Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom
Abstract
1. Introduction
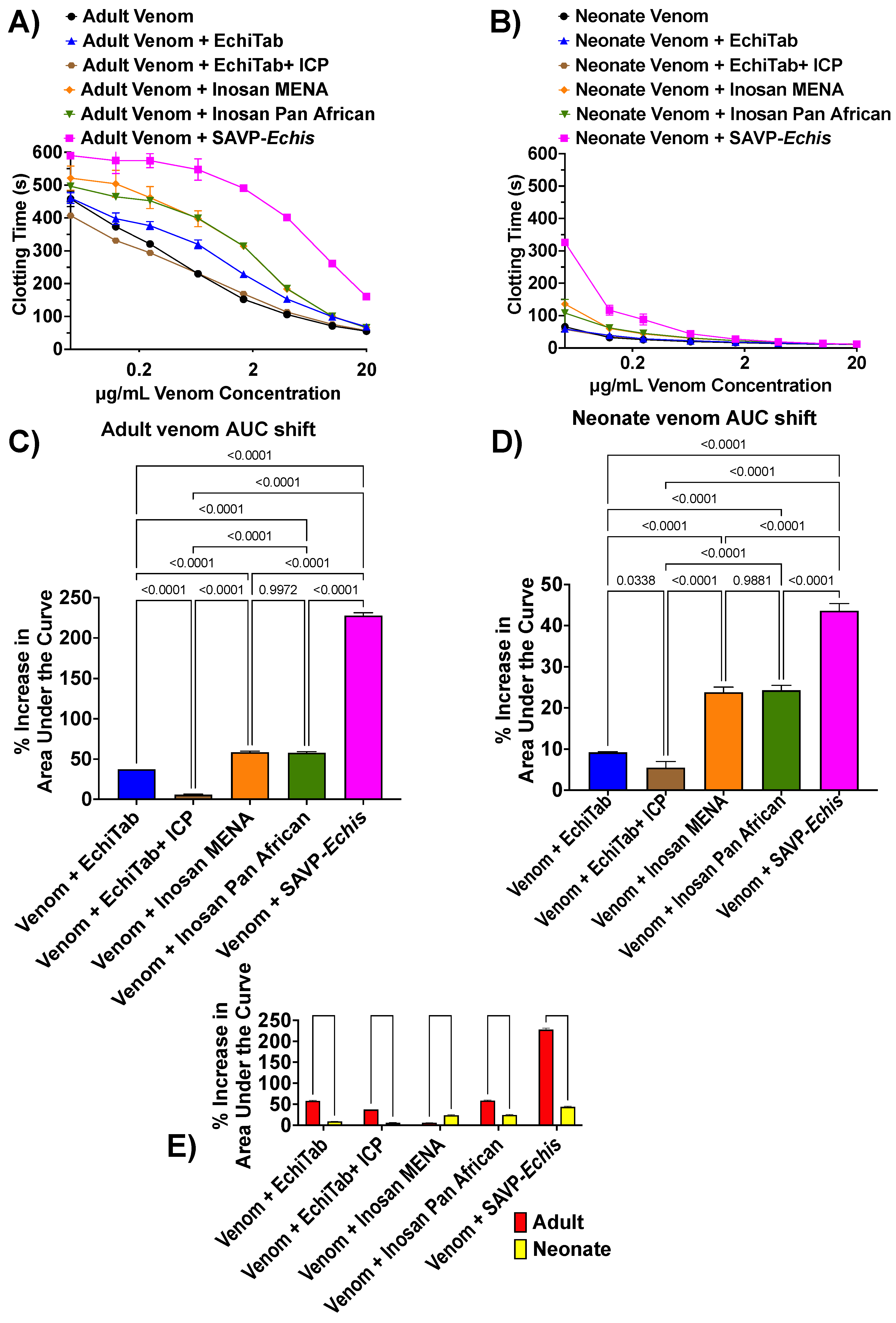
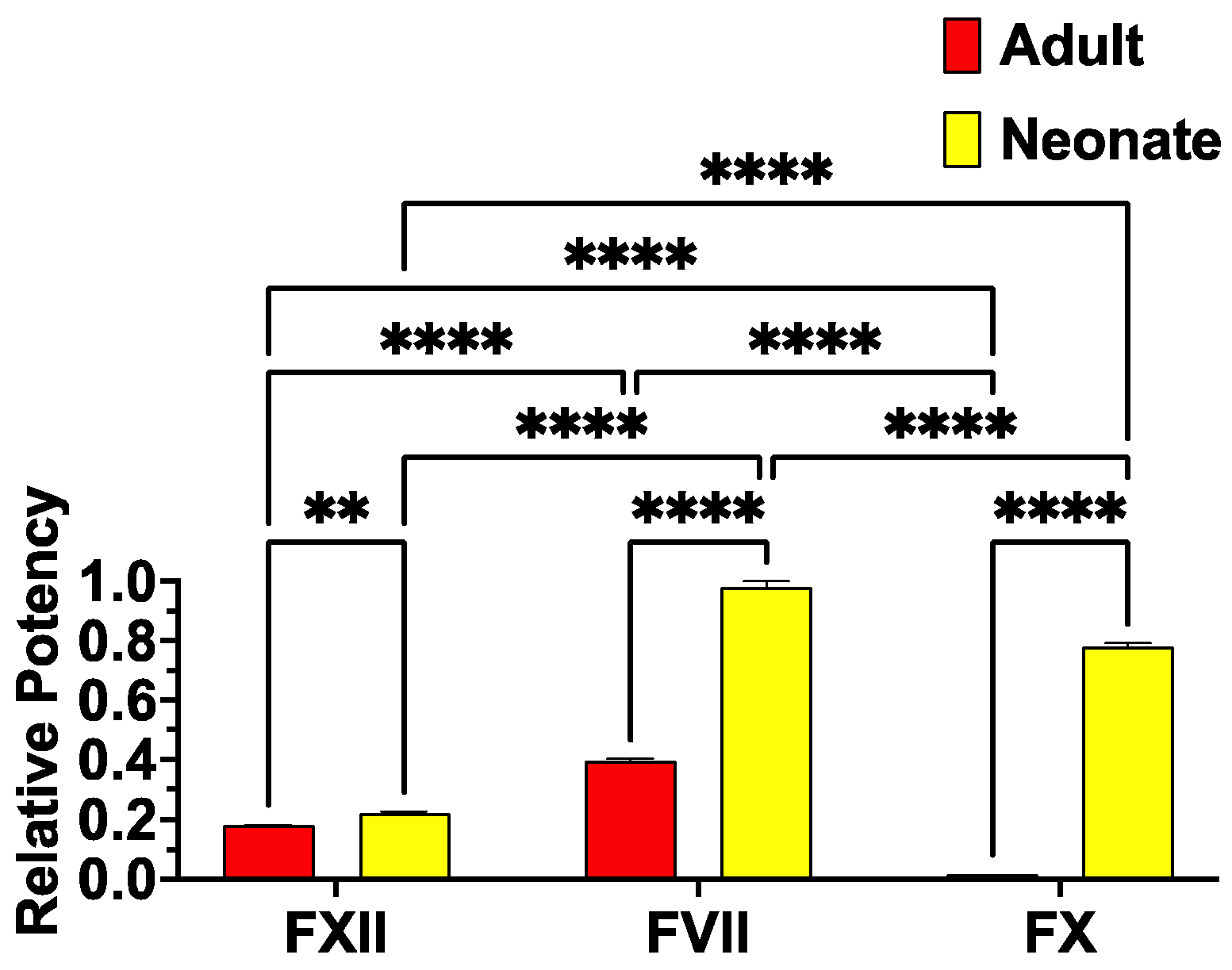
2. Results and Discussion
3. Materials and Methods
3.1. Venom
3.2. Plasma
3.3. Thromboelastography (TEG)
3.4. Coagulation Testing
3.5. Antivenom Neutralization Studies
3.6. Factor Activation Studies
- Reagents were manually plated in 384-well plates (black, lot#1171125, Nunc™ Thermo Scientific, Rochester, NY, USA).
