Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review
Abstract
:1. Introduction
2. Search Methodology
2.1. Review Planning
2.2. Conducting the Review
2.3. Data Screening
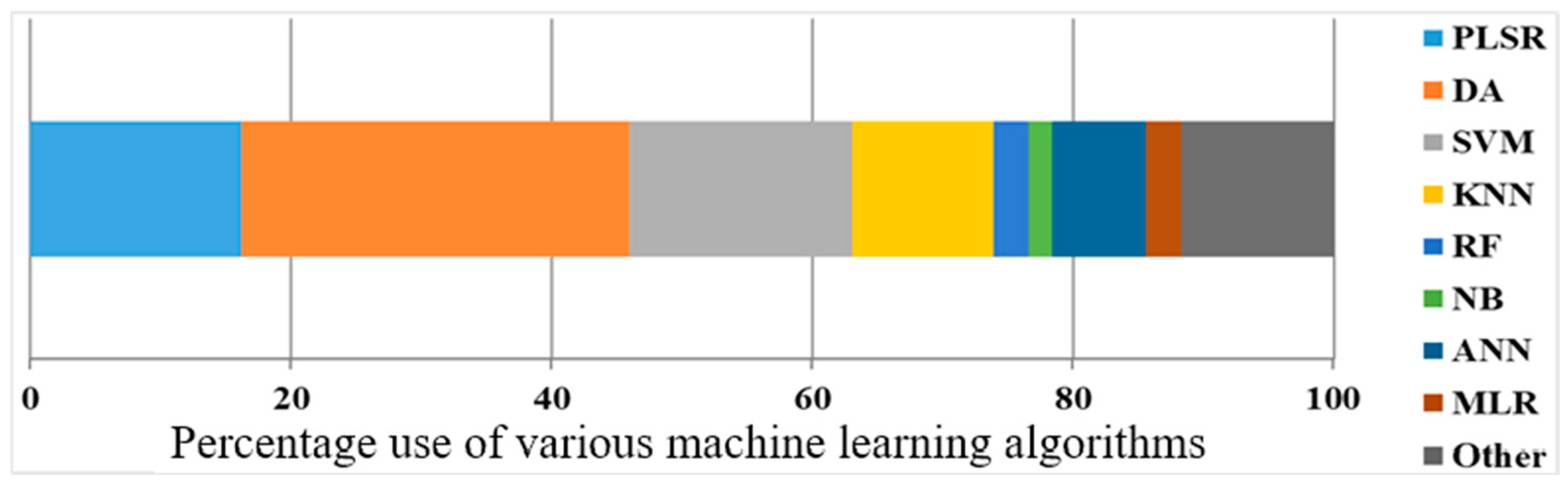
2.4. Result Analysis
3. Hyperspectral Analysis of Mycotoxins in Cereal and Nuts
3.1. Hyperspectral Response of Different Mycotoxins
3.2. Detection of Mycotoxins in Wheat
3.3. Detection of Mycotoxins in Corn
3.4. Detection of Mycotoxin in Other Cereals and Grains
3.5. Detection of Mycotoxins in Peanuts
| Nuts | Mycotoxin | Sample Number | Wavelength (nm) | Preprocessing | ML Methods | Performance | Reference |
|---|---|---|---|---|---|---|---|
| Peanut | AFB1 | 20 | 415–799 | Noise fraction | CNN | Acc: 91.3% | [82] |
| Peanut | AFB1 | 1260 | 900–2500 | SG, 1st dev | PLS, PCR | Acc: 100% | [83] |
| Peanut | AFB1 | 73 | 292–865 | N-FINDR | ResNet18, LeNet, AlexNet | R2: 0.88 | [81] |
| Peanut | AFB1 | 150 | 1000–2500 | SNV, 1st and 2nd dev | SVR, PLSR | R2: 0.95 | [37] |
| Peanut | AFB1 | 150 | 400–1000 | SG, SNV, 1st dev, MSC | PCA-LDA, SVM | Acc: 93% | [84] |
| Peanut | AFB1 | 250 | 400–720 | RI, DRI, RRI, NDRI | SVM | Acc: 93.1% | [87] |
| Peanut | AFB1 | 210 | 400–2500 | SNV | PLS-DA | Acc: 94.8% | [88] |
| Peanut | AFB1 | 73 | 292–865 | Raw Image | CNN, | Acc: 95% | [89] |
| Peanut | AFB1 | 13,000 | 415–799 | PCA | DCNN | Acc: 97.87% | [90] |
| Almond | AFB1 | 400 | 900–1700 | SNV, SG, 1st and 2nd dev | SVM, LR, LDA, QDA | Acc: 95% | [91] |
| Almond | AFB1 | 500 | 900–1700 | SNV, SG, 1st and 2nd dev | RF, QDA | ACC: 96. 37% | [92] |
| Almond | AFB1 | 1520 | 900–1700 | SNV, SG, 1st and 2nd dev | ResNet, InceptionNet, Inception ResNet | ACC: 95.91% | [93] |
| Almond | AFB1 | 1832 | 900–1700 | SNV, SG, 1st and 2nd dev | SVM, Subspace, Rusboost | ACC: 96% | [94] |
| Almond | AFB1 | 3596 | 900–1700 | Raw Image | 3D CNN | ACC: 90.81% | [95] |
| Almond | AFB1 | 500 | 900–1700 | SNV, SG, 1st and 2nd dev | SVR, GPR | R2: 0.966 | [85] |
| Almond | AFB1 | 936 | 900–1700 | SNV, SG, 1st and 2nd dev | SVM | Acc: 98.7% | [96] |
| Almond | AFB1 | 465 | 900–1700 | SNV, SG, 1st and 2nd dev | PLSR, MLR | R2: 0.958 | [36] |
| Pistachio | AFB1 | 300 | 694–988 | SNV, SG | LDA, SMLR | Acc: 90% | [38] |
| Pistachio | AFB1 | 300 | 408–1007 | SG | SVM, PLSR | Acc: 98% | [86] |
| Pistachio | AFB | 300 | 400–1000 | Raw Image | ResNet | Acc: 96.67% | [97] |
4. ML Techniques for Detection of Mycotoxin in Cereals and Nuts Using HSI
4.1. Partial Least Squares Regression
4.2. Multiple Linear Regression
4.3. Support Vector Machine
4.4. Discriminant Analysis
4.5. Decision Tree
4.6. Random Forest
4.7. K-Nearest Neighbors
4.8. Naive Bayes
4.9. Ensemble Boosting
4.10. Artificial Neural Network
5. Research Scope
5.1. Enhancing Model Accuracy and Generalization
5.2. Optimized Feature Selection for Mycotoxin Detection
5.3. Integration of Advanced Algorithms
5.4. High-Dimensional Imbalanced Data
5.5. Real-Time Mycotoxin Detection Systems
