An Elastase Inhibitor ShSPI from Centipede Attenuates Bleomycin-Induced Pulmonary Fibrosis
Abstract
1. Introduction
2. Results
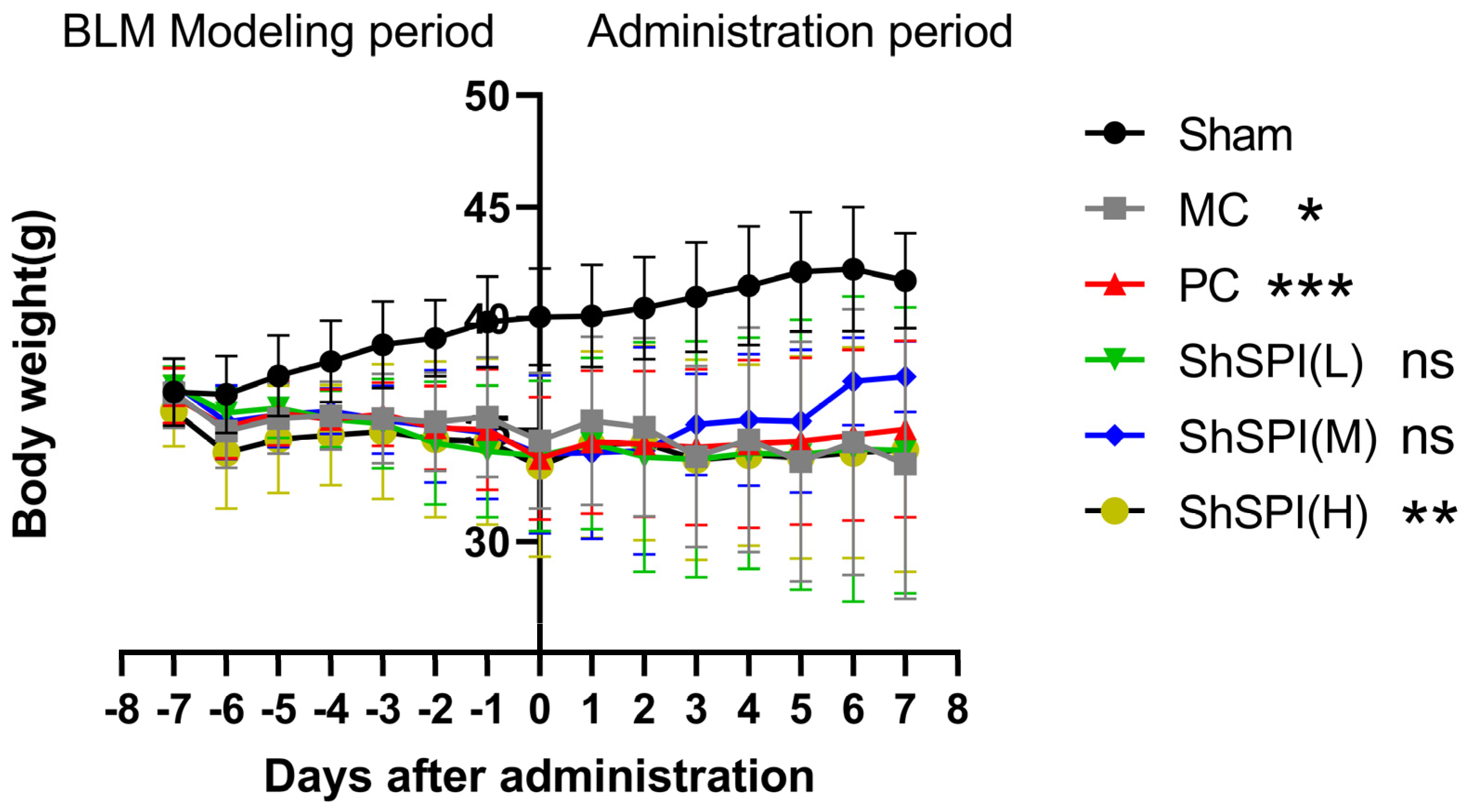
2.1. Body Weight Changes in IPF Mice
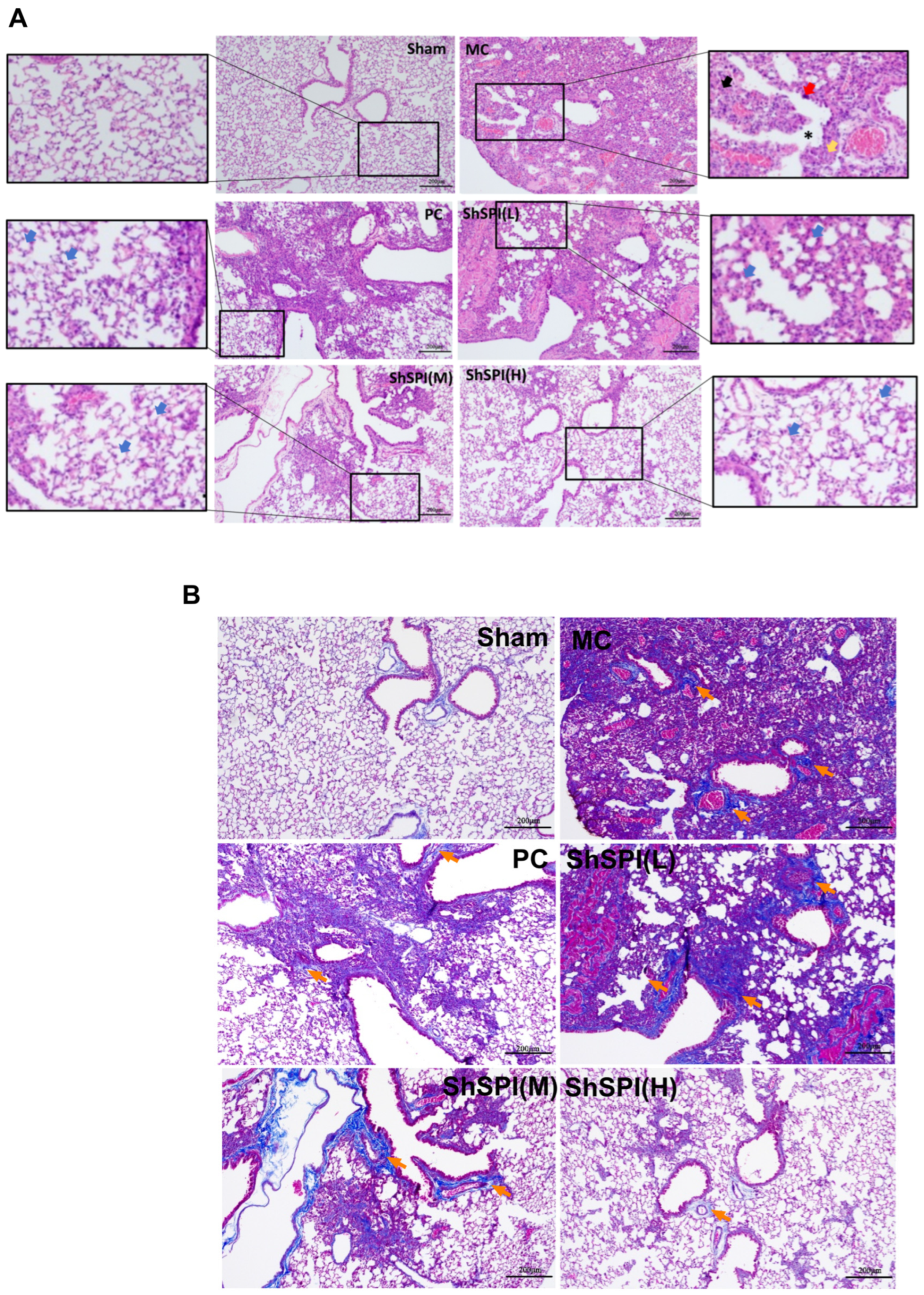
2.2. ShSPI on Bleomycin-Induced Histopathological Changes in Lungs of Mouse Models
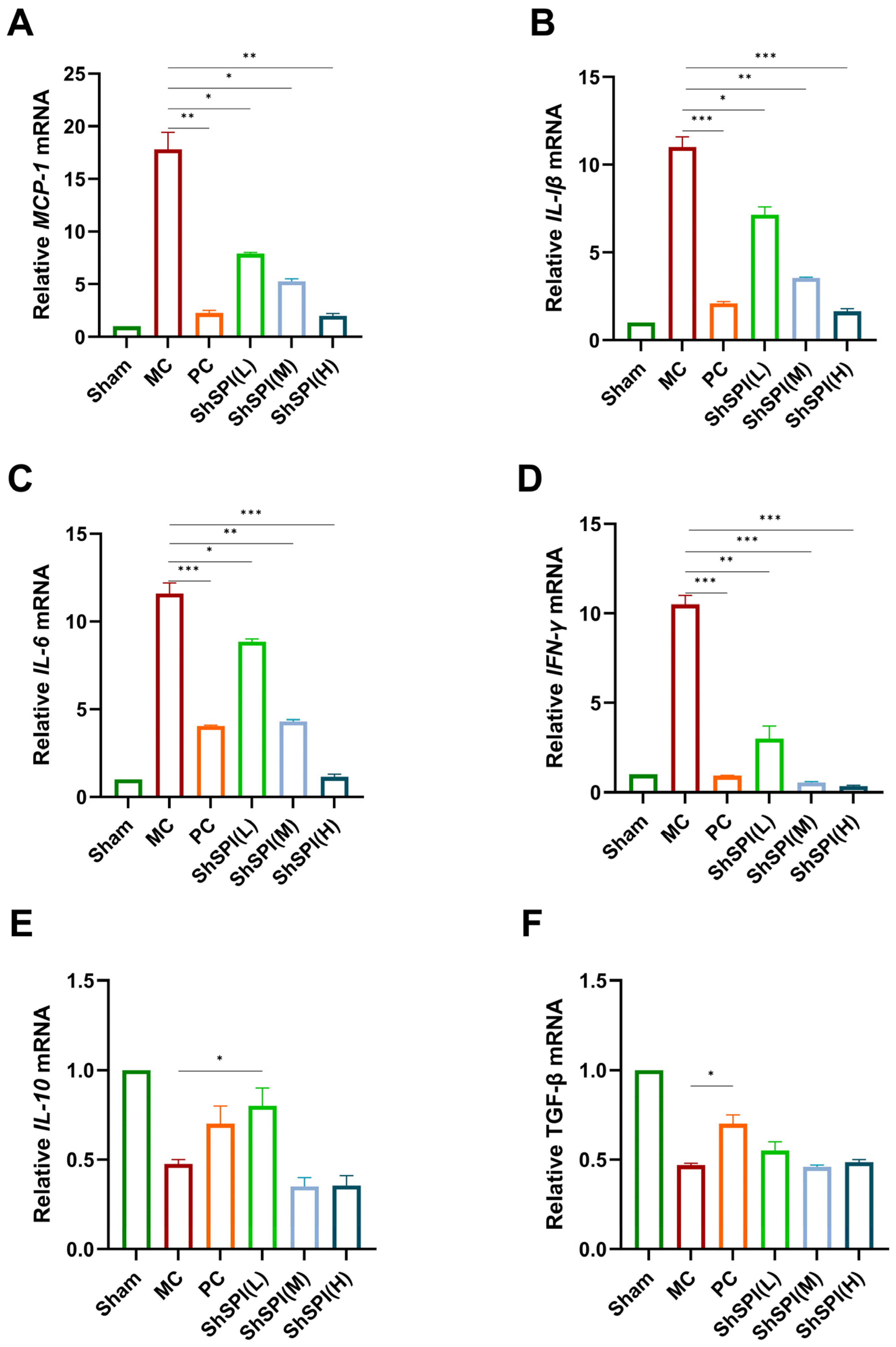
2.3. Effect of ShSPI on Inflammation in Bleomycin-Induced Mouse Models
2.4. Toxicity Study of ShSPI Single Administration on Mice
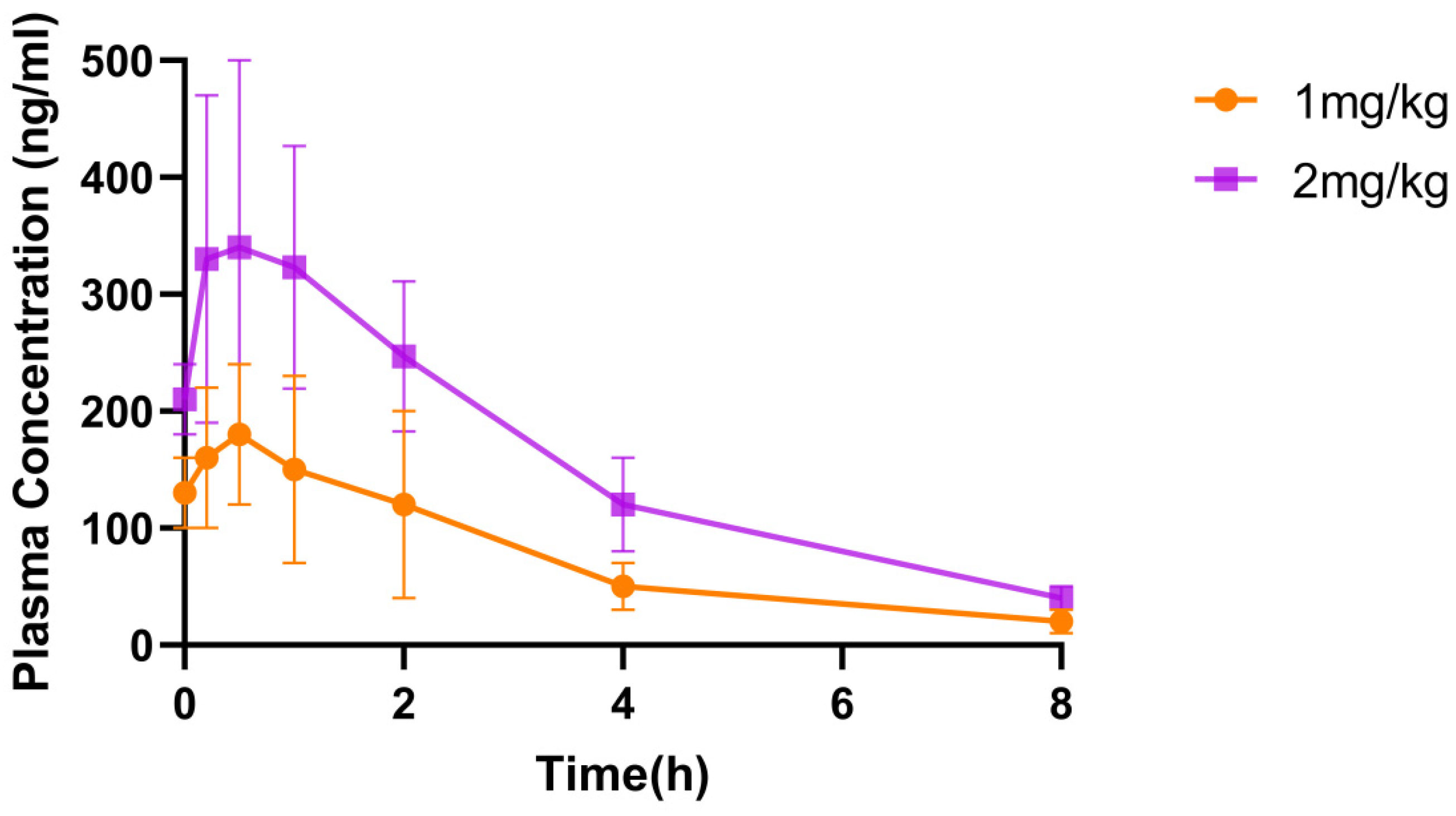
2.5. Pharmacokinetic Study of ShSPI in Mice
3. Discussion and Conclusions
4. Materials and Methods
4.1. Peptide Synthesis and Refolding
4.2. Grouping and Administration of Model Mice
4.3. Histopathologic Analysis of the Lungs
4.4. Total RNA Extraction and RT-qPCR
4.5. Toxicity Study of ShSPI Single Administration in Mice
4.6. Pharmacokinetic Study of ShSPI in Rats
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Zhao, H.; Li, B.-L.; Gao, F.; Liu, H.; Cai, J.-M.; Zheng, M. CpG-oligodeoxynucleotides may be effective for preventing ionizing radiation induced pulmonary fibrosis. Toxicol. Lett. 2018, 292, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, Z.; Zhou, Q.; Chang, M.; Wang, Y.; Guan, Y.; Li, H.; Zhao, Y.; Liu, N.; Jin, Y.; et al. Metformin alleviates crystalline silica-induced pulmonary fibrosis by remodeling endothelial cells to mesenchymal transition via autophagy signaling. Ecotoxicol. Environ. Saf. 2022, 245, 114100. [Google Scholar] [CrossRef]