- Loaded into a Fluoroskan Ascent™ (Thermo Scientific, Vantaa, Finland), followed by automated pipetting of 70 μL of buffer containing 5 mM CaCl2, 150 mM NaCl, and 50 mM Tris-HCl (pH 7.3) and Fluorogenic Peptide Substrate (ES011Boc-Val-Pro-Arg-AMC. Boc: t-Butyloxycarbonyl; 7-Amino-4-methylcoumarin; R & D systems, Cat# ES011, Minneapolis, Minnesota) in 500:1 ratio to start the reaction.
- The plate was warmed up at 37 °C.
- The plate was shaken for 3 s before each measurement.
- The reaction was carried out 300 times at 390/460 nm (excitation/emission), and every 10 s, the fluorescence generated by the cleavage of the substrate was measured by Ascent® Software v2.6 (Thermo Scientific, Vantaa, Finland).
- To obtain final results, subtraction of “venom without zymogen” values from “venom with zymogen” values was calculated to nullify artificial increment of the fluorescence values caused by some venoms that work directly on the substrate.
- Finally, the resultant values from the subtractions were normalized as a percentage relative to the activated factors/enzyme (kaolin in case of FXII) by organizing in Excel and then analyzing in GraphPad PRISM 8.1.1 (GraphPad Prism Inc., La Jolla, CA, USA).
3.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ediriweera, D.S.; Kasturiratne, A.; Pathmeswaran, A.; Gunawardena, N.K.; Wijayawickrama, B.A.; Jayamanne, S.F.; Isbister, G.K.; Dawson, A.; Giorgi, E.; Diggle, P.J.; et al. Mapping the risk of snakebite in sri lanka—A national survey with geospatial analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004813. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Rathuwithana, A.C.; Kasturiratne, A.; Lalloo, D.G.; de Silva, H.J. Underestimation of snakebite mortality by hospital statistics in the Monaragala District of Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Katewa, S.S.; Sharma, S.K.; Galav, P.; Jain, V. Snakelore and indigenous snakebite remedies practiced by some tribals of Rajasthan. Indian J. Tradit. Knowl. 2010, 10, 258–268. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Mohapatra, B.; Warrell, D.A.; Suraweera, W.; Bhatia, P.; Dhingra, N.; Jotkar, R.M.; Rodriguez, P.S.; Mishra, K.; Whitaker, R.; Jha, P.; et al. Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl. Trop. Dis. 2011, 5, e1018. [Google Scholar] [CrossRef]
- Schioldann, E.; Mahmood, M.A.; Kyaw, M.M.; Halliday, D.; Thwin, K.T.; Chit, N.N.; Cumming, R.; Bacon, D.; Alfred, S.; White, J.; et al. Why snakebite patients in Myanmar seek traditional healers despite availability of biomedical care at hospitals? Community perspectives on reasons. PLoS Negl. Trop. Dis. 2018, 12, e0006299. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bovier, P.; Jha, N.; Alirol, E.; Loutan, L.; Chappuis, F. Effectiveness of rapid transport of victims and community health education on snake bite fatalities in rural Nepal. Am. J. Trop. Med. Hyg. 2013, 89, 145–150. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Vaiyapuri, R.; Ashokan, R.; Ramasamy, K.; Nattamaisundar, K.; Jeyaraj, A.; Chandran, V.; Gajjeraman, P.; Baksh, M.F.; Gibbins, J.M.; et al. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PLoS ONE 2013, 8, e80090. [Google Scholar] [CrossRef]
- Michael, G.; Grema, B.; Aliyu, I.; Alhaji, M.; Lawal, T.; Ibrahim, H.; Fikin, A.; Gyaran, F.; Kane, K.; Thacher, T.; et al. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 47–56. [Google Scholar] [CrossRef]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. WHO 1998, 76, 515–524. [Google Scholar] [PubMed]