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whetton, R.L.; Waine, T.W.; Mouazen, A.M. Evaluating management zone maps for variable rate fungicide application and selective harvest. Comput. Electron. Agric. 2018, 153, 202–212. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I. Aflatoxin Biosynthesis, Genetic Regulation, Toxicity, and Control Strategies: A Review. J. Fungi 2021, 7, 606. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Borzekowski, A.; Anggriawan, R.; Auliyati, M.; Kunte, H.-J.; Koch, M.; Rohn, S.; Karlovsky, P.; Maul, R. Formation of Zearalenone Metabolites in Tempeh Fermentation. Molecules 2019, 24, 2697. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 82—Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2002; Volume 86. [Google Scholar]
- Almoujahed, M.B.; Rangarajan, A.K.; Whetton, R.L.; Vincke, D.; Eylenbosch, D.; Vermeulen, P.; Mouazen, A.M. Detection of fusarium head blight in wheat under field conditions using a hyperspectral camera and machine learning. Comput. Electron. Agric. 2022, 203, 107456. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems; Aflatoxins, R.-A.M., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 12. [Google Scholar]
- European Union. Commission Regulation (EU) No 165/2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins (Text with EEA Relevance). 2010. Available online: http://data.europa.eu/eli/reg/2010/165/oj (accessed on 24 April 2025).
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans, Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; GraslKraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2022/1370 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ochratoxin A in Certain Foodstuffs. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R1370 (accessed on 24 April 2025).
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef]
- Verbrugghe, E.; Vandenbroucke, V.; Dhaenens, M.; Shearer, N.; Goossens, J.; De Saeger, S.; Eeckhout, M.; D’Herde, K.; Thompson, A.; Deforce, D.; et al. T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions. Veter-Res. 2012, 43, 22. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2024/1038 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food. 2024. Available online: http://data.europa.eu/eli/reg/2024/1038/oj (accessed on 24 April 2025).
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E. Chapter Fourteen—Toxins and Venoms. In Progress in Molecular Biology and Translational Science; Hodgson, E., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 373–415. [Google Scholar]
- European Union. Commission Regulation (EU) 2024/1808 Amending Regulation (EU) 2023/915 as Regards the Application Date of Lower Maximum Levels for Ergot Sclerotia and Ergot Alkaloids in Food. 2024. Available online: http://data.europa.eu/eli/reg/2024/1808/oj (accessed on 24 April 2025).
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2024/1022 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. 2024. Available online: http://data.europa.eu/eli/reg/2024/1022/oj (accessed on 24 April 2025).
- Puvača, N.; Avantaggiato, G.; Merkuri, J.; Vuković, G.; Bursić, V.; Cara, M. Occurrence and Determination of Alternaria Mycotoxins Alternariol, Alternariol Monomethyl Ether, and Tentoxin in Wheat Grains by QuEChERS Method. Toxins 2022, 14, 791. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2022/553 on Monitoring the Presence of Alternaria toxins in Food. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H0553 (accessed on 24 April 2025).
- WHO. Food Safety. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 1 April 2023).
