Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins
Abstract
:1. Introduction
2. Results
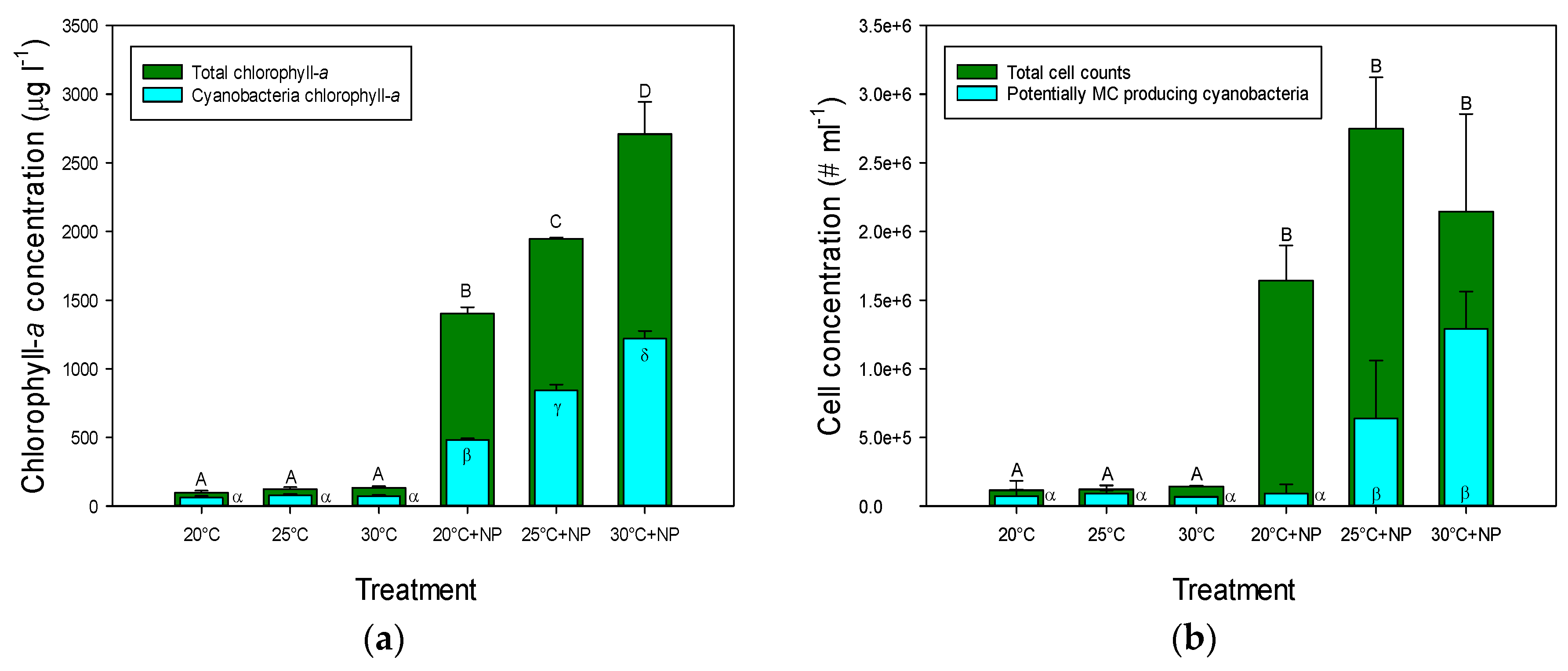
2.1. Chlorophyll-a Concentrations and Cell Concentrations in Incubated Natural Seston
2.2. Growth Rates of Incubated Natural Seston
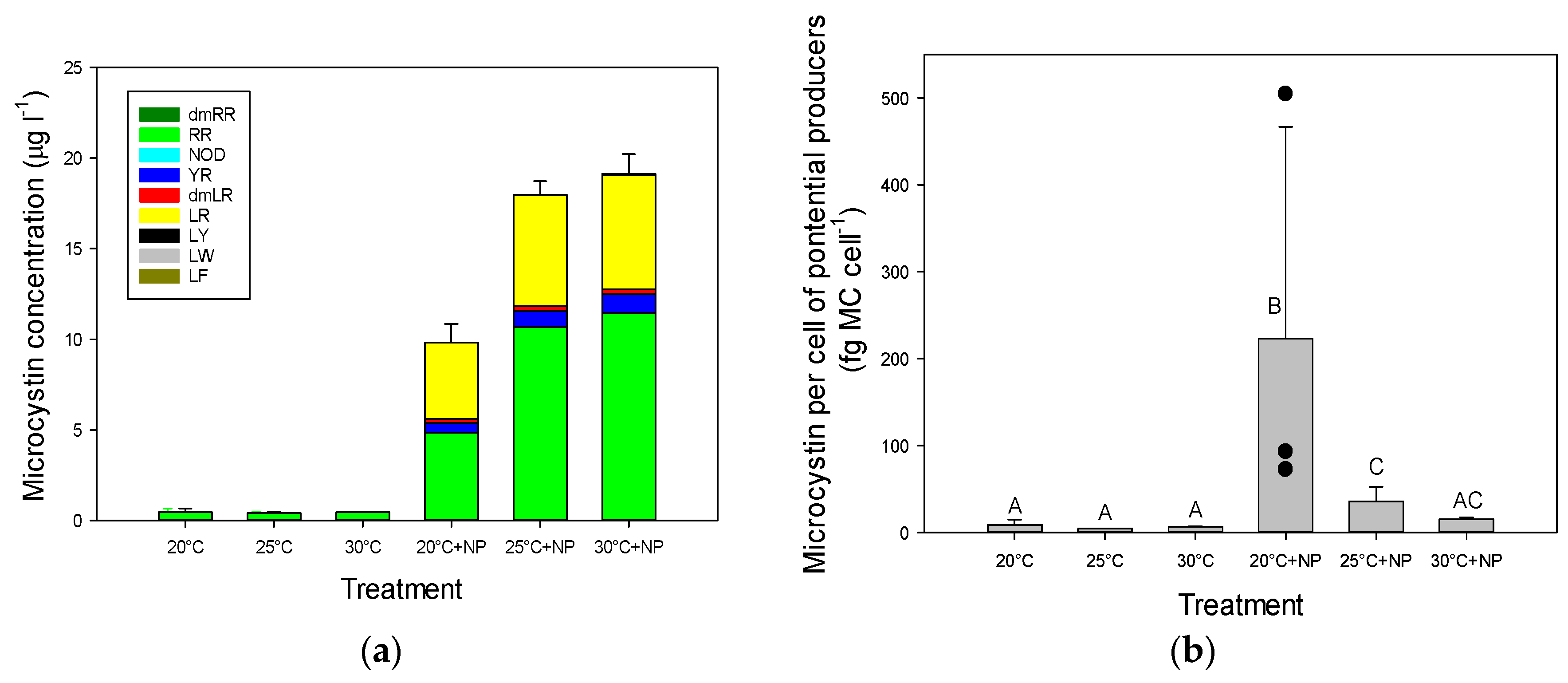
2.3. Microcystins in Incubated Seston
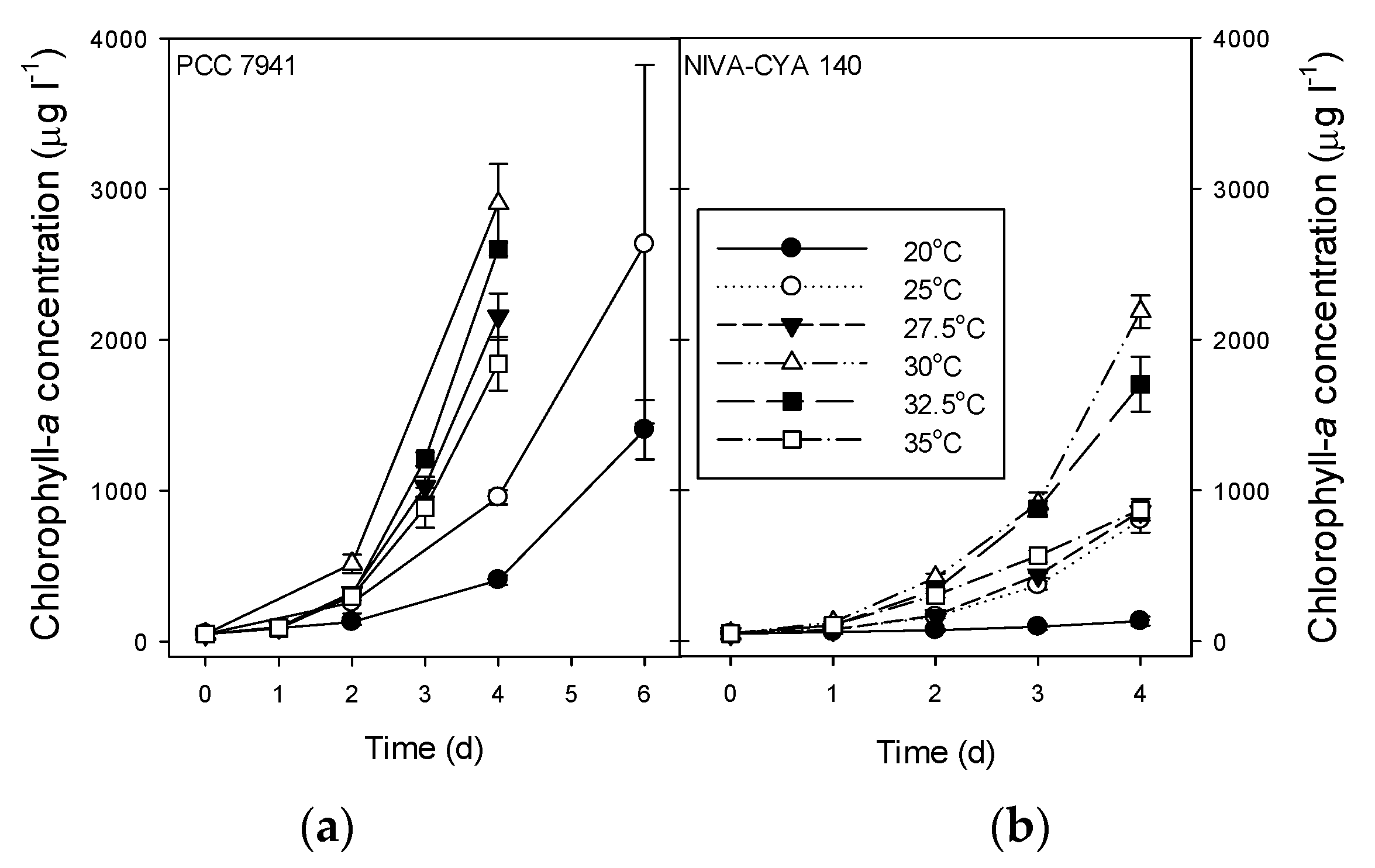
2.4. Growth Rates, Chlorophyll-a, and Microcystins in M. Aeruginosa Strains
3. Discussion
4. Conclusions
5. Materials and Methods
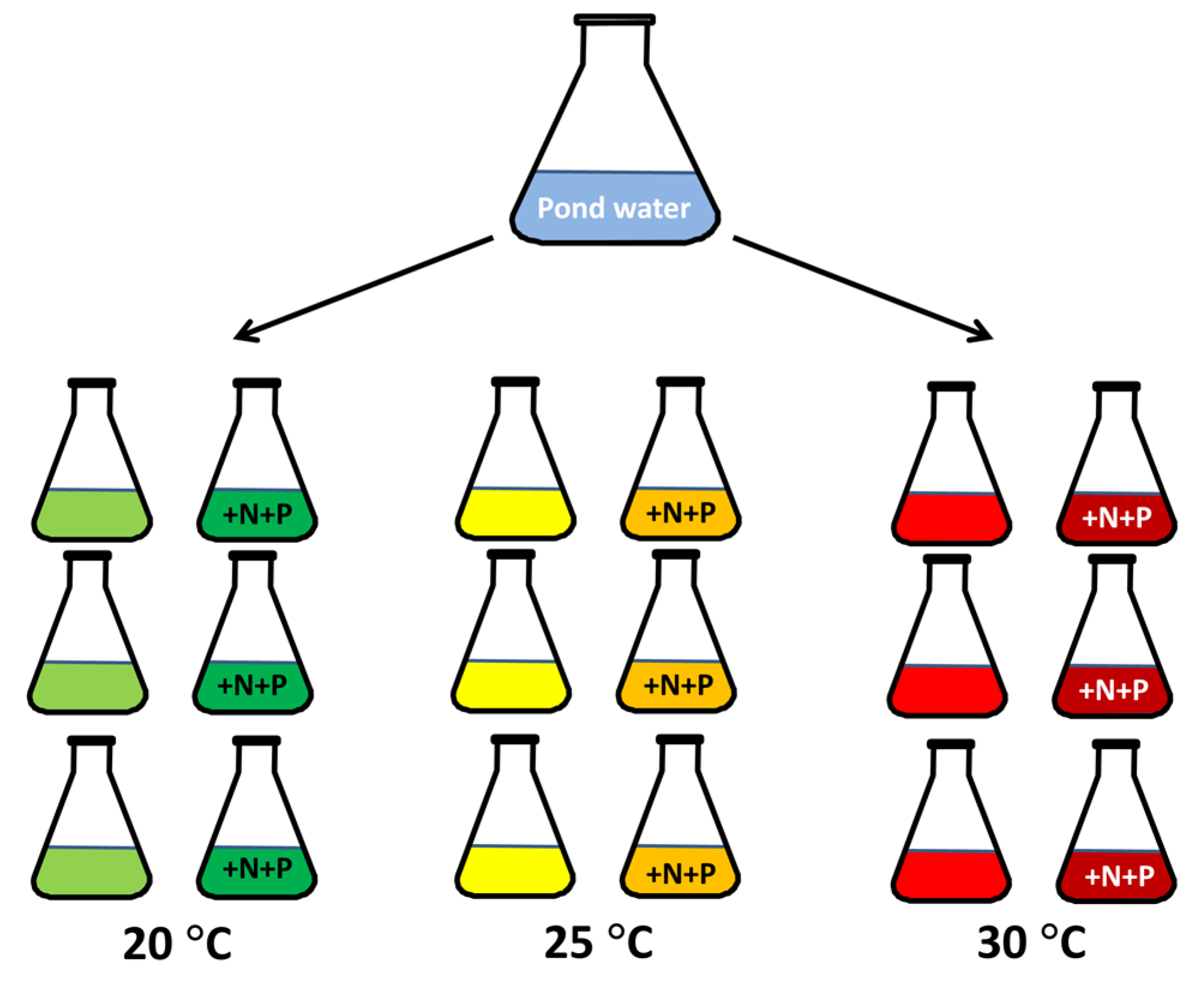
5.1. Sampling
5.2. Experiment Pond Water
5.3. Experiment with Two Microcystis Aeruginosa Strains
5.4. Microcystin (MC) Analysis
5.5. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| 20 °C | 25 °C | 30 °C | 20 °C | 25 °C | 30 °C | |
|---|---|---|---|---|---|---|
| Organisms | +N + P | +N + P | +N + P | |||
| Pico-cyanobacteria | 29,212 A (22,948) | 13,000 A (10,476) | 51,167 A (6371) | 1,323,333 B (304,737) | 1,921,667 B (319,074) | 571,667 AB (770,460) |
| Aphnanizomenon flos-aquae | 1964 (1418) | 3200 (3464) | 2400 (2078) | 42,000 (72,746) | - | - |
| Dolichospermum sp. | 1667 (2082) | 2000 (1732) | 1000(-) | 3333 (5774) | 20,000 (10,000) | 16,667 (11,547) |
| Microcystis sp. | 41,439 A (45,991) | 48,000 A (24,434) | 47,000 A (8352) | 74,167 A (68,298) | 498,333 B (205,933) | 1,275,000 B (277,804) |
| Woronichinia naegeliana | 28,364 (630) | 41,333 (2309) | 18,667 (10,066) | 13,333 (23,094) | 120,000 (207,846) | - |
| Chlorophytes | 10,030 A (4371) | 12,867 A (3523) | 16,467 A (1361) | 181,333 B (35,388) | 180,000 B (68,440) | 258,667 B (43,143) |
| Diatoms | 755(342) | 967 (611) | 5367 (1756) | 3333 (4163) | 9667 (10,017) | 22,000 (15,716) |
| Cryptophytes | 648 (89) | 400 (265) | 333 (252) | - | - | - |
| Others | 315 (223) | 33 (58) | - | - | - | - |
| Potential MC producing | 71,470 (47,698) | 91,333 (24,214) | 66,667 (3014) | 90,833 (67,376) | 638,333 (421,733) | 1,291,667 (270,386) |