- Chippaux, J.P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A.; Davidson, N.; Greenwood, B.M.; Ormerod, L.D.; Pope, H.M.; Watkins, B.J.; Prentice, C.R. Poisoning by bites of the saw-scaled or carpet viper (Echis carinatus) in Nigeria. Q. J. Med. 1977, 46, 33–62. [Google Scholar]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Alape-Giron, A.; Sanz, L.; Escolano, J.; Flores-Diaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef]
- Ali, S.A.; Jackson, T.N.; Casewell, N.R.; Low, D.H.; Rossi, S.; Baumann, K.; Fathinia, B.; Visser, J.; Nouwens, A.; Hendrikx, I.; et al. Extreme venom variation in Middle Eastern vipers: A proteomics comparison of Eristicophis macmahonii, Pseudocerastes fieldi and Pseudocerastes persicus. J. Proteom. 2015, 116, 106–113. [Google Scholar] [CrossRef]
- Alirol, E.; Lechevalier, P.; Zamatto, F.; Chappuis, F.; Alcoba, G.; Potet, J. Antivenoms for snakebite envenoming: What is in the research pipeline? PLoS Negl. Trop. Dis. 2015, 9, e0003896. [Google Scholar] [CrossRef]
- Avella, I.; Calvete, J.J.; Sanz, L.; Wüster, W.; Licata, F.; Quesada-Bernat, S.; Rodríguez, Y.; Martínez-Freiría, F. Interpopulational variation and ontogenetic shift in the venom composition of Lataste’s viper (Vipera latastei, Boscá 1878) from northern Portugal. J. Proteom. 2022, 263, 104613. [Google Scholar] [CrossRef]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wuster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. Biol. Sci. R. Soc. 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Tanaka-Azevedo, A.M.; Silveira, G.P.M.; Sant’Anna, S.S.; Grego, K.F.; Rodrigues, C.F.B.; Fry, B.G. Clinical and evolutionary implications of dynamic coagulotoxicity divergences in Bothrops (lancehead pit viper) venoms. Toxins 2022, 14, 297. [Google Scholar] [CrossRef]
- Calvete, J.J.; Arias, A.S.; Rodriguez, Y.; Quesada-Bernat, S.; Sanchez, L.V.; Chippaux, J.P.; Pla, D.; Gutierrez, J.M. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: Neutralization of toxic activities and antivenomics. Toxicon 2016, 119, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Cook, D.A.; Wagstaff, S.C.; Nasidi, A.; Durfa, N.; Wuster, W.; Harrison, R.A. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 2010, 4, e851. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Wuster, W.; Cook, D.A.; Bolton, F.M.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteom. 2011, 74, 2169–2179. [Google Scholar] [CrossRef]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. Biol. Sci. R. Soc. 2016, 283, 20152841. [Google Scholar] [CrossRef]
- Madrigal, M.; Sanz, L.; Flores-Diaz, M.; Sasa, M.; Nunez, V.; Alape-Giron, A.; Calvete, J.J. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of Lachesis stenophrys and comparative proteomics of the venoms of adult Lachesis melanocephala and Lachesis acrochorda. J. Proteom. 2012, 77, 280–297. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Sasa, M.; Acevedo, M.E.; Dwyer, Q.; Durban, J.; Perez, A.; Rodriguez, Y.; Lomonte, B.; Calvete, J.J. Proteomic analysis of venom variability and ontogeny across the arboreal palm-pitvipers (genus Bothriechis). J. Proteom. 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Rodrigues, C.F.B.; Zdenek, C.N.; Bourke, L.A.; Seneci, L.; Chowdhury, A.; Freitas-de-Sousa, L.A.; de Alcantara Menezes, F.; Moura-da-Silva, A.M.; Tanaka-Azevedo, A.M.; Fry, B.G. Clinical implications of ontogenetic differences in the coagulotoxic activity of Bothrops jararacussu venoms. Toxicol. Lett. 2021, 348, 59–72. [Google Scholar] [CrossRef]
