Venom and Its Components Strongly Affect Osteoclasts’ Maturation and Protein Patterns. Toxins 2021, 13, 459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Pathway Description | Database | Adjusted p-Value | Gene/Complex Involved |
|---|---|---|---|
| Complement cascade | Bioplanet | 0.00360 | IGHG3; IGHG4; IGHG1; IGHG2 |
| ASCC1 | Bioplex | 0.00834 | IGHG1; IGHG2 |
| ZNF354C | Bioplex | 0.00834 | IGHG1; IGHG2 |
| SUSD3 | Bioplex | 0.01085 | IGHG1; IGHG2 |
| FAM175B | Bioplex | 0.01085 | IGHG1; IGHG2 |
| GDPD1 | Bioplex | 0.01596 | IGHG1; IGHG2 |
| ZFP41 | Bioplex | 0.02269 | AKAP1; FBN1 |
| PDE4DIP | Bioplex | 0.04499 | IGHG1; IGHG2 |
| ZSCAN20 | Bioplex | 0.04499 | IGHG1; IGHG2 |
| PRKAR2B | HuMAP | 0.02100 | AKAP1 |
| LDLRAD4 | HuMAP | 0.02100 | HECW1 |
| PAX9-MSX1 complex | CORUM | 0.00991 | MSX1 |
| ITK-SLP-76 complex, anti-TCR stimulated | CORUM | 0.00991 | ITK |
| AMY-1-S-AKAP84-RII-beta complex | CORUM | 0.00991 | AKAP1 |
| SLP-76-PLC-gamma-1-ITK complex, alpha-TCR stimulated | CORUM | 0.00991 | ITK |
| Sam68-p85 P13K-IRS-1-IR signaling complex | CORUM | 0.01055 | IRS1 |
| Doramapimod | HMS | 0.00441 | MK14; MK11 |
| QL-XI-92 | HMS | 0.03307 | MK14; MK11 |
| CG-930 | HMS | 0.04648 | MK14; MK11 |
| ALW-II-38-3 | HMS | 0.04648 | MK14; MK11 |
| Pathway Description | Database | Adjusted p-Value | Gene/Complex Involved |
|---|---|---|---|
| ALL-1 supercomplex | CORUM | 0.04903 | SYMPK; CHD3 |
| NRD complex (nucleosome remodeling and deacetylation complex) | CORUM | 0.04903 | CHD3 |
| Polyadenylation complex (CSTF1, CSTF2, CSTF3, SYMPK CPSF1, CPSF2, CPSF3) | CORUM | 0.04903 | SYMPK |
| HDAC2-asscociated core complex | CORUM | 0.04903 | CHD3 |
| HDAC1-associated protein complex | CORUM | 0.04903 | CHD3 |
| KCNQ1 macromolecular complex | CORUM | 0.04903 | AKAP9 |
| HDAC1-associated core complex cII | CORUM | 0.04903 | CHD3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amélio, F.; Vigerelli, H.; Batista, I.d.F.C.; Araldi, R.P.; Prieto-da-Silva, Á.R.B.; Pimenta, D.C.; Kerkis, I. Selective Modulation of Osteoclast Function by Bothrops moojeni Venom and Its Fractions: Implications for Therapeutic Targeting in Bone Diseases. Toxins 2025, 17, 141. https://doi.org/10.3390/toxins17030141
D’Amélio F, Vigerelli H, Batista IdFC, Araldi RP, Prieto-da-Silva ÁRB, Pimenta DC, Kerkis I. Selective Modulation of Osteoclast Function by Bothrops moojeni Venom and Its Fractions: Implications for Therapeutic Targeting in Bone Diseases. Toxins. 2025; 17(3):141. https://doi.org/10.3390/toxins17030141
Chicago/Turabian StyleD’Amélio, Fernanda, Hugo Vigerelli, Isabel de Fátima Correia Batista, Rodrigo Pinheiro Araldi, Álvaro R. B. Prieto-da-Silva, Daniel Carvalho Pimenta, and Irina Kerkis. 2025. "Selective Modulation of Osteoclast Function by Bothrops moojeni Venom and Its Fractions: Implications for Therapeutic Targeting in Bone Diseases" Toxins 17, no. 3: 141. https://doi.org/10.3390/toxins17030141
APA StyleD’Amélio, F., Vigerelli, H., Batista, I. d. F. C., Araldi, R. P., Prieto-da-Silva, Á. R. B., Pimenta, D. C., & Kerkis, I. (2025). Selective Modulation of Osteoclast Function by Bothrops moojeni Venom and Its Fractions: Implications for Therapeutic Targeting in Bone Diseases. Toxins, 17(3), 141. https://doi.org/10.3390/toxins17030141