- Chen, I.-C.; Liu, Y.-C.; Wu, Y.-H.; Lo, S.-H.; Dai, Z.-K.; Hsu, J.-H.; Tseng, Y.-H. Evaluation of Proteasome Inhibitors in the Treatment of Idiopathic Pulmonary Fibrosis. Cells 2022, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Lee, Y.-J.; Choi, Y.-H.; Park, E.-M.; Kang, J.L. Gas6 Ameliorates Inflammatory Response and Apoptosis in Bleomycin-Induced Acute Lung Injury. Biomedicines 2021, 9, 1674. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Cox, I.A.; Arriagada, N.B.; de Graaff, B.; Corte, T.J.; Glaspole, I.; Lartey, S.; Walters, E.H.; Palmer, A.J. Health-related quality of life of patients with idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Eur. Respir. Rev. 2020, 29, 200154. [Google Scholar] [CrossRef]
- Kreuter, M.; Swigris, J.; Pittrow, D.; Geier, S.; Klotsche, J.; Prasse, A.; Wirtz, H.; Koschel, D.; Andreas, S.; Claussen, M.; et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: Longitudinal data from the INSIGHTS-IPF registry. Respir. Res. 2019, 20, 59. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Jia, Q.; Bo, C.; Shao, H.; Zhang, Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol. Lett. 2019, 300, 59–66. [Google Scholar] [CrossRef]
- Elazab, S.T.; Eldin, R.E.A.G. α-Bisabolol and royal jelly differentially mitigate thioacetamide-induced hepatic fibrosis in rats associated with the inhibition of TGF-β1/FAK/α-SMA signaling. Food Chem. Toxicol. 2024, 193, 115069. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.S.; Kuhbandner, I.; Gehrig, S.; Rickert-Zacharias, V.; Twigg, M.; Wege, S.; Taggart, C.C.; Herth, F.; Schultz, C.; Mall, M.A. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respir. J. 2018, 51, 1701910. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, Y.; Sun, H.; Zhang, Y.; Yang, J.; Brasier, A.R. BRD4 mediates NF-kappaB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1183–L1201. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Xu, G.; Wang, Q. Artesunate Protects LPS-Induced Acute Lung Injury by Inhibiting TLR4 Expression and Inducing Nrf2 Activation. Inflammation 2017, 40, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Shahzeidi, S.; Laurent, G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991, 65, 81–94. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 170057. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Luan, N.; Zhao, Q.; Duan, Z.; Ji, M.; Xing, M.; Zhu, T.; Mwangi, J.; Rong, M.; Liu, J.; Lai, R. Identification and Characterization of ShSPI, a Kazal-Type Elastase Inhibitor from the Venom of Scolopendra Hainanum. Toxins 2019, 11, 708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruwanpura, S.M.; Thomas, B.J.; Bardin, P.G. Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease. Am. J. Respir. Cell Mol. Biol. 2020, 62, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Cuello-Garcia, C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19, Erratum in Am. J. Respir. Crit. Care Med. 2015, 192, 644. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; De Los Santos, G.F.; Phan, S.H. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol. Biol. 2017, 1627, 27–42. [Google Scholar] [CrossRef]
- Della Latta, V.; Cecchettini, A.; Del Ry, S.; Morales, M.A. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol. Res. 2015, 97, 122–130. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.H.A.; Vieira, J.B.; Cabral, M.R.; Antunes, M.A.; Lee, D.; Cruz, F.F.; Hanes, J.; Rocco, P.R.M.; Morales, M.M.; Suk, J.S. Development of nintedanib nanosuspension for inhaled treatment of experimental silicosis. Bioeng. Transl. Med. 2023, 8, e10401. [Google Scholar]
- Ruggiero, V.; Aquino, G.; Del Gaudio, P.; Mencherini, T.; Tedesco, C.; Russo, P. Development and characterization of nintedanib inhalable powders as a potential pulmonary fibrosis treatment. J. Drug Deliv. Sci. Technol. 2024, 92, 105340. [Google Scholar] [CrossRef]
- Chaudhuri, N.; Azuma, A.; Sroka-Saidi, K.; Erhardt, E.; Ritter, I.; Harari, S. Safety and Tolerability of Nintedanib in Patients with Fibrosing Interstitial Lung Diseases: Post-marketing Data. Adv. Ther. 2024, 41, 4581–4590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Salar-Behzadi, S. Oral inhalation for delivery of proteins and peptides to the lungs. Eur. J. Pharm. Biopharm. 2021, 163, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Yang, F.; Xie, C.; Du, W.; Mohammadtursun, N.; Wang, B.; Le, J.; Dong, J. Pulmonary fibrosis model of mice induced by different administration methods of bleomycin. BMC Pulm. Med. 2023, 23, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, B.; Ge, C.; Wu, F.; Fan, Y.; Kong, J.; Li, K.; Bian, H.; Miao, Q. Wogonin Attenuates Bleomycin-Induced Pulmonary Fibrosis and Oxidative Stress Injury via the MAPK Signaling Pathway. Biol. Pharm. Bull. 2024, 47, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Wu, Y.; Ai, F.; Li, W.; Peng, H.; Gui, F.; Yu, B.; Chen, Z. Sinomenine attenuates bleomycin-induced pulmonary fibrosis, inflammation, and oxidative stress by inhibiting TLR4/NLRP3/TGFβ signaling. Inhal. Toxicol. 