- Rogalski, A.; Soerensen, C.; Op den Brouw, B.; Lister, C.; Dashevsky, D.; Arbuckle, K.; Gloria, A.; Zdenek, C.N.; Casewell, N.R.; Gutierrez, J.M.; et al. Differential procoagulant effects of saw-scaled viper (Serpentes: Viperidae: Echis) snake venoms on human plasma and the narrow taxonomic ranges of antivenom efficacies. Toxicol. Lett. 2017, 280, 159–170. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef]
- Visser, L.E.; Kyei-Faried, S.; Belcher, D.W.; Geelhoed, D.W.; van Leeuwen, J.S.; van Roosmalen, J. Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: The importance of quality surveillance. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 445–450. [Google Scholar] [CrossRef]
- Warrell, D.A.; Warrell, M.J.; Edgar, W.; Prentice, C.R.; Mathison, J.; Mathison, J. Comparison of Pasteur and Behringwerke antivenoms in envenoming by the carpet viper (Echis carinatus). Br. Med. J. 1980, 280, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Bdolah, A.; Kinamon, S.; Batzri-Izraeli, R. The neurotoxic complex from the venom of Pseudocerastes fieldi. Contribution of the nontoxic subunit. Biochem. Int. 1985, 11, 627–636. [Google Scholar]
- Batzri-Izraeli, R.; Bdolah, A. Isolation and characterization of the main toxic fraction from the venom of the false horned viper (Pseudocerastes fieldi). Toxicon 1982, 20, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Bdolah, A.; Kaiser, I.I. Amino acid sequences of a heterodimeric neurotoxin from the venom of the false horned viper (Pseudocerastes fieldi). Toxicon 1995, 33, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Shabo-Shina, R.; Bdolah, A. Interactions of the neurotoxic complex from the venom of the false horned viper (Pseudocerastes fieldi) with rat striatal synaptosomes. Toxicon 1987, 25, 253–266. [Google Scholar] [CrossRef]
- Tsai, M.C.; Lee, C.Y.; Bdolah, A. Mode of neuromuscular blocking action of a toxic phospholipase A2 from Pseudocerastes fieldi (Field’s horned viper) snake venom. Toxicon 1983, 21, 527–534. [Google Scholar] [CrossRef]
- Op den Brouw, B.; Coimbra, F.C.P.; Bourke, L.A.; Huynh, T.M.; Vlecken, D.H.W.; Ghezellou, P.; Visser, J.C.; Dobson, J.S.; Fernandez-Rojo, M.A.; Ikonomopoulou, M.P.; et al. Extensive variation in the activities of Pseudocerastes and Eristicophis viper venoms suggests divergent envenoming strategies are used for prey capture. Toxins 2021, 13, 112. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal viperid snake genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef]
- Coimbra, F.C.P.; Dobson, J.; Zdenek, C.N.; Op den Brouw, B.; Hamilton, B.; Debono, J.; Masci, P.; Frank, N.; Ge, L.; Kwok, H.F.; et al. Does size matter? Venom proteomic and functional comparison between night adder species (Viperidae: Causus) with short and long venom glands. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 211, 7–14. [Google Scholar] [CrossRef]
- Dobson, J.; Yang, D.C.; Op den Brouw, B.; Cochran, C.; Huynh, T.; Kurrupu, S.; Sanchez, E.E.; Massey, D.J.; Baumann, K.; Jackson, T.N.W.; et al. Rattling the border wall: Pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2018, 205, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) venoms from Brazil: Differential biochemistry and antivenom efficacy resulting from prey-driven venom variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; Chowdhury, A.; Haw, G.Y.H.; Violette, A.; Fourmy, R.; Christ, T.; Vonk, F.J.; Fry, B.G. Taxon-selective venom variation in adult and neonate Daboia russelii (Russell’s Viper), and antivenom efficacy. Toxicon 2022, 205, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Seneci, L.; Zdenek, C.N.; Chowdhury, A.; Rodrigues, C.F.B.; Neri-Castro, E.; Benard-Valle, M.; Alagon, A.; Fry, B.G. A Clot Twist: Extreme variation in coagulotoxicity mechanisms in Mexican neotropical rattlesnake venoms. Front. Immunol. 2021, 12, 612846. [Google Scholar] [CrossRef]
