Protective Effect of Lipoic Acid on Oxidative Stress and Tissue Damage Induced by Aflatoxin B1 in Young Laying Hens
Abstract
:1. Introduction
2. Results
2.1. Changes in Production Performance and Organ Weight
2.2. Changes in Serum Biochemical Indicators
2.3. Changes in Serum Antioxidant Enzyme Levels
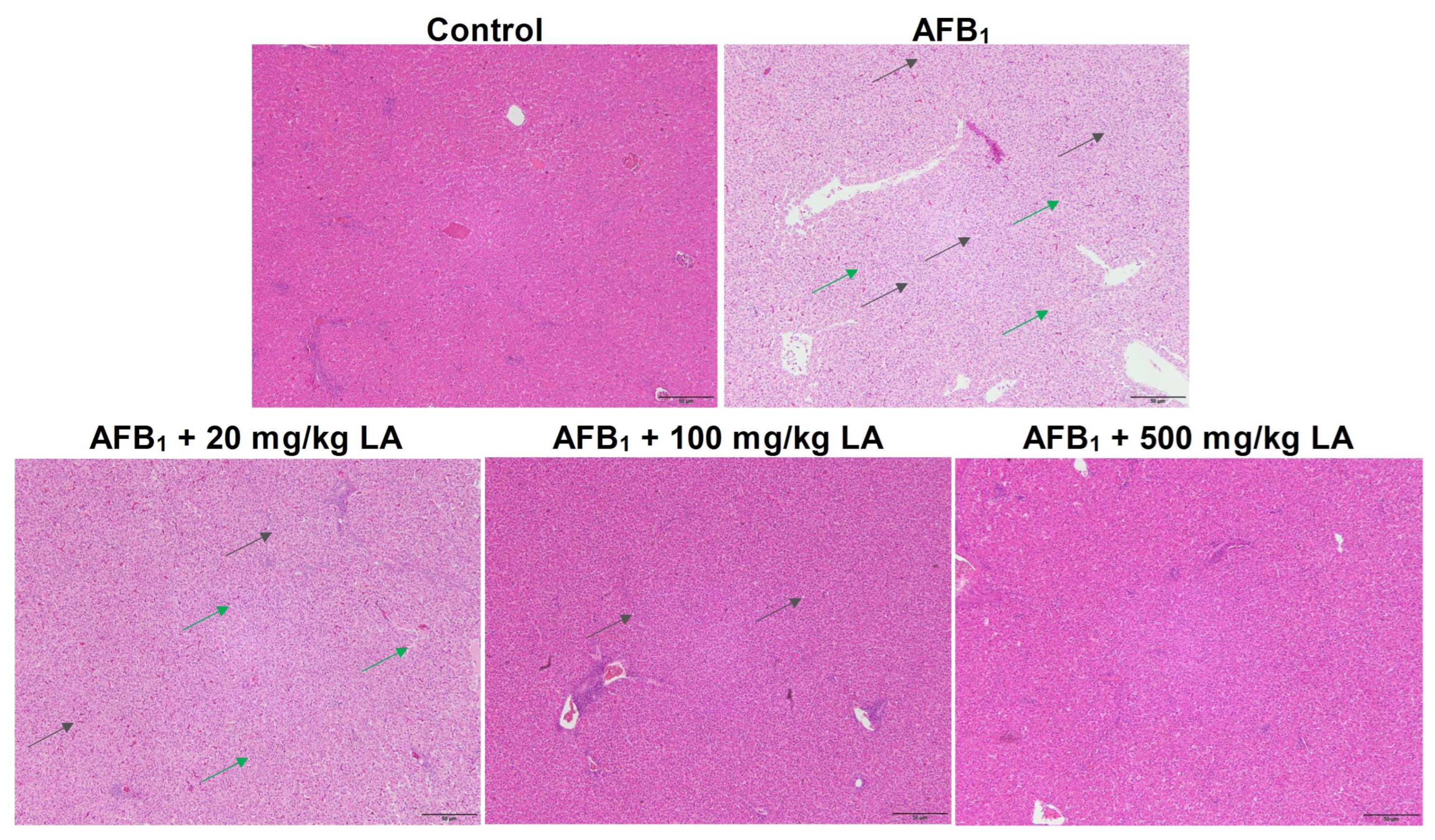
2.4. Changes in Liver Tissue Structure
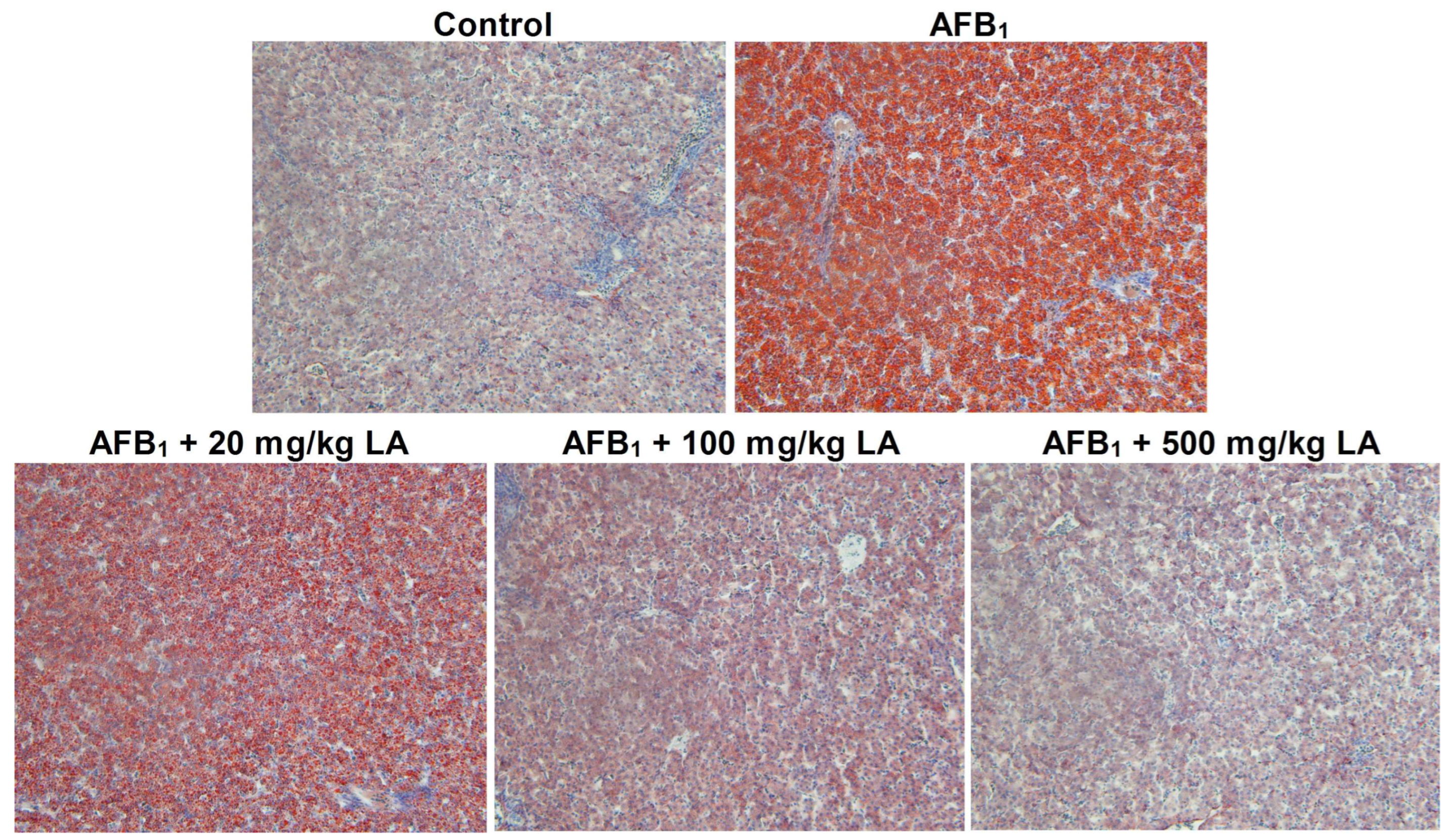
2.5. The Condition of Hepatic Steatosis
2.6. Changes in Liver Ultrastructure
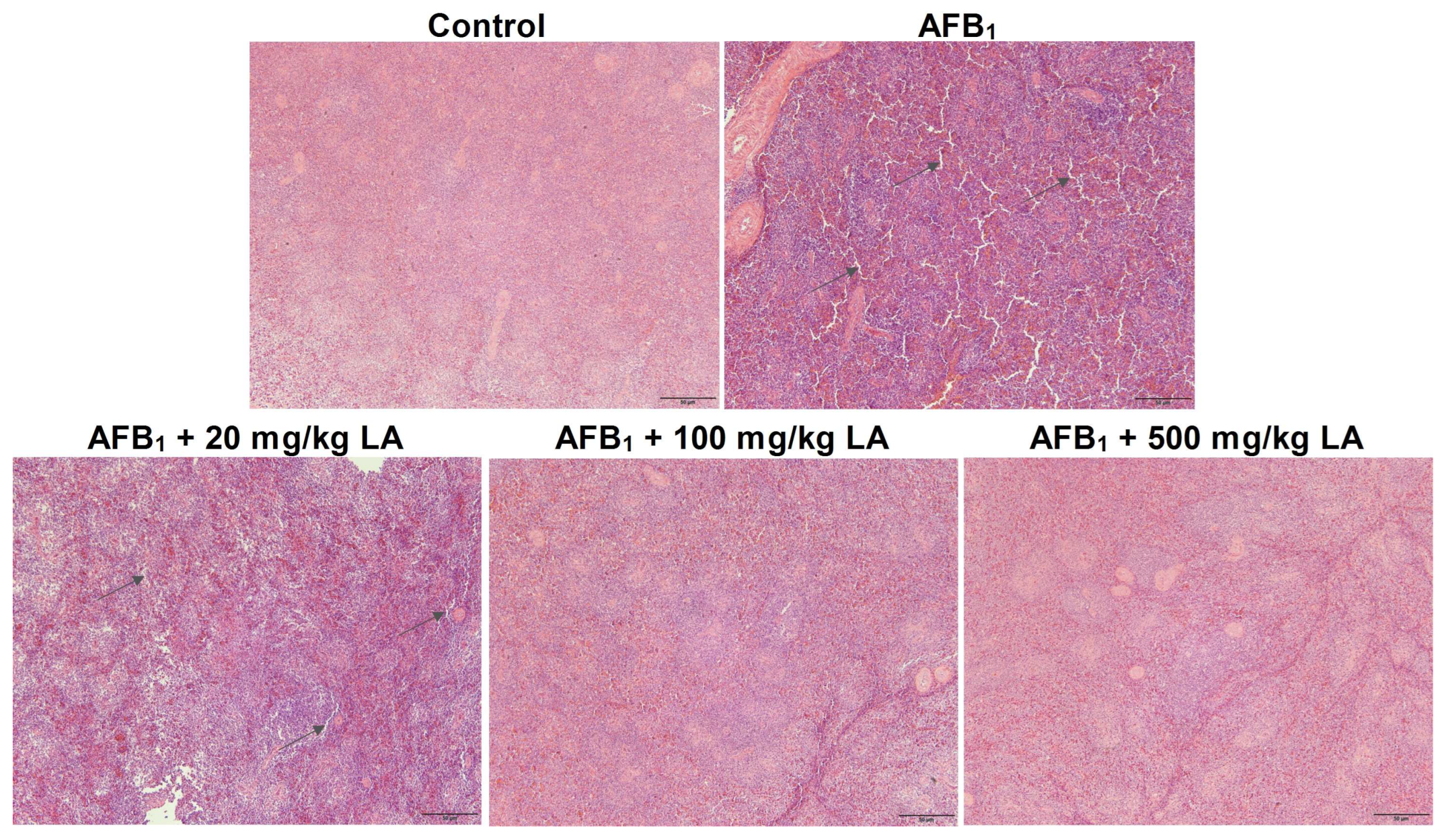
2.7. Changes in the Structure of Spleen Tissue
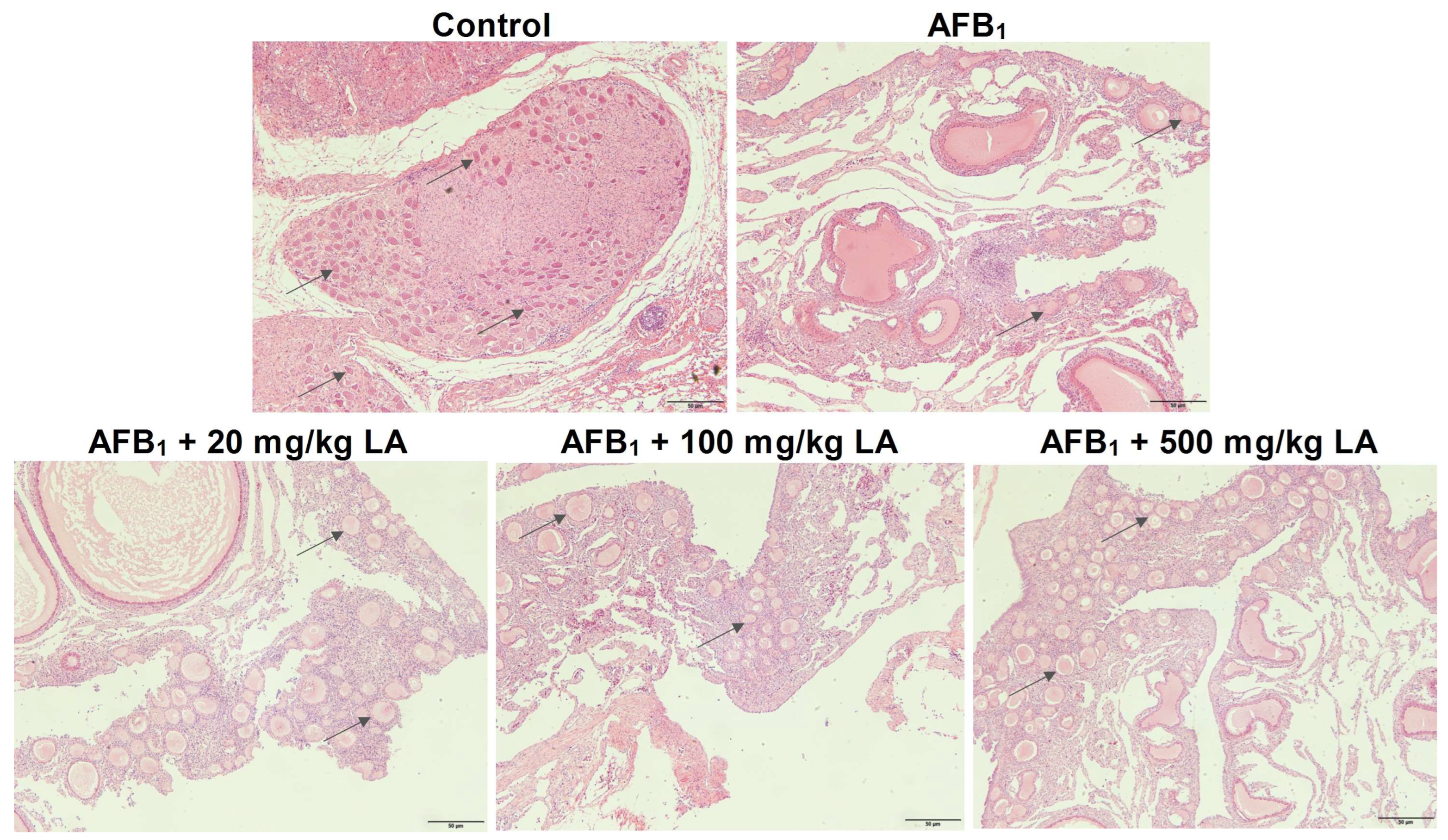
2.8. Changes in Ovarian Tissue Structure
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Sample Collection Methods
5.2. Determination of Growth Performance
5.3. Determination of Organ Weight
5.4. Determination of Serum Biochemical Indicators
5.5. Determination of Serum Antioxidant Indicators
5.6. H&E Staining of the Liver, Spleen, and Ovary
5.7. Liver Oil Red O Staining
5.8. Observation of Liver Ultrastructure
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Wang, Y.; Feng, T.; Yan, Z.; Zhu, D.; Lin, H.; Iqbal, M.; Deng, D.; Kulyar, M.F.; Shen, Y. Hedyotis diffusa alleviate aflatoxin B1-induced liver injury in ducks by mediating Nrf2 signaling pathway. Ecotox. Environ. Safe. 2023, 249, 114339. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Garavito-Duarte, Y.; Gormley, A.R.; Kim, S.W. Aflatoxin B1: Challenges and Strategies for the Intestinal Microbiota and Intestinal Health of Monogastric Animals. Toxins 2025, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Karimi, T.M.; Sedaghat, A. A consortium of detoxifying bacteria mitigates the aflatoxin B1 toxicosis on performance, health, and blood constituents of laying hens. Poult. Sci. 2023, 102, 102601. [Google Scholar] [CrossRef] [PubMed]
- Nazir, U.; Fu, Z.; Zheng, X.; Zafar, M.H.; Yang, Z.; Wang, Z.; Yang, H. Transcriptomic analysis of ileal adaptations and growth responses in growing hens supplemented with alanyl-glutamine dipeptide. Poult. Sci. 2024, 103, 104479. [Google Scholar] [CrossRef]
- Qing, H.; Huang, S.; Zhan, K.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Combined Toxicity Evaluation of Ochratoxin A and Aflatoxin B1 on Kidney and Liver Injury, Immune Inflammation, and Gut Microbiota Alteration Through Pair-Feeding Pullet Model. Front. Immunol. 2022, 13, 920147. [Google Scholar] [CrossRef]
- Pandey, I.; Chauhan, S.S. Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. Br. Poult. Sci. 2007, 48, 713–723. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Niu, Y.J.; Wu, J.; Ren, W.; Liu, G.; Wu, G.; Peng, Y.; Zheng, D.; Jin, K.; Zuo, Q.; Li, G.; et al. Aflatoxin B1 impairs the growth and development of chicken PGCs through oxidative stress and mitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2025, 290, 117727. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins 2022, 14, 876. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef]