2024, 36, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, M.; Ai, Y.; Zheng, S.; Chen, Y.; Du, H.; Yuan, S.; Guo, X.; Yuan, Y.; Li, G.; et al. The compound artemisinin-hydroxychloroquine ameliorates bleomycin-induced pulmonary fibrosis in rats by inhibiting TGF-β1/Smad2/3 signaling pathway. Pulm. Pharmacol. Ther. 2023, 83, 102268. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Long, T.; Zhang, H.; Li, M.; Sun, Q.; Zhai, X.; Sun, L. Anti-fibrosis attributes: UHPLC-MS/MS-based pharmacokinetics profiling of a novel autotaxin inhibitor with excellent in vivo efficacy in rat. Biomed. Chromatogr. 2022, 36, e5301. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elaziz, K.S.; Cheng, R.; Chen, J.; Maarse, H.; Lee, Y.; Yang, W.; Chien, B.; Diamant, Z.; Kosterink, J.; Touw, D.J. Validation of a method for the determination of Aderamastat (FP-025) in K2EDTA human plasma by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2024, 1245, 124244. [Google Scholar] [CrossRef] [PubMed]

| Name | 40 mg/kg | 80 mg/kg | 160 mg/kg | Control |
|---|---|---|---|---|
| WBC (109/L) | 8.22 ± 3.30 | 6.66 ± 2.04 | 11.5 ± 4.13 | 6.48 ± 2.37 |
| Neu (109/L) | 1.41 ± 0.69 | 1.35 ± 0.40 | 2.23 ± 1.28 | 1.30 ± 0.50 |
| Lym (109/L) | 6.40 ± 2.49 | 4.66 ± 1.68 | 8.63 ± 2.79 | 4.80 ± 1.94 |
| Mon (109/L) | 0.28 ± 0.13 | 0.38 ± 0.05 | 0.42 ± 0.21 | 0.25 ± 0.11 |
| Eos (109/L) | 0.09 ± 0.05 | 0.22 ± 0.20 | 0.21 ± 0.19 | 0.10 ± 0.04 |
| Bas (109/L) | 0.02 ± 0.01 | 0.03 ± 0.04 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Neu% (%) | 16.78 ± 1.90 | 21.23 ± 5.92 | 18.46 ± 5.30 | 20.73 ± 4.53 |