- Rasch, C.; Kumke, M.; Löhmannsröben, H.-G. Sensing of Mycotoxin Producing Fungi in the Processing of Grains. Food Bioprocess Technol. 2010, 3, 908–916. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.; Lee, I. Research advancements in optical imaging and spectroscopic techniques for nondestructive detection of mold infection and mycotoxins in cereal grains and nuts. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4612–4651. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.B.; Slate, A.; Birmingham, T.; Adams, J.; Jacobs, M.; Gray, G. Correlation Between Aflatoxin Contamination and Various USDA Grade Categories of Shelled Almonds. J. AOAC Int. 2010, 93, 943–947. [Google Scholar] [CrossRef]
- Santiago, M.; Strobel, S. Chapter Twenty-Four—Thin Layer Chromatography, in Methods in Enzymology; Lorsch, J., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 303–324. [Google Scholar]
- Li, P.; Zhou, Q.; Wang, T.; Zhou, H.; Zhang, W.; Ding, X.; Zhang, Z.; Chang, P.-K.; Zhang, Q. Development of an Enzyme-Linked Immunosorbent Assay Method Specific for the Detection of G-Group Aflatoxins. Toxins 2015, 8, 5. [Google Scholar] [CrossRef]
- Gorran, A.; Farzaneh, M.; Shivazad, M.; Rezaeian, M.; Ghassempour, A. Aflatoxin B1-reduction of Aspergillus flavus by three medicinal plants (Lamiaceae). Food Control 2013, 31, 218–223. [Google Scholar] [CrossRef]
- Krska, R.; Molinelli, A. Rapid test strips for analysis of mycotoxins in food and feed. Anal. Bioanal. Chem. 2009, 393, 67–71. [Google Scholar] [CrossRef]
- Chavez, R.A.; Cheng, X.; Stasiewicz, M.J. A Review of the Methodology of Analyzing Aflatoxin and Fumonisin in Single Corn Kernels and the Potential Impacts of These Methods on Food Security. Foods 2020, 9, 297. [Google Scholar] [CrossRef]
- Wang, W.; Heitschmidt, G.W.; Ni, X.; Windham, W.R.; Hawkins, S.; Chu, X. Identification of aflatoxin B1 on maize kernel surfaces using hyperspectral imaging. Food Control 2014, 42, 78–86. [Google Scholar] [CrossRef]
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of insect-damaged vegetable soybeans using hyperspectral transmittance image. J. Food Eng. 2013, 116, 45–49. [Google Scholar] [CrossRef]
- Tu, K.; Wu, W.; Cheng, Y.; Zhang, H.; Xu, Y.; Dong, X.; Wang, M.; Sun, Q. AIseed: An automated image analysis software for high-throughput phenotyping and quality non-destructive testing of individual plant seeds. Comput. Electron. Agric. 2023, 207, 107740. [Google Scholar] [CrossRef]
- Du, C.-J.; Sun, D.-W. Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci. Technol. 2004, 15, 230–249. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.-H.; Lee, I. Application of SWIR hyperspectral imaging coupled with chemometrics for rapid and non-destructive prediction of Aflatoxin B1 in single kernel almonds. LWT 2022, 155, 112954. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, B.; Lu, Y.; Yoon, S.-C.; Ni, X.; Zhuang, H.; Guo, X.; Le, W.; Wang, W. Detection of Aflatoxin B1 in Single Peanut Kernels by Combining Hyperspectral and Microscopic Imaging Technologies. Sensors 2022, 22, 4864. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H. Detection of Aflatoxin B1 in Pistachio Kernels Using Visible/Near-Infrared Hyperspectral Imaging. Trans. ASABE 2019, 62, 1065–1074. [Google Scholar] [CrossRef]
- Nadimi, M.; Brown, J.; Morrison, J.; Paliwal, J. Examination of wheat kernels for the presence of Fusarium damage and mycotoxins using near-infrared hyperspectral imaging. Meas. Food 2021, 4, 100011. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Kincaid, R.; Rajasekaran, K. Near-infrared hyperspectral imaging for evaluation of aflatoxin contamination in corn kernels. Biosyst. Eng. 2022, 221, 181–194. [Google Scholar] [CrossRef]
- Sendin, K.; Williams, P.J.; Manley, M. Near infrared hyperspectral imaging in quality and safety evaluation of cereals. Crit. Rev. Food Sci. Nutr. 2017, 58, 575–590. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, L.; Xu, H. Determination of toxigenic fungi and aflatoxins in nuts and dried fruits using imaging and spectroscopic techniques. Food Chem. 2018, 252, 228–242. [Google Scholar] [CrossRef]
- Chu, X.; Wang, W.; Yoon, S.-C.; Ni, X.; Heitschmidt, G.W. Detection of aflatoxin B1 (AFB1) in individual maize kernels using short wave infrared (SWIR) hyperspectral imaging. Biosyst. Eng. 2017, 157, 13–23. [Google Scholar] [CrossRef]
- Pearson, T.C.; Wicklow, D.T.; Pasikatan, M.C. Reduction of aflatoxin and fumonisin contamination in yellow corn by high-speed dual-wavelength sorting. Cereal Chem. 2004, 81, 490–498. [Google Scholar] [CrossRef]