- Cipriani, V.; Debono, J.; Goldenberg, J.; Jackson, T.N.; Arbuckle, K.; Dobson, J.; Koludarov, I.; Li, B.; Hay, C.; Dunstan, N. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 197, 53–60. [Google Scholar] [CrossRef]
- Jackson, T.N.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef]
- Pook, C.E.; Joger, U.; Stumpel, N.; Wuster, W. When continents collide: Phylogeny, historical biogeography and systematics of the medically important viper genus Echis (Squamata: Serpentes: Viperidae). Mol. Phylogenet. Evol. 2009, 53, 792–807. [Google Scholar] [CrossRef]
- Alencar, L.R.V.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 2016, 105, 50–62. [Google Scholar] [CrossRef]
- Gillissen, A.; Theakston, R.D.; Barth, J.; May, B.; Krieg, M.; Warrell, D.A. Neurotoxicity, haemostatic disturbances and haemolytic anaemia after a bite by a Tunisian saw-scaled or carpet viper (Echis ‘pyramidum’-complex): Failure of antivenom treatment. Toxicon 1994, 32, 937–944. [Google Scholar] [CrossRef]
- Casewell, N.R.; Harrison, R.A.; Wuster, W.; Wagstaff, S.C. Comparative venom gland transcriptome surveys of the saw-scaled vipers (Viperidae: Echis) reveal substantial intra-family gene diversity and novel venom transcripts. BMC Genom. 2009, 10, 564. [Google Scholar] [CrossRef]
- Bhatia, S.; Vasudevan, K. Comparative proteomics of geographically distinct saw-scaled viper (Echis carinatus) venoms from India. Toxicon X 2020, 7, 100048. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Senthilvadivel, V.; Velmurugan, D. Inhibitory effects of ascorbic acid toward snake venom metalloproteinase (SVMP) from Indian Echis carinatus venom: Insights from molecular modeling and binding studies. J. Biochem. Mol. Toxicol. 2018, 32, e22224. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.M.; Wahby, A.F. Purification and characterization of five snake venom metalloproteinases from Egyptian Echis pyramidum pyramidum venom. J. Toxicol. Sci. 2014, 39, 523–536. [Google Scholar] [CrossRef]
- Conlon, J.M.; Attoub, S.; Arafat, H.; Mechkarska, M.; Casewell, N.R.; Harrison, R.A.; Calvete, J.J. Cytotoxic activities of [Ser(4)(9)]phospholipase A(2) from the venom of the saw-scaled vipers Echis ocellatus, Echis pyramidum leakeyi, Echis carinatus sochureki, and Echis coloratus. Toxicon 2013, 71, 96–104. [Google Scholar] [CrossRef]
- Hemker, H.C.; van Dam-Mieras, M.C.; Devilee, P.P. The action of Echis carinatus venom on the blood coagulation system. Demonstration of an activator of factor X. Thromb. Res. 1984, 35, 1–9. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Theakston, R.D.; Tomy, S.C. An investigation of the coagulant activity of the venom of the saw-scaled viper (Echis carinatus) from Saudi Arabia. Ann. Trop. Med. Parasitol. 1988, 82, 503–509. [Google Scholar] [CrossRef]
- Khodadadi, S.; Rabiei, H.; Sardari, S.; Mahboudi, H.; Bayatzadeh, M.A.; Vazifeh Shiran, N.; Sardabi, M.; Akbari Eidgahi, M.R.; Madanchi, H.; Mohammadpour, N. Purification, and characterization of a new pro-coagulant protein from Iranian Echis carinatus venom. Biochem. Biophys. Rep. 2024, 38, 101701. [Google Scholar] [CrossRef]
- Morita, T. Proteases which activate factor X. In Enzymes from Snake Venom; Bailey, G.S., Ed.; Alaken Inc.: Fort Collins, CO, USA, 1998; pp. 179–208. [Google Scholar]
- Morita, T.; Iwanaga, S.; Suzuki, T. The mechanism of activation of bovine prothrombin by an activator isolated from Echis carinatus venom and characterization of the new active intermediates. J. Biochem. 1976, 79, 1089–1108. [Google Scholar] [CrossRef]