| Lym% (%) | 78.20 ± 2.18 | 68.93 ± 6.78 | 75.90 ± 6.54 | 73.00 ± 5.83 |
| Mon% (%) | 3.35 ± 0.74 | 6.16 ± 1.76 * | 3.58 ± 0.74 | 4.03 ± 0.86 |
| Eos% (%) | 131 ± 0.77 | 3.10 ± 2.15 | 1.85 ± 1.23 | 1.80 ± 1.13 |
| Bas% (%) | 0.35 ± 0.28 | 0.56 ± 0.60 | 0.20 ± 0.12 | 0.43 ± 0.21 |
| RBC (1012/L) | 7.78 ± 0.23 | 7.49 ± 0.34 | 7.90 ± 0.31 | 7.52 ± 0.61 |
| HGB (g/L) | 123.50 ± 5.64 | 120.33 ± 6.83 | 126.66 ± 4.36 | 121.5 ± 10.55 |
| HCT (%) | 36.98 ± 1.32 | 35.93 ± 2.11 | 37.81 ± 1.30 | 36.23 ± 2.85 |
| MCV (fL) | 47.56 ± 0.95 | 47.95 ± 0.38 | 47.86 ± 65 | 48.16 ± 0.46 |
| MCH (pg) | 15.86 ± 0.43 | 16.08 ± 0.40 | 16.05 ± 0.40 | 16.16 ± 0.17 |
| MCHC (g/L) | 333.50±3.98 | 335.33 ± 6.28 | 335.33 ± 4.13 | 335.66 ± 4.63 |
| RDW-CV (%) | 12.70 ± 0.76 | 12.18 ± 0.22 | 12.83 ± 0.74 | 12.40 ± 40.41 |
| RDW-SD (fL) | 26.68 ± 1.26 | 25.75 ± 1.03 | 27.20 ± 1.81 | 26.21 ± 0.68 |
| PLT (109/L) | 1326.50 ± 494.34 | 1272 ± 206.81 | 1511.2 ± 178.44 | 1319.8 ± 47.06 |
| MPV (fL) | 5.35 ± 0.18 | 5.48 ± 0.48 | 5.21 ± 0.21 | 5.31 ± 0.47 |
| PDW | 15.21 ± 0.17 | 15.33 ± 0.10 | 15.21 ± 0.07 | 15.18 ± 0.11 |
| PCT (%) | 0.71 ± 0.04 | 0.69 ± 0.09 | 0.78 ± 0.07 | 0.70 ± 10.07 |
| Name | 40 mg/kg | 80 mg/kg | 160 mg/kg | Control |
|---|---|---|---|---|
| ALT (U/L) | 44.95 ± 22.02 | 38.58 ± 7.12 | 39.80 ± 7.79 | 49.76 ± 33.81 |
| AST (U/L) | 97.20 ± 13.33 | 92.95 ± 20.45 | 80.6 ± 16.79 | 83.03 ± 26.05 |
| ALP (U/L) | 85.25 ± 18.91 | 94.75 ± 22.33 | 98.6 ± 27.17 | 88.16 ± 23.88 |
| r-GT (U/L) | 0.38 ± 0.20 | 0.00 ± 1.01 | 0.31 ± 0.35 | 0.43 ± 0.49 |
| ALBII (g/L) | 29.13 ± 1.27 | 28.05 ± 1.53 | 30.1 ± 1.28 | 29.20 ± 2.56 |