| Total cyanobacteria cells | 102,645 (65,134) | 107,533 (32,305) | 120,233 (5301) | 1,456,167 (292,218) | 2,560,000 (377,227) | 1,863,333 (762,534) |
| Diazotrophs | 3630 (2220) | 5200 (4583) | 3400 (2078) | 45,333 (70,038) | 20,000 (10,000) | 16,667 (11,547) |
| Total cells | 114,394 (68,915) | 121,800 (28,574) | 142,400 (6075) | 1,640,833 (255,225) | 2,749,667 (373,305) | 2,144,000 (710,762) |
Appendix B
Appendix C
| Temperature: | 19.5 °C |
| pH: | 8.13 |
| Conductivity: | 392 μS·cm−1 |
| O2 concentration: | 9.1 mg·L−1 |
| O2 saturation: | 96% |
| Turbidity: | 28.5 NTU |
| Secchi depth: | 40 cm |
| Cyano CHL-a: | 19.6 μg·L−1 |
| Total CHL-a: | 65.5μg·L−1 |
| Total-P: | 76 μg·P·L−1 |
| Phosphate-P: | 5.6 μg·SRP·L−1 |
| DIN: | 36 μg·N·L−1 |

Appendix D
References
- Figueiredo de, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environ. Microbiol. 2016, 18, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; McCauley, E.; Downing, J.A. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol. Oceanogr. 1997, 42, 487–495. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Trolle, D.; Nielsen, A.; Rolighed, J.; Thodsen, H.; Andersen, H.E.; Karlsson, I.B.; Refsgaard, J.C.; Olesen, J.E.; Bolding, K.; Kronvang, B.; et al. Projecting the future ecological state of lakes in Denmark in a 6 degree warming scenario. Clim. Res. 2015, 64, 55–72. [Google Scholar] [CrossRef]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008; p. 210. [Google Scholar]
- Elliot, J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.; Steffen, M.; Gobler, C.; Otten, T.; Wilhelm, S.; Wood, S.A.; Pearl, H. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Lürling, M. Inventarisation of Cyanotoxins at Bathing Sites under Jurisdiction of The Netherlands National Water Authority; Report M354; Wageningen University: Wageningen, The Netherlands, 2010; p. 73. (In Dutch) [Google Scholar]