- Superti, F.; Russo, R. Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits. Antioxidants 2024, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Padmalayam, I. Targeting mitochondrial oxidative stress through lipoic acid synthase: A novel strategy to manage diabetic cardiovascular disease. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liao, S.; Guo, S.; Li, H.; Yang, M.; Tang, Z. Protective effects of selenium on aflatoxin B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver. Biol. Trace Elem. Res. 2015, 163, 162–168. [Google Scholar] [CrossRef]
- Yu, A.; Wang, H.; Cheng, Q.; Rajput, S.A.; Qi, D. The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks. Toxins 2024, 16, 239. [Google Scholar] [CrossRef]
- Chu, Y.; Yu, A.; Wang, H.; Rajput, S.A.; Yu, Q.; Qi, D. Biological Mechanisms of Aflatoxin B1-Induced Bile Metabolism Abnormalities in Ducklings. Animals 2024, 14, 2996. [Google Scholar] [CrossRef]
- Seifi, S.; Sadighara, P.; Mohajer, A. Protective effects of Aloe vera powder supplementation on some quantitative and qualitative characteristics of egg, histopathological changes and serum biochemistry of laying hens fed by Aflatoxin B1. Vet. Res. Forum. 2022, 13, 507–512. [Google Scholar] [CrossRef]
- Wu, K.; Liu, M.; Wang, H.; Rajput, S.A.; Shan, Y.; Qi, D.; Wang, S. The Mechanism Underlying the Extreme Sensitivity of Duck to Aflatoxin B1. Oxid. Med. Cell. Longev. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Raj, J.; Farkas, H.; Jakovcevic, Z.; Vasiljevic, M.; Kumar, R.; Asrani, R.K. Effects of supplemented multicomponent mycotoxin detoxifying agent in laying hens fed aflatoxin B1 and T2-toxin contaminated feeds. Poult. Sci. 2023, 102, 102795. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; et al. Effect of dietary protein on growth performance, and serum biochemical index in late pregnant Hu ewes and their offspring. Anim. Biotechnol. 2023, 34, 97–105. [Google Scholar] [CrossRef]
- Mojgani, N.; Razmgah, N.; Torshizi, M.A.K.; Sanjabi, M.R. Effects of three Bacillus specious on hatchability, growth performance and serum biochemistry in Japanese quails fed diet contaminated with Aflatoxin B1. Acta Sci. Anim. Sci. 2020, 42, e50184. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.G.; Zhao, L.H.; Guo, Y.Q.; Duan, G.X.; Zhang, J.Y.; Ji, C. Protective Efficacy of Alpha-lipoic Acid against AflatoxinB1-induced Oxidative Damage in the Liver. Asian-Australas. J. Anim. Sci. 2014, 27, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Ge, B.; Li, H.; Zhou, H.; Yan, K.; Wang, W.; Cui, Q.; Zhang, X. Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy. Ecotoxicol. Environ. Saf. 2023, 251, 114546. [Google Scholar] [CrossRef]
- Hatipoglu, D.; Keskin, E. The effect of curcumin on some cytokines, antioxidants and liver function tests in rats induced by Aflatoxin B1. Heliyon 2022, 8, e9890. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Zhang, L.; Zhang, Z.; Zhao, Y.; Wang, Y.; Ou, J. New Insights into the Persistent Effects of Acute Exposure to AFB1 on Rat Liver. Front. Microbiol. 2022, 13, 911757. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zebrowska, E.; Nesterowicz, M.; Zendzian-Piotrowska, M.; Zalewska, A. Alpha-Lipoic Acid Strengthens the Antioxidant Barrier and Reduces Oxidative, Nitrosative, and Glycative Damage, as well as Inhibits Inflammation and Apoptosis in the Hypothalamus but Not in the Cerebral Cortex of Insulin-Resistant Rats. Oxid. Med. Cell. Longev. 2022, 2022, 7450514. [Google Scholar] [CrossRef]
- Skibska, B.; Kochan, E.; Stanczak, A.; Lipert, A.; Skibska, A. Antioxidant and Anti-inflammatory Effects of alpha-Lipoic Acid on Lipopolysaccharide-induced Oxidative Stress in Rat Kidney. Arch. Immunol. Ther. Exp. 2023, 71, 16. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Pang, F.; Zhang, L.; Fu, T.; Wang, L.; Liu, K.; Gao, T. Effects of alpha-lipoic acid on growth performance, antioxidant capacity, and immune function in sheep. J. Anim. Sci. 2023, 101, skad092. [Google Scholar] [CrossRef]
- Longhitano, L.; Distefano, A.; Musso, N.; Bonacci, P.; Orlando, L.; Giallongo, S.; Tibullo, D.; Denaro, S.; Lazzarino, G.; Ferrigno, J.; et al. (+)-Lipoic acid reduces mitochondrial unfolded protein response and attenuates oxidative stress and aging in an in vitro model of non-alcoholic fatty liver disease. J. Transl. Med. 2024, 22, 82. [Google Scholar] [CrossRef]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-lipoic Acid: An Antioxidant with Anti-aging Properties for Disease Therapy. Curr. Med. Chem. 2025, 32, 23–54. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Distefano, A.; Amorini, A.M.; Orlando, L.; Giallongo, S.; Tibullo, D.; Lazzarino, G.; Nicolosi, A.; Alanazi, A.M.; Saoca, C.; et al. (+)-Lipoic Acid Reduces Lipotoxicity and Regulates Mitochondrial Homeostasis and Energy Balance in an In Vitro Model of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 14491. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, F.; Dehghani, M.A.; Vanani, A.R.; Mahdavinia, M. Protective Effects of Alpha Lipoic Acid Against Arsenic Induced Oxidative Stress in Isolated Rat Liver Mitochondria. Biol. Trace Elem. Res. 2022, 200, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Lin, J.; Zhang, H.; Guo, Y.; Mao, Y.; Liu, Z.; Li, G.; Wang, Y. Long-Term Sleep Deprivation-Induced Myocardial Remodeling and Mitochondrial Dysfunction in Mice Were Attenuated by Lipoic Acid and N-Acetylcysteine. Pharmaceuticals 2022, 16, 51. [Google Scholar] [CrossRef]
- Dieckmann, C.L. A hub for regulation of mitochondrial metabolism: Fatty acid and lipoic acid biosynthesis. Iubmb Life 2024, 76, 332–344. [Google Scholar] [CrossRef]
- Zhu, P.; Zuo, Z.; Zheng, Z.; Wang, F.; Peng, X.; Fang, J.; Cui, H.; Gao, C.; Song, H.; Zhou, Y.; et al. Aflatoxin B1 affects apoptosis and expression of death receptor and endoplasmic reticulum molecules in chicken spleen. Oncotarget 2017, 8, 99531–99540. [Google Scholar] [CrossRef]
- Wu, K.; Liu, M.; Wang, H.; Rajput, S.A.; Al, Z.O.; Wang, S.; Qi, D. Effect of zearalenone on aflatoxin B1-induced intestinal and ovarian toxicity in pregnant and lactating rats. Ecotoxicol. Environ. Saf. 2023, 258, 114976. [Google Scholar] [CrossRef]

| Items | Control | AFB1 | AFB1 + 20 mg/kg LA | AFB1 + 100 mg/kg LA | AFB1 + 500 mg/kg LA |
|---|---|---|---|---|---|