| TC (mmol/L) | 2.09 ± 0.33 | 2.21 ± 0.61 | 2.26 ± 0.33 | 2.21 ± 0.45 |
| CREA-S (μmol/L) | 21.70 ± 4.99 | 23.10 ± 2.24 | 19.1 ± 3.98 | 18.7 ± 2.77 |
| UREA (mmol/L) | 17.07 ± 3.07 | 14.79 ± 3.07 | 13.6 ± 4.00 | 12.85 ± 1.75 |
| CK (U/L) | 236.4 ± 108.76 | 175.4 ± 69.9 | 156.4 ± 75.0 | 155.50 ± 104.86 |
| Glu-G (mmol/L) | 9.93 ± 1.48 | 9.71 ± 1.69 | 9.24 ± 1.33 | 9.90 ± 1.36 |
| TG (mmol/L) | 1.35 ± 0.50 | 1.21 ± 0.32 | 1.23 ± 0.35 | 1.33 ± 0.28 |
| T-Bil-VII (μmol/L) | 1.73 ± 0.80 | 1.47 ± 0.43 | 209 ± 0.75 | 1.68 ± 0.60 |
| TPII (g/L) | 46.81 ± 1.48 | 45.26 ± 1.62 | 48.2 ± 1.79 | 46.69 ± 2.61 |
| AST/ALT | 2.45 ± 0.79 | 2.45 ± 0.64 | 2.16 ± 0.81 | 2.21 ± 1.20 |
| GLO2 (g/L) | 17.67 ± 0.80 | 17.21 ± 0.82 | 18.0 ± 1.06 | 17.49 ± 1.03 |
| A/G2 (g/L) | 1.65 ± 0.11 | 1.63 ± 0.13 | 1.67 ± 0.12 | 1.67 ± 0.18 |
| potassium ion (mmol/L) | 4.82 ± 0.27 | 5.01 ± 0.46 | 4.68 ± 0.30 | 4.77 ± 0.33 |
| sodium ion (mmol/L) | 152.39 ± 1.75 | 150.7 ± 1.89 | 152.9 ± 1.60 | 152.75 ± 1.82 |
| chloride ion (mmol/L) | 106.80 ± 1.14 | 106.5 ± 1.26 | 106.5 ± 1.44 | 108.33 ± 0.68 |
| calcium ionomer (mmol/L) | 1.23 ± 0.01 * | 1.24 ± 0.05 * | 1.28 ± 0.03 | 1.33 ± 0.03 |
| total calcium (mmol/L) | 2.48 ± 0.02 * | 2.49 ± 0.10 * | 2.5 ± 0.06 * | 2.67 ± 0.05 |
| calcium binding (mmol/L) | 1.23 ± 0.011 | 1.24 ± 0.05 | 1.28 ± 0.03 | 1.33 ± 0.03 |
| Pharmacokinetic Parameters | 1 mg/kg ShSPI (Inhalation) | 2 mg/kg ShSPI (Inhalation) |
|---|---|---|
| T1/2 (h) | 1.98 ± 0.70 | 2.14 ± 0.663 |
| Cmax (ng·mL−1) | 188.00 ± 64.40 | 347.00 ± 151.00 |
| MRTinf (h) | 2.86 ± 0.725 | 3.1 ± 0.898 |