- Kosten, S.; Kardinaal, E.; Faassen, E.; Netten, J.; Lürling, M. Klimaat & Waterkwaliteit, Klimaatinvloed op Waterkwaliteit en Het Voorkomen van Cyanobacteriële Toxines; Kennis voor Klimaat rapport; Programmabureau Kennis voor Klimaat: Utrecht, Wageningen, 2011; KvK/043/2011, ISBN/EAN 9789490070489. (In Dutch) [Google Scholar]
- Faassen, E.J.; Lürling, M. Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar. Drugs 2013, 11, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Verspagen, J.M.H.; Lürling, M.; Van Donk, E.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Phosphorus enrichment and carbon depletion contribute to high Microcystis biomass and microcystin concentrations in Ugandan lakes. Limnol. Oceanogr. 2013, 58, 1075–1088. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Horst, G.P.; Sarnelle, O.; White, J.D.; Hamilton, S.K.; Kaul, R.B.; Bressie, J.D. Nitrogen availability increases the toxin quota of a harmful cyanobacterium, Microcystis aeruginosa. Water Res. 2014, 54, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Van Oosterhout, E.; Faassen, E.J.; Lürling, M.; Helmsing, N.R.; Van de Waal, D.B. Elevated pCO2 causes a shift towards more toxic microcystin variants in nitrogen-limited Microcystis aeruginosa. FEMS Microbiol. Ecol. 2015, 92, fiv159. [Google Scholar] [CrossRef] [PubMed]
- Gianuzzi, L.; Krock, B.; Crettaz Minaglia, M.C.; Rosso, L.; Houghton, C.; Sedan, D.; Malanga, G.; Espinosa, M.; Andrinolo, D.; Hernando, M. Growth, toxin production, active oxygen species and catalase activity of Microcystis aeruginosa (Cyanophyceae) exposed to temperature stress. Comp. Biochem. Physiol. Part C 2016, 189, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutemark, A.; Engström-Öst, J.; Vehmaa, A.; Gorokhova, E. Growth, toxicity and oxidative stress of a cultured cyanobacterium (Dolichospermum sp.) under different CO2/pH and temperature conditions. Phycol. Res. 2015, 63, 56–63. [Google Scholar] [CrossRef]