- Peiris, K.H.; Pumphrey, M.O.; Dowell, F.E. NIR absorbance characteristics of deoxynivalenol and of sound and Fusarium-damaged wheat kernels. J. Near Infrared Spectrosc. 2009, 17, 213–221. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Jayas, D.; White, N.; Fields, P.; Gräfenhan, T. Detection of fungal infection and Ochratoxin A contamination in stored wheat using near-infrared hyperspectral imaging. J. Stored Prod. Res. 2016, 65, 30–39. [Google Scholar] [CrossRef]
- Teixido-Orries, I.; Molino, F.; Gatius, F.; Sanchis, V.; Marín, S. Near-infrared hyperspectral imaging as a novel approach for T-2 and HT-2 toxins estimation in oat samples. Food Control 2023, 153, 109952. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Baek, I.; Lee, K.-M.; Kim, G.; Kim, S.; Kim, S.-Y.; Chan, D.; Herrman, T.J.; Kim, N.; Kim, M.S. Rapid Detection of Single- and Co-Contaminant Aflatoxins and Fumonisins in Ground Maize Using Hyperspectral Imaging Techniques. Toxins 2023, 15, 472. [Google Scholar] [CrossRef]
- Shen, G.; Cao, Y.; Yin, X.; Dong, F.; Xu, J.; Shi, J.; Lee, Y.-W. Rapid and nondestructive quantification of deoxynivalenol in individual wheat kernels using near-infrared hyperspectral imaging and chemometrics. Food Control 2022, 131, 108420. [Google Scholar] [CrossRef]
- Sirisomboon, C.D.; Wongthip, P.; Sirisomboon, P. Potential of near infrared spectroscopy as a rapid method to detect aflatoxins in brown rice. J. Near Infrared Spectrosc. 2019, 27, 232–240. [Google Scholar] [CrossRef]
- da Conceição, R.R.P.; Simeone, M.L.F.; Queiroz, V.A.V.; de Medeiros, E.P.; de Araújo, J.B.; Coutinho, W.M.; da Silva, D.D.; Miguel, R.d.A.; Lana, U.G.d.P.; Stoianoff, M.A.d.R. Application of near-infrared hyperspectral (NIR) images combined with multivariate image analysis in the differentiation of two mycotoxicogenic Fusarium species associated with maize. Food Chem. 2021, 344, 128615. [Google Scholar] [CrossRef]
- Visconti, A.; Pascale, M. REVIEW: An Overview on Fusarium Mycotoxins in the Durum Wheat Pasta Production Chain. Cereal Chem. 2010, 87, 21–27. [Google Scholar] [CrossRef]
- Jaillais, B.; Roumet, P.; Pinson-Gadais, L.; Bertrand, D. Detection of Fusarium head blight contamination in wheat kernels by multivariate imaging. Food Control 2015, 54, 250–258. [Google Scholar] [CrossRef]
- Femenias, A.; Llorens-Serentill, E.; Ramos, A.J.; Sanchis, V.; Marín, S. Near-infrared hyperspectral imaging evaluation of Fusarium damage and DON in single wheat kernels. Food Control 2022, 142, 109239. [Google Scholar] [CrossRef]
- Femenias, A.; Bainotti, M.B.; Gatius, F.; Ramos, A.J.; Marín, S. Standardization of near infrared hyperspectral imaging for wheat single kernel sorting according to deoxynivalenol level. Food Res. Int. 2021, 139, 109925. [Google Scholar] [CrossRef] [PubMed]
- Vicens-Sans, A.; Pascari, X.; Molino, F.; Ramos, A.; Marín, S. Near infrared hyperspectral imaging as a sorting tool for deoxynivalenol reduction in wheat batches. Food Res. Int. 2024, 178, 113984. [Google Scholar] [CrossRef]
- Wang, C.; Fu, X.; Zhou, Y.; Fu, F. Deoxynivalenol Detection beyond the Limit in Wheat Flour Based on the Fluorescence Hyperspectral Imaging Technique. Foods 2024, 13, 897. [Google Scholar] [CrossRef]
- Liang, K.; Huang, J.; He, R.; Wang, Q.; Chai, Y.; Shen, M. Comparison of Vis-NIR and SWIR hyperspectral imaging for the non-destructive detection of DON levels in Fusarium head blight wheat kernels and wheat flour. Infrared Phys. Technol. 2020, 106. [Google Scholar] [CrossRef]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Sanchis, V.; Marín, S. Standardisation of near infrared hyperspectral imaging for quantification and classification of DON contaminated wheat samples. Food Control 2020, 111, 107074. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, Q. Comparison of wavelength selected methods for improving of prediction performance of PLS model to determine aflatoxin B1 (AFB1) in wheat samples during storage. Microchem. J. 2021, 170. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Jayas, D.; White, N.; Fields, P.; Gräfenhan, T. Detection of ochratoxin A contamination in stored wheat using near-infrared hyperspectral imaging. Infrared Phys. Technol. 2017, 81, 228–235. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Rajasekaran, K.; Qin, J.; Kim, M.; Chao, K. Raman Hyperspectral Imaging as a Potential Tool for Rapid and Nondestructive Identification of Aflatoxin Contamination in Corn Kernels. J. Food Prot. 2024, 87, 100335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, T.; Wang, W.; Fan, S.; Chen, L.; Tian, X. Qualitative and quantitative detection of aflatoxins B1 in maize kernels with fluorescence hyperspectral imaging based on the combination method of boosting and stacking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122679. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yao, J.; Yu, H.; Wang, W.; Huang, W. Early contamination warning of Aflatoxin B1 in stored maize based on the dynamic change of catalase activity and data fusion of hyperspectral images. Comput. Electron. Agric. 2024, 217, 108615. [Google Scholar] [CrossRef]