- Morita, T.; Iwanaga, S.; Suzuki, T. Activation of bovine prothrombin by an activator isolated from Echis carinatus venom. Thromb. Res. 1976, 8, 59–65. [Google Scholar] [CrossRef]
- Petrovan, R.J.; Govers-Riemslag, J.W.; Nowak, G.; Hemker, H.C.; Rosing, J.; Tans, G. Purification and characterization of multisquamase, the prothrombin activator present in Echis multisquamatus venom. Thromb. Res. 1997, 88, 309–316. [Google Scholar] [CrossRef]
- Solov’ev, D.A.; Platonova, T.N.; Ugarova, T.P. Isolation and characteristics of ekamulin—A prothrombin activator from multiscaled viper (Echis multisquamatus) venom. Biokhimiia 1996, 61, 1094–1105. [Google Scholar] [PubMed]
- Solovjov, D.A.; Platonova, T.N.; Ugarova, T.P. Purification and characterization of ecamulin—A prothrombin activator from the venom of multi-scaled viper (Echis multisquamatus). Ukr. Biokhim. Zh. 1996, 68, 18–19. [Google Scholar] [PubMed]
- Stocker, K.; Fischer, H.; Brogli, M. Chromogenic assay for the prothrombin activator ecarin from the venom of the saw-scaled viper (Echis carinatus). Toxicon 1986, 24, 81–89. [Google Scholar] [CrossRef]
- Tans, G.; Rosing, J. Snake venom activators of factor X: An overview. Haemostasis 2001, 31, 225–233. [Google Scholar] [CrossRef]
- Yamada, D.; Morita, T. Purification and characterization of a Ca2+-dependent prothrombin activator, multactivase, from the venom of Echis multisquamatus. J. Biochem. 1997, 122, 991–997. [Google Scholar] [CrossRef]
- Yamada, D.; Sekiya, F.; Morita, T. Isolation and characterization of carinactivase, a novel prothrombin activator in Echis carinatus venom with a unique catalytic mechanism. J. Biol. Chem. 1996, 271, 5200–5207. [Google Scholar] [CrossRef]
- Yamada, D.; Sekiya, F.; Morita, T. Prothrombin and factor X activator activities in the venoms of Viperidae snakes. Toxicon 1997, 35, 1581–1589. [Google Scholar] [CrossRef]
- Yukelson, L.Y.; Tans, G.; Thomassen, M.C.; Hemker, H.C.; Rosing, J. Procoagulant activities in venoms from central Asian snakes. Toxicon 1991, 29, 491–502. [Google Scholar] [CrossRef]
- Abdeldayem, A.; Alanazi, A.A.; Aljabri, J.N.; Abid, I. Challenges in the management of an Echis coloratus adult snakebite victim at a tertiary care hospital: A case report. Am. J. Case Rep. 2021, 22, e931532. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Warrell, D.A.; Arnett, C. The importance of bites by the saw-scaled or carpet viper (Echis carinatus): Epidemiological studies in Nigeria and a review of the world literature. Acta Trop. 1976, 33, 307–341. [Google Scholar] [PubMed]
- Katkar, G.D.; Sundaram, M.S.; Hemshekhar, M.; Sharma, D.R.; Santhosh, M.S.; Sunitha, K.; Rangappa, K.S.; Girish, K.S.; Kemparaju, K. Melatonin alleviates Echis carinatus venom-induced toxicities by modulating inflammatory mediators and oxidative stress. J. Pineal Res. 2014, 56, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Kempson, K.; Chowdhury, A.; Violette, A.; Fourmy, R.; Soria, R.; Fry, B.G. Age is just a number: Ontogenetic conservation in activation of blood clotting factors VII, X, and XII by Caucasus Blunt-Nosed Viper (Macrovipera lebetina obtusa) venoms. Toxins 2024, 16, 520. [Google Scholar] [CrossRef]
- Qiao, Z.; Jones, L.; Bourke, L.A.; Seneci, L.; Chowdhury, A.; Violette, A.; Fourmy, R.; Soria, R.; Aldridge, M.; Fry, B.G. Tiny but mighty: Vipera ammodytes meridionalis (Eastern Long-Nosed Viper) ontogenetic venom variations in procoagulant potency and the impact on antivenom efficacies. Toxins 2024, 16, 396. [Google Scholar] [CrossRef]