- Rapala, J.; Sivonen, K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb. Ecol. 1998, 36, 181–192. [Google Scholar] [PubMed]
- Rapala, J.; Sivonen, K.; Lyra, C.; Niemela, S.I. Variation of microcystin, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimulation. App. Environ. Microbiol. 1997, 63, 2206–2212. [Google Scholar]
- Mowe, M.A.D.; Porojan, C.; Abbas, F.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Rising temperatures may increase growth rates and microcystin production in tropical Microcystis species. Harmful Algae 2015, 50, 88–98. [Google Scholar] [CrossRef]
- Song, L.; Sano, T.; Li, R.; Watanabe, M.M.; Liu, Y.; Kaya, K. Microcystin production of Microcystis viridis (cyanobacteria) under different culture conditions. Phycol. Res. 1998, 46, 19–23. [Google Scholar] [CrossRef]
- Van der Westhuizen, A.J.; Eloff, J.N. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006). Planta 1985, 163, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.F.; Oishi, S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [PubMed]
- Tonk, L.; Welker, M.; Huisman, J.; Visser, P.M. Production of cyanopeptolins, anabaenopeptins, and microcystins by the harmful cyanobacteria Anabaena 90 and Microcystis PCC 7806. Harmful Algae 2009, 8, 219–224. [Google Scholar] [CrossRef]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshwater Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Ger, K.A.; Hansson, L.A.; Lürling, M. Understanding cyanobacteria–zooplankton interactions in a more eutrophic world. Freshwater Biol. 2014, 59, 1783–1798. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Groen, G. Extreme Zomerneerslag 2006 en Klimaatscenario’s 2007; KNMI publicatie 215: De Bilt, The Netherlands, 2007; p. 26. (In Dutch) [Google Scholar]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shan, K.; Lin, L.; Shen, W.; Huang, L.; Gan, N.; Song, L. Multi-year assessment of toxic genotypes and microcystin concentration in northern Lake Taihu, China. Toxins 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Conradie, K.R.; Barnard, S. The dynamics of toxic microcystis strains and microcystin production in two hypertrophic South African reservoirs. Harmful Algae 2012, 20, 1–10. [Google Scholar] [CrossRef]