- Guo, X.; Jia, B.; Zhang, H.; Ni, X.; Zhuang, H.; Lu, Y.; Wang, W. Evaluation of Aspergillus flavus Growth and Detection of Aflatoxin B1 Content on Maize Agar Culture Medium Using Vis/NIR Hyperspectral Imaging. Agriculture 2023, 13, 237. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, B.; Yoon, S.-C.; Zhuang, H.; Ni, X.; Guo, B.; Gold, S.E.; Fountain, J.C.; Glenn, A.E.; Lawrence, K.C.; et al. Spatio-temporal patterns of Aspergillus flavus infection and aflatoxin B1 biosynthesis on maize kernels probed by SWIR hyperspectral imaging and synchrotron FTIR microspectroscopy. Food Chem. 2022, 382, 132340. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Baek, I.; Lee, K.-M.; Qin, J.; Kim, G.; Shin, B.K.; Chan, D.E.; Herrman, T.J.; Cho, S.-K.; Kim, M.S. Investigation of reflectance, fluorescence, and Raman hyperspectral imaging techniques for rapid detection of aflatoxins in ground maize. Food Control 2021, 132, 108479. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Z.; Cheng, F. Application of hyperspectral imaging in the detection of aflatoxin B1 on corn seed. J. Food Meas. Charact. 2021, 16, 448–460. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, W.; Liang, D.; Tian, X. Classification of Aflatoxin B1 Concentration of Single Maize Kernel Based on Near-Infrared Hyperspectral Imaging and Feature Selection. Sensors 2021, 21, 4257. [Google Scholar] [CrossRef]
- Gao, J.; Ni, J.; Wang, D.; Deng, L.; Li, J.; Han, Z. Pixel-level aflatoxin detecting in maize based on feature selection and hyperspectral imaging. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020, 234, 118269. [Google Scholar] [CrossRef]
- Borràs-Vallverdú, B.; Marín, S.; Sanchis, V.; Gatius, F.; Ramos, A.J. NIR-HSI as a tool to predict deoxynivalenol and fumonisins in maize kernels: A step forward in preventing mycotoxin contamination. J. Sci. Food Agric. 2024, 104, 5495–5503. [Google Scholar] [CrossRef]
- Parrag, V.; Gillay, Z.; Kovács, Z.; Zitek, A.; Böhm, K.; Hinterstoisser, B.; Krska, R.; Sulyok, M.; Felföldi, J.; Firtha, F.; et al. Application of hyperspectral imaging to detect toxigenic Fusarium infection on cornmeal. Prog. Agric. Eng. Sci. 2020, 16, 51–60. [Google Scholar] [CrossRef]
- Conceição, R.R.P.; Queiroz, V.A.V.; Medeiros, E.P.; Araújo, J.B.; Araújo, D.D.S.; Miguel, R.A.; Stoianoff, M.A.R.; Simeone, M.L.F. Determination of fumonisin content in maize using near-infrared hyperspectral imaging (NIR-HSI) technology and chemometric methods. Braz. J. Biol. 2024, 84, e277974. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Deng, H.; Shi, Y.; Liu, C.; Zheng, L. Application of multispectral imaging combined with machine learning methods for rapid and non-destructive detection of zearalenone (ZEN) in maize. Measurement 2022, 203, 111944. [Google Scholar] [CrossRef]
- Fan, K.-J.; Liu, B.-Y.; Su, W.-H. Discrimination of Deoxynivalenol Levels of Barley Kernels Using Hyperspectral Imaging in Tandem with Optimized Convolutional Neural Network. Sensors 2023, 23, 2668. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-H.; Yang, C.; Dong, Y.; Johnson, R.; Page, R.; Szinyei, T.; Hirsch, C.D.; Steffenson, B.J. Hyperspectral imaging and improved feature variable selection for automated determination of deoxynivalenol in various genetic lines of barley kernels for resistance screening. Food Chem. 2021, 343, 128507. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Jayas, D.S.; White, N.D.; Fields, P.G.; Gräfenhan, T. Detection of fungal infection and Ochratoxin A contamination in stored barley using near-infrared hyperspectral imaging. Biosyst. Eng. 2016, 147, 162–173. [Google Scholar] [CrossRef]
- Tekle, S.; Måge, I.; Segtnan, V.H.; Bj, Å. Near-Infrared Hyperspectral Imaging of Fusarium-Damaged Oats (Avena sativa L.). Cereal Chem. 2015, 92, 73–80. [Google Scholar] [CrossRef]
- Janila, P.; Manohar, S.S.; Patne, N.; Variath, M.T.; Nigam, S.N. Genotype × Environment Interactions for Oil Content in Peanut and Stable High-Oil-Yielding Sources. Crop Sci. 2016, 56, 2506–2515. [Google Scholar] [CrossRef]