| CI/F (ng·min−1·kg−1) | 30.10 ± 15.20 | 29.00 ± 11.90 |
| AUC0–t (h·ng·mL−1) | 580.00 ± 294.00 | 1169.00 ± 415.00 |
| AUC0–iaf (h·ng·mL−1) | 641.00 ± 243.00 | 1265.00 ± 422.00 |
| Tmax (h) | 1.00 | 1.00 |
| Name | Upstream Primers (5′-3′) | Downstream Primers (5′-3′) |
|---|---|---|
| IL-10 | GGTGAGAAGCTGAAGACCCT | ACACCTTGGTCTTGGAGCTT |
| MCP-1 | GTCCCTGTCATGCTTCTGG | GCGTTAACTGCATCTGGCT |
| TGF-β | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| IFN-γ | TGGCTGTTTCTGGCTGTTACTG | AATCAGCAGCGACTCCTTTTCC |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| β-actin | CACCATGTACCCAGGCATTG | CCTGCTTGCTGATCCACATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, X.; Liu, B.; Li, D.; Wang, X.; Long, C.; Feng, X.; Liao, Q.; Rong, M. An Elastase Inhibitor ShSPI from Centipede Attenuates Bleomycin-Induced Pulmonary Fibrosis. Toxins 2025, 17, 213. https://doi.org/10.3390/toxins17050213
Lian X, Liu B, Li D, Wang X, Long C, Feng X, Liao Q, Rong M. An Elastase Inhibitor ShSPI from Centipede Attenuates Bleomycin-Induced Pulmonary Fibrosis. Toxins. 2025; 17(5):213. https://doi.org/10.3390/toxins17050213
Chicago/Turabian StyleLian, Xi, Bin Liu, Dan Li, Xinyao Wang, Chengbo Long, Xing Feng, Qiong Liao, and Mingqiang Rong. 2025. "An Elastase Inhibitor ShSPI from Centipede Attenuates Bleomycin-Induced Pulmonary Fibrosis" Toxins 17, no. 5: 213. https://doi.org/10.3390/toxins17050213
APA StyleLian, X., Liu, B., Li, D., Wang, X., Long, C., Feng, X., Liao, Q., & Rong, M. (2025). An Elastase Inhibitor ShSPI from Centipede Attenuates Bleomycin-Induced Pulmonary Fibrosis. Toxins, 17(5), 213. https://doi.org/10.3390/toxins17050213