- Wood, S.A.; Borges, H.; Puddick, J.; Biessy, L.; Atalah, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P. Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: Insights into potential effects of climate change. Hydrobiologia 2017, 785, 71–89. [Google Scholar] [CrossRef]
- Pacheco, A.B.F.; Guedes, I.A.; Azevedo, S.M.F.O. Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 2016, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Ferreruela, G.; Tonk, L.; Van Donk, E.; Huisman, J.; Visser, P.M.; Matthijs, H.C.P. Pulsed nitrogen supply induces dynamic changes in the amino acid composition and microcystin production of the harmful cyanobacterium Planktothrix agardhii. FEMS Microbiol. Ecol. 2010, 74, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lyck, S. Simultaneous changes in cell quotas of microcystin, chlorophyll a, protein and carbohydrate during different growth phases of a batch culture experiment with Microcystis aeruginosa. J. Plankton Res. 2004, 26, 727–736. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Fastner, J.; Dittmann, E. Microcystin production revisited: Conjugate formation makes a major contribution. Environ. Microbiol. 2013, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Steinhauser, D.; Dittmann, E. Metabolomic analysis indicates a pivotal role of the hepatotoxin microcystin in high light adaptation of Microcystis. Environ. Microbiol. 2015, 17, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Kondo, F.; Ikai, Y.; Oka, H.; Okumura, M.; Ishikawa, N.; Harada, K.-I.; Matsuura, K.; Murata, H.; Suzuki, M. Formation, characterization, and toxicity of the glutathione and cystein conjugates of toxic hepatopeptide microcystins. Chem. Res. Toxicol. 