- Wang, K.-H.; Lai, Y.-H.; Chang, J.-C.; Ko, T.-F.; Shyu, S.-L.; Chiou, R.Y.-Y. Germination of Peanut Kernels to Enhance Resveratrol Biosynthesis and Prepare Sprouts as a Functional Vegetable. J. Agric. Food Chem. 2005, 53, 242–246. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, L.; Gao, J.; Gao, M.; Han, Z. Quantitative detection of Aflatoxin B1 by subpixel CNN regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120633. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, L.; Han, Z. Quantitative aflatoxin B1 detection and mining key wavelengths based on deep learning and hyperspectral imaging in subpixel level. Comput. Electron. Agric. 2023, 206, 107561. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Dong, H.; Sun, J.; Huang, J.; Li, S.; Ma, C.; Guo, Y.; Sun, X. Spatio-temporal distribution patterns and quantitative detection of aflatoxin B1 and total aflatoxin in peanut kernels explored by short-wave infrared hyperspectral imaging. Food Chem. 2023, 424, 136441. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yan, C.; Jiang, X.; Shen, F.; You, J.; Fang, Y. Classification of aflatoxin B1 naturally contaminated peanut using visible and near-infrared hyperspectral imaging by integrating spectral and texture features. Infrared Phys. Technol. 2021, 114, 103652. [Google Scholar] [CrossRef]
- Kabir, A.; Lee, I.; Mishra, G.; Singh, C.B.; Panda, B.K.; Lee, S.-H. Quantifying Aflatoxin B1 Contamination Levels in Almonds Using Hyperspectral Imaging Utilizing Gaussian Process and Support Vector Regression. In Proceedings of the 2024 IEEE Symposium on Wireless Technology & Applications (ISWTA), Kuala Lumpur, Malaysia, 20–21 July 2024. [Google Scholar]
- Wu, Q.; Tian, H.; Xu, H. Laser Induced Fluorescence Hyperspectral Imaging (LIF-HSI) for Aflatoxin B1 Detection in Pistachio Kernels. In Proceedings of the 2019 ASABE Annual International Meeting, St. Joseph, MI, USA, 7–10 July 2019; ASABE: St. Joseph, MI, USA; p. 1. [Google Scholar]
- Zhongzhi, H.; Limiao, D. Aflatoxin contaminated degree detection by hyperspectral data using band index. Food Chem. Toxicol. 2020, 137, 111159. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Liu, Y.; Rajasekaran, K.; Bhatnagar, D. Use of Visible–Near-Infrared (Vis-NIR) Spectroscopy to Detect Aflatoxin B1 on Peanut Kernels. Appl. Spectrosc. 2019, 73, 415–423. [Google Scholar] [CrossRef]
- Han, Z.; Gao, J. Pixel-level aflatoxin detecting based on deep learning and hyperspectral imaging. Comput. Electron. Agric. 2019, 164, 104888. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, Y.; Gu, Q.; Zhao, L.; Yang, R.; Han, Z. Spectral intelligent detection for aflatoxin B1 via contrastive learning based on Siamese network. Food Chem. 2024, 449, 139171. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.-H.; Lee, I. Detection of aflatoxin contamination in single kernel almonds using multispectral imaging system. J. Food Compos. Anal. 2024, 125, 105701. [Google Scholar] [CrossRef]
- Kabir, A.; Lee, I.; Mishra, G.; Panda, B.K.; Singh, C.B.; Lee, S.-H. Classification of Aflatoxin B1 Contamination Level Using Hyperspectral Images with Random Forest and QDA. In Proceedings of the 2024 IEEE International Conference on Big Data, Cloud Computing, and Data Science (BCD), Kitakyushu, Japan, 16–18 July 2024; IEEE: Piscataway, NJ, USA, 2024. [Google Scholar]
- Kabir, A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Aflatoxin B1 Contamination Level Detection in Almond Kernels Through Short Wave Infrared Hyperspectral Image Analysis. Adv. Agrochem. 2025, in press. [CrossRef]
- Kabir, A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Correlation awareness evolutionary sparse hybrid spectral band selection algorithm to detect aflatoxin B1 contaminated almonds using hyperspectral images. Food Chem. 2025, 476, 143381. [Google Scholar] [CrossRef]
- Kabir, A.; Lee, I.; Lee, S.-H. Deep Learning-Based Detection of Aflatoxin B1 Contamination in Almonds Using Hyperspectral Imaging: A Focus on Optimized 3D Inception–ResNet Model. Toxins 2025, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Multilayer Perception-Based Hybrid Spectral Band Selection Algorithm for Aflatoxin B1 Detection Using Hyperspectral Imaging. Appl. Sci. 2024, 14, 9313. [Google Scholar] [CrossRef]
- Williams, L.; Shukla, P.; Sheikh-Akbari, A.; Mahroughi, S.; Mporas, I. Measuring the Level of Aflatoxin Infection in Pistachio Nuts by Applying Machine Learning Techniques to Hyperspectral Images. Sensors 2025, 25, 1548. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ibañez, V.; Soldado, A.; Martínez-Fernández, A.; de la Roza-Delgado, B. Application of near infrared spectroscopy for rapid detection of aflatoxin B1 in maize and barley as analytical quality assessment. Food Chem. 2009, 113, 629–634. [Google Scholar] [CrossRef]