- Jones, L.; Youngman, N.J.; Neri-Castro, E.; Guadarrama-Martinez, A.; Lewin, M.R.; Carter, R.; Frank, N.; Fry, B.G. Differential antivenom and small-molecule inhibition of novel coagulotoxic variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium American viperid snake venoms. Toxins 2022, 14, 511. [Google Scholar] [CrossRef]
- Nakagaki, T.; Lin, P.; Kisiel, W. Activation of human factor VII by the prothrombin activator from the venom of Oxyuranus scutellatus (Taipan snake). Thromb. Res. 1992, 65, 105–116. [Google Scholar] [CrossRef]
- Chandrasekara, U.; Chowdhury, A.; Seneci, L.; Zdenek, C.N.; Dunstan, N.; Fry, B.G. From venom to vein: Factor VII activation as a major pathophysiological target for procoagulant Australian elapid snake venoms. Toxins 2024, 16, 430. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Carter, R.W.; Casewell, N.R.; Fry, B.G. Keel venom: Rhabdophis subminiatus (Red-Necked Keelback) venom pathophysiologically affects diverse blood clotting pathways. Toxicon 2022, 218, 19–24. [Google Scholar] [CrossRef]
- Dobson, J.; Chowdhury, A.; Tai, A.P.J.; van der Ploeg, H.; Gillett, A.; Fry, B.G. The clot thickens: Differential coagulotoxic and cardiotoxic activities of Anguimorpha lizard venoms. Toxins 2024, 16, 283. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Benard-Valle, M.; Alagon, A.; Gutierrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American lancehead pit-vipers: Coagulotoxic venom effects and antivenom neutralisation of Bothrops asper and B. atrox geographical variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef]

| Antivenom | Company | Country of Production | Species Used in Immunizing Mixture |
|---|---|---|---|
| EchiTabPlus (ET-Plus) | ICP | Costa Rica | Bitis arietans, Echis ocellatus, Naja nigricollis |
| SAVP-Echis | SAVP (formerly SAIMR) | South Africa | Echis ocellatus and E. pyramidum |
| EchiTabG (ET-G) | MicroPharm | UK | Echis ocellatus |
| Inoserp Pan-Africa (Inoserp-P) | Inosan | Mexico, Spain | Echis ocellatus, E. leucoaster, E. pyramidum, Bitis arietans, B. nasicornis, B. gabonica, Dendroaspus polylepis, D. viridis, D. angusticeps, D. jamesoni, Naja niricollis, N. melanoleuca, Naja haje, and Naja pallida. |
| Inoserp MENA | Inosan | Mexico, Spain | Bitis arietans, Cerastes cerastes, C. gasperettii, Daboia deserti, D. mauritanica, D. palestinae, Echis carinatus sochureki, E. coloratus, E. leucogaster, E. megalocephalus, E. pyramidum, Macrovipera lebetina obtusa, M. lebetina turanica, Montivipera bornmuelleri, M. raddei kurdistanica, Pseudocerastes persicus, Vipera latastei, Naja haje, N. nubiae, N. pallida, and Walterinnesia aeyptia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, A.; Jones, L.; Bourke, L.A.; Seneci, L.; Chowdhury, A.; Violette, A.; Fourmy, R.; Soria, R.; Aldridge, M.; Fry, B.G. Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom. Toxins 2025, 17, 149. https://doi.org/10.3390/toxins17030149
Barker A, Jones L, Bourke LA, Seneci L, Chowdhury A, Violette A, Fourmy R, Soria R, Aldridge M, Fry BG. Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom. Toxins. 2025; 17(3):149. https://doi.org/10.3390/toxins17030149
Chicago/Turabian StyleBarker, Alex, Lee Jones, Lachlan A. Bourke, Lorenzo Seneci, Abhinandan Chowdhury, Aude Violette, Rudy Fourmy, Raul Soria, Matt Aldridge, and Bryan G. Fry. 2025. "Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom" Toxins 17, no. 3: 149. https://doi.org/10.3390/toxins17030149
APA StyleBarker, A., Jones, L., Bourke, L. A., Seneci, L., Chowdhury, A., Violette, A., Fourmy, R., Soria, R., Aldridge, M., & Fry, B. G. (2025). Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom. Toxins, 17(3), 149. https://doi.org/10.3390/toxins17030149