1992, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 323–347. [Google Scholar]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Mazzeo, N.; Scheffer, M.; Sternberg, L.D.S.L.; Jeppesen, E. Lake and watershed characteristics rather than climate influence nutrient limitation in shallow lakes. Ecol. Appl. 2009, 19, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Brookes, J.D.; Carey, C.C. Resilience to blooms. Science 2011, 334, 46–47. [Google Scholar] [CrossRef] [PubMed]
- NEN. Water: Photometric Determination of the Content of Dissolved Orthophosphate and the Total Content of Phosphorous Compounds by Continuous Flow Analysis; NEN 6663; Netherlands Normalization Institute: Delft, The Netherlands, 1986. [Google Scholar]
- NEN. Water: Photometric Determination of the Content of Ammonium Nitrogen and the Sum of the Contents of Ammoniacal and Organically Bound Nitrogen According to Kjeldahl by Continuous Flow Analysis; NEN 6646; Netherlands Normalization Institute: Delft, The Netherlands, 1990. [Google Scholar]
- NEN. Bepaling van het Stikstofgehalte in de Vorm van Nitriet en in de Vorm van Nitraat en de som van Beide Met Doorstroomanalyse (CFA en FIA) en Spectrometrische Detectie; NEN-EN-ISO 13395; Netherlands Normalization Institute: Delft, The Netherlands, 1997. (In Dutch) [Google Scholar]
- Lambou, V.W.; Taylor, W.D.; Hern, S.C.; Williams, L.R. Comparisons of trophic state measurements. Water Res. 1983, 17, 1619–1626. [Google Scholar] [CrossRef]
- Lürling, M.; Beekman, W. Palmelloids formation in Chlamydomonas reinhardtii: Defence against rotifer predators? Ann. Limnol. 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Lürling, M.; Faassen, E.J. Dog poisonings associated with a Microcystis aeruginosa bloom in the Netherlands. Toxins 2013, 5, 556–567. [Google Scholar] [CrossRef] [PubMed]
| Growth Rates (d−1) | |||
|---|---|---|---|
| Treatment | Cyanobacteria | Algae | Pairwise Comparison |