| Week 2 | |||||
| Weight (kg) | 1.42 ± 0.03 | 1.37 ± 0.04 | 1.41 ± 0.06 | 1.40 ± 0.09 | 1.39 ± 0.10 |
| Daily feed intake (g) | 93.80 ± 2.73 a | 89.82 ± 2.38 ab | 87.28 ± 6.96 b | 89.91 ± 3.19 ab | 88.77 ± 4.81 b |
| Week 4 | |||||
| Weight (kg) | 1.49 ± 0.03 | 1.47 ± 0.06 | 1.49 ± 0.09 | 1.49 ± 0.09 | 1.44 ± 0.09 |
| Daily feed intake (g) | 94.00 ± 3.71 | 92.80 ± 4.76 | 90.03 ± 10.08 | 91.62 ± 3.27 | 89.24 ± 5.27 |
| Heart (g) | 6.28 ± 1.13 | 6.93 ± 1.41 | 6.34 ± 0.82 | 6.87 ± 1.35 | 6.35 ± 0.81 |
| Liver (g) | 34.43 ± 6.69 ab | 39.71 ± 8.45 a | 35.10 ± 5.47 ab | 32.03 ± 4.85 b | 33.12 ± 4.34 ab |
| Spleen (g) | 3.15 ± 0.59 | 3.06 ± 0.60 | 2.81 ± 0.47 | 3.26 ± 0.40 | 3.02 ± 0.43 |
| Bioindicators | Control | AFB1 | AFB1 + 20 mg/kg LA | AFB1 + 100 mg/kg LA | AFB1 + 500 mg/kg LA |
|---|---|---|---|---|---|
| ALT (U/L) | 1.43 ± 0.27 d | 3.36 ± 0.55 a | 3.39 ± 0.44 a | 2.61 ± 0.38 b | 1.96 ± 0.37 c |
| AST (U/L) | 195.78 ± 19.37 c | 293.73 ± 18.44 a | 281.29 ± 16.10 a | 250.90 ± 18.60 b | 211.11 ± 13.72 c |
| ALP (U/L) | 288.23 ± 47.06 c | 474.99 ± 40.13 a | 470.80 ± 45.79 a | 381.59 ± 30.33 b | 315.33 ± 61.57 c |
| TP (g/L) | 47.79 ± 6.17 a | 16.69 ± 3.70 d | 17.53 ± 3.98 d | 26.80 ± 5.51 c | 36.48 ± 4.31 b |
| ALB (g/L) | 22.44 ± 3.87 a | 10.35 ± 1.97 d | 11.18 ± 2.85 d | 14.24 ± 1.91 c | 17.71 ± 3.02 b |
| TBA (μmol/L) | 37.39 ± 4.93 | 41.01 ± 7.19 | 40.88 ± 7.21 | 39.20 ± 9.16 | 36.49 ± 5.85 |
| Antioxidant Enzymes | Control | AFB1 | AFB1 + 20 mg/kg LA | AFB1 + 100 mg/kg LA | AFB1 + 500 mg/kg LA |
|---|---|---|---|---|---|