- Putthang, R.; Sirisomboon, P.; Sirisomboon, C.D. Shortwave Near-Infrared Spectroscopy for Rapid Detection of Aflatoxin B1 Contamination in Polished Rice. J. Food Prot. 2019, 82, 796–803. [Google Scholar] [CrossRef]
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Cleveland, T.; Bhatnagar, D. Correlation and classification of single kernel fluorescence hyperspectral data with aflatoxin concentration in corn kernels inoculated with Aspergillus flavus spores. Food Addit. Contam. Part A 2010, 27, 701–709. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Integration of Fluorescence and Reflectance Visible Near-Infrared (VNIR) Hyperspectral Images for Detection of Aflatoxins in Corn Kernels. Trans. ASABE 2016, 59, 785–794. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Liu, Y.; Rajasekaran, K.; Bhatnagar, D. Detection of aflatoxin B1 on corn kernel surfaces using visible-near infrared spectroscopy. J. Near Infrared Spectrosc. 2020, 28, 59–69. [Google Scholar] [CrossRef]
- Firrao, G.; Torelli, E.; Gobbi, E.; Raranciuc, S.; Bianchi, G.; Locci, R. Prediction of milled maize fumonisin contamination by multispectral image analysis. J. Cereal Sci. 2010, 52, 327–330. [Google Scholar] [CrossRef]
- Zhongzhi, H.; Limiao, D. Application driven key wavelengths mining method for aflatoxin detection using hyperspectral data. Comput. Electron. Agric. 2018, 153, 248–255. [Google Scholar] [CrossRef]
- Cheng, X.; Vella, A.; Stasiewicz, M.J. Classification of aflatoxin contaminated single corn kernels by ultraviolet to near infrared spectroscopy. Food Control 2019, 98, 253–261. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, B.; Ji, H.; Wang, H.; Liu, Y.; Zhang, X.; Ren, C. Nondestructive Prediction of Mechanical Parameters to Apple Using Hyperspectral Imaging by Support Vector Machine. Food Anal. Methods 2022, 15, 1397–1406. [Google Scholar] [CrossRef]
- Kim, J.; Kang, J.; Sohn, M. Ensemble learning-based filter-centric hybrid feature selection framework for high-dimensional imbalanced data. Knowl.-Based Syst. 2021, 220, 106901. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Yang, Z.; Zhou, C.; Wang, R.; Zhang, K.; Lv, X.; Yang, L.; Wang, Z.; Li, P.; et al. Pixel-level regression for UAV hyperspectral images: Deep learning-based quantitative inverse of wheat stripe rust disease index. Comput. Electron. Agric. 2023, 215, 108434. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Falade, T.D.O.; Mutuma, M.; Mutiga, S.K.; Harvey, J.J.W.; Fox, G.; Pearson, T.C.; Muthomi, J.W.; Nelson, R.J. Multi-spectral kernel sorting to reduce aflatoxins and fumonisins in Kenyan maize. Food Control 2017, 78, 203–214. [Google Scholar] [CrossRef]
- Levasseur-Garcia, C. Updated Overview of Infrared Spectroscopy Methods for Detecting Mycotoxins on Cereals (Corn, Wheat, and Barley). Toxins 2018, 10, 38. [Google Scholar] [CrossRef]
- Quan, Y.; Zhong, X.; Feng, W.; Chan, J.C.-W.; Li, Q.; Xing, M. SMOTE-Based Weighted Deep Rotation Forest for the Imbalanced Hyperspectral Data Classification. Remote. Sens. 2021, 13, 464. [Google Scholar] [CrossRef]
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Detecting maize inoculated with toxigenic and atoxigenic fungal strains with fluorescence hyperspectral imagery. Biosyst. Eng. 2013, 115, 125–135. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Lee, S.; Kim, M.S.; Bae, H.; Cho, B.-K. Short wave infrared (SWIR) hyperspectral imaging technique for examination of aflatoxin B1 (AFB1) on corn kernels. Food Control 2015, 51, 171–176. [Google Scholar] [CrossRef]
- Wynants, L.; Vergouwe, Y.; Van Huffel, S.; Timmerman, D.; Van Calster, B. Does ignoring clustering in multicenter data influence the performance of prediction models? A simulation study. Stat. Methods Med. Res. 2016, 27, 1723–1736. [Google Scholar] [CrossRef]

| Mycotoxin | Main Producers | Commonly Affected Foods | Possible Health Issues | MRLs in Cereals and Nuts (µg/kg) |
|---|---|---|---|---|
| Aflatoxin | Aspergillus flavus, A. parasiticus | Peanut, pistachio, almond, wheat, corn, and rice | Liver damage, high hemorrhage, DNA damage, impaired body immune function, etc. [8] | Total aflatoxins (nuts): EU: 4–10 [9]; US: 20 [10] |
| Ochratoxin A | Aspergillus ochraceus, A. carbonarius | Wheat, corn, and barley | Kidney toxicity in both animals and humans, kidney tumor in rodents [11] | EU: 5–10 (pistachio); 3–5 (cereals) [10,12] |
| Patulin | Penicillium and Byssochlamys | Wheat bread | Damaged liver, kidney, immune system, and intestinal tissues [13] | -- |