| 20 °C | 0.16 ± 0.03 A | −0.04 ± 0.02 | t4 = 10.0; p < 0.001 |
| 25 °C | 0.20 ± 0.02 B | −0.01 ± 0.03 | t4 = 10.0; p < 0.001 |
| 30 °C | 0.19 ± 0.02 AB | 0.04 ± 0.01 | T3 = 10.0; p = 0.100 # |
| 20 °C + NP | 0.46 ± 0.01 C | 0.43 ± 0.01 | t4 = 8.04; p = 0.001 |
| 25 °C + NP | 0.54 ± 0.01 D | 0.45 ± 0.01 | t4 = 16.8; p < 0.001 |
| 30 °C + NP | 0.59 ± 0.01 E | 0.50 ± 0.02 | t4 = 9.03; p < 0.001 |
| Treatment | MC:Chlorophyll |
|---|---|
| 20 °C | 0.008 ± 0.005 A |
| 25 °C | 0.005 ± 0.002 A |
| 30 °C | 0.006 ± 0.001 A |
| 20 °C + NP | 0.020 ± 0.003 B |
| 25 °C + NP | 0.021 ± 0.001 B |
| 30 °C + NP | 0.016 ± 0.001 B |
| Growth Rate (d−1) | Chlorophyll-a (μg·L−1) | Total MC (μg·L−1) | ||||
|---|---|---|---|---|---|---|
| Treatment | PCC7941 | CYA140 | PCC7941 | CYA140 | PCC7941 | CYA140 |
| 20 °C | 0.52 ± 0.02 A | 0.24 ± 0.06 A | 1404 ± 197 # | 133 ± 30 | 178 ± 7.1 A # | 43 ± 11 A |
| 25 °C | 0.74 ± 0.01 B | 0.69 ± 0.03 B | 2635 ± 1188 # | 803 ± 82 | 49 ± 7.6 AB # | 79 ± 19 B |
| 27.5 °C | 0.94 ± 0.02 CE | 0.71 ± 0.01 B | 2156 ± 153 | 859 ± 41 | 36 ± 2.5 AB | 36 ± 3.1 A |
| 30 °C | 1.01 ± 0.02 D | 0.94 ± 0.01 C | 2905 ± 262 | 2186 ± 107 | ND | 16 ± 1.2 C |
| 32.5 °C | 0.99 ± 0.01 CD | 0.88 ± 0.03 D | 2603 ± 46 | 1704 ± 181 | 3.6 ± 2.4 B | 5.9 ± 0.6 D |
| 35 °C | 0.90 ± 0.02 E | 0.71 ± 0.02 B | 1842 ± 179 | 871 ± 71 | 2.6 ± 0.6 B | 1.6 ± 0.4 E |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lürling, M.; Van Oosterhout, F.; Faassen, E. Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins. Toxins 2017, 9, 64. https://doi.org/10.3390/toxins9020064
Lürling M, Van Oosterhout F, Faassen E. Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins. Toxins. 2017; 9(2):64. https://doi.org/10.3390/toxins9020064
Chicago/Turabian StyleLürling, Miquel, Frank Van Oosterhout, and Elisabeth Faassen. 2017. "Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins" Toxins 9, no. 2: 64. https://doi.org/10.3390/toxins9020064