| T-SOD (U/mL) | 89.02 ± 7.88 a | 60.06 ± 6.03 d | 60.18 ± 4.52 d | 67.48 ± 4.19 c | 77.64 ± 5.80 b |
| MDA (nmol/mL) | 7.80 ± 0.53 c | 16.36 ± 1.36 a | 15.78 ± 1.57 a | 12.69 ± 1.13 b | 8.80 ± 1.69 c |
| GSH-Px (U/mL) | 1862.84 ± 63.03 a | 1371.62 ± 137.74 c | 1397.30 ± 81.93 c | 1642.57 ± 126.95 b | 1775.68 ± 61.16 a |
| CAT (U/mL) | 3.34 ± 0.46 a | 1.82 ± 0.56 c | 1.91 ± 0.52 c | 2.25 ± 0.35 bc | 2.58 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Wang, H.; Xu, X.; Ji, Y.; Zhao, Y.; Yu, Q.; Rajput, S.A.; Xue, Y.; Qi, D. Protective Effect of Lipoic Acid on Oxidative Stress and Tissue Damage Induced by Aflatoxin B1 in Young Laying Hens. Toxins 2025, 17, 184. https://doi.org/10.3390/toxins17040184
Chu Y, Wang H, Xu X, Ji Y, Zhao Y, Yu Q, Rajput SA, Xue Y, Qi D. Protective Effect of Lipoic Acid on Oxidative Stress and Tissue Damage Induced by Aflatoxin B1 in Young Laying Hens. Toxins. 2025; 17(4):184. https://doi.org/10.3390/toxins17040184
Chicago/Turabian StyleChu, Yihong, Huanbin Wang, Xinyu Xu, Yun Ji, Yiting Zhao, Qianqian Yu, Shahid Ali Rajput, Yi Xue, and Desheng Qi. 2025. "Protective Effect of Lipoic Acid on Oxidative Stress and Tissue Damage Induced by Aflatoxin B1 in Young Laying Hens" Toxins 17, no. 4: 184. https://doi.org/10.3390/toxins17040184
APA StyleChu, Y., Wang, H., Xu, X., Ji, Y., Zhao, Y., Yu, Q., Rajput, S. A., Xue, Y., & Qi, D. (2025). Protective Effect of Lipoic Acid on Oxidative Stress and Tissue Damage Induced by Aflatoxin B1 in Young Laying Hens. Toxins, 17(4), 184. https://doi.org/10.3390/toxins17040184