| T-2 toxin | Fusarium, Myrotecium | Wheat, barley, rye, oats, and maize | Reduces weight, weakens the immune system [14] | EU: 50 (UP cereal); 150 (UP barley); 100 (UP maize); 1250 (UP oats) [15] |
| Fumonisins | Fusarium verticillioides | Corn and corn-based food products | Can create defects in the neural tube and esophageal cancer [16] | EU: 1000–4000; US: 2000–4000 (cereal) [10] |
| Ergot alkaloids | Claviceps spp. | Wheat, oats, barley, and rye | It can create pulse variation, decrease blood circulation, etc. [17] | EU: 50 (barley, oats, and wheat) [18] |
| Deoxynivalenol | Fusarium graminearum | Wheat, corn, oats, barley, and other cereal grains | It causes vomiting and short-term nausea issues [19] | EU: 150 (P cereal); 1000 (UP cereal), 1500 (UP wheat), 1750 (UP oats) [20] |
| Alternaria toxins | Alternaria | Wheat, corn, and other cereal | DNA strands can be broken, reducing cell proliferation [21] | EU (recommended in cereal, not MRLs): 2 (Alternariol); 500 (Tenuazonic acid) [22] |
| Zearalenone | Fusarium graminearum and F. culmorum | Wheat, rice, oats, barley, and rye | Can have an impact on hormonal balance [5] | EU: 100–350 (cereal) [10] |
| Mycotoxin | Sample Number | Wavelength (nm) | Preprocessing | ML Methods | Performance | Reference |
|---|---|---|---|---|---|---|
| DON | 600 | 1000–16,000 | SNV, MSC | LDA | Acc: 83% | [56] |
| DON | 72 | 250–2500 | SG, MSC | RF, SVM, CNN | Acc: 98.95% | [57] |
| DON | 300 | 900–1700 | MSC, SG, 1st and 2nd dev, SNV | PLSR | R2: 0.88 | [54] |
| DON | 120 | 970–1623 | MSC, 1st and 2nd dev, SNV | PLSR, SVR | R2: 0.88 | [49] |
| DON | 480 | 960–1700 | Raw | KNN | Acc: 80% | [39] |
| DON | 50 | 900–1700 | MSC, 1st and 2nd dev, SNV | LDA, NB, KNN | Acc: 100% | [55] |
| DON | 96 | 400–2500 | SNV, MSC | SVM, SAE | Acc: 96% | [58] |
| DON | 150 | 900–1700 | MSC, 1st and 2nd dev, SNV | PLSR, LDA | Acc: 62.7% | [59] |
| AFB1 | 150 | 899–1725 | SG, SNV | PLSR | R2: 0.99 | [60] |
| OTA | 20 kg | 1000–1600 | Raw | DA | Acc: 99.8% | [61] |
| OTA | 10 kg | 1000–1600 | Raw | DA | Acc: 100% | [46] |
| Mycotoxin | Sample Number | Wavelength (nm) | Preprocessing | ML Methods | Performance | Reference |
|---|---|---|---|---|---|---|
| AFB1 | 900 | 786–824 | SG | LDA | Acc: 89.6% | [62] |
| AFB1 | 958 | 380–814 | SNV | SVR | Acc: 98% | [63] |
| AFB1 | 228 | 900–2500 | SNV, SG | SVM, PLS-DA | Acc: 95.7% | [48] |
| AFB1 | 968 | 327–1098 930–2548 | Raw | PLSR | R2: 0.924 | [64] |
| AFB1 | 26 | 400–1000 | SNV | SVM, PLSR | R2: 0.95 | [65] |
| AFB1 | 300 | 900–2500 | SNV, SG, 1st and 2nd dev | PLS-DA | Acc: 90.4% | [40] |
| AFB1 | 320 | 1000–2500 | SNV | PLSR, SVR | R2: 0.97 | [66] |
| AFB1 | 122 | 785 | SG, SNV | SVM | Acc: 95.7% | [67] |
| AFB1 | 958 | 460–929 | SNV, MSC | KNN, SVM, LDA | Acc: 87.3% | [68] |
| AFB1 | 250 | 1100–2000 | SG, 1st and 2nd dev | LDA | Acc: 95.56% | [69] |
| AFB1 | 6 | 292–865 | MSC, PCA, | RF, KNN | Acc: 99.38% | [70] |
| DON | 98 | 893–1730 | MSC, SNV, SG | RF, NN, LR, KNN | Acc: 98.6% R2: 81.9 | [71] |
| DON | 176 | 900–1700 | SNV, SG smoothing | PLSR, PLS-DA | R2: 0.974, Acc: 98.8% | [72] |
| FM | 58 | 1000–2100 | SNV | PLSR | R2: 0.98 | [73] |
| ZEN | 1890 | 400–1000 | Raw | SVM, DA, PLSR, NN | Acc: 93.33 % | [74] |
| Other Cereal | Mycotoxin | Sample Number | Wavelength (nm) | Preprocessing | ML Methods | Performance | Reference |
|---|---|---|---|---|---|---|---|
| Barley | DON | 590 | 367–1048 | MAF | CNN | Acc: 89.81% | [75] |
| Barley | DON | 1004 | 382–1030 | Raw | PLDA | R2: 0.931 | [76] |
| Barley | OTA | 101 kg | 1000–1600 | Raw | DA | Acc: 100% | [77] |
| Oats | DON | 119 | 900–1700 | SNV, 1st dev | PLSR, RF, KNN, NB | Acc: 77.8% | [47] |
| Oats | DON | 220 | 1000–2500 | Normalization | PLSR, LDA | R2: 0.8 | [78] |
| Oats | T-2 | 119 | 900–1700 | SNV, 1st dev | PLSR, RF, KNN, NB | Acc: 94.1% | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, M.A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review. Toxins 2025, 17, 219. https://doi.org/10.3390/toxins17050219
Kabir MA, Lee I, Singh CB, Mishra G, Panda BK, Lee S-H. Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review. Toxins. 2025; 17(5):219. https://doi.org/10.3390/toxins17050219
Chicago/Turabian StyleKabir, Md. Ahasan, Ivan Lee, Chandra B. Singh, Gayatri Mishra, Brajesh Kumar Panda, and Sang-Heon Lee. 2025. "Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review" Toxins 17, no. 5: 219. https://doi.org/10.3390/toxins17050219
APA StyleKabir, M. A., Lee, I., Singh, C. B., Mishra, G., Panda, B. K., & Lee, S.-H. (2025). Detection of Mycotoxins in Cereal Grains and Nuts Using Machine Learning Integrated Hyperspectral Imaging: A Review. Toxins, 17(5), 219. https://doi.org/10.3390/toxins17050219






