Engineering a Bi-Conical Microchip as Vascular Stenosis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
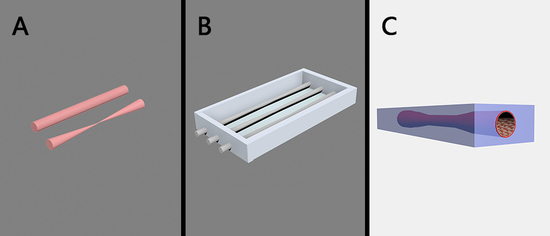
2.2. Fabrication of Bi-Conical Blood-Vessel-Like Microchip
2.3. Characterization of the Morphology of Bi-Conical Blood-Vessel-Like Microchip and Investigation of Liquid Transport Properties
2.4. Investigation of Microchannel Permeability
2.5. Cell Culture
2.6. Investigation of the Cytocompatibility and Endothelialization of Blood-Vessel-Like Microchip
2.7. Investigation of Cell Morphology and Alignment Changes at the Bi-Conical Region
2.8. Measurement of Endothelial Cell-Derived von Willebrand Factor
2.9. Computational Simulation of Wall Shear Rates
3. Results
3.1. Construction of Bi-Conical Vascular Hydrogel Models with Different Stenosis Structures
3.2. Liquid Transport and Microchannel Permeability Assay of the Blood-Vessel-Like Microchip
3.3. Cytocompatibility and Endothelialization of the Artificial Blood Vessel
3.4. Shear Stress Caused Cell Morphology and Alignment Changes at the Bi-Conical Region
3.5. Measurement of Endothelial Cell-Derived vWF
3.6. Computational Simulation of Hydrodynamics on the Blood-Vessel-Like Microchip Geometries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Craven, T.E.; Ryu, J.E.; Espeland, M.A.; Kahl, F.R.; Mckinney, W.M.; Toole, J.F.; Mcmahan, M.R.; Thompson, C.J.; Heiss, G.; Crouse, J.R. Evaluation of the association between carotid artery atherosclerosis and coronary artery stenosis: A case-controlled study. Circulation 1990, 82, 1230–1242. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Savage, P.J.; Borhani, N.O.; Kittner, S.J.; Tracy, R.; Gardin, J.M.; Price, T.R.; Furberg, C.D. Thickening of the carotid wall: A marker for atherosclerosis in the elderly? Stroke 1996, 27, 224–231. [Google Scholar]
- Santos, M.J.; Pedro, L.M.; Canhão, H.; Fernandes, J.F.E.; Silva, J.C.D.; Fonseca, J.E.; Saldanha, C. Hemorheological parameters are related to subclinical atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis patients. Atherosclerosis 2011, 219, 821–826. [Google Scholar] [CrossRef]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, W.S.; Mangin, P.; Salem, H.H.; Jackson, S.P. The impact of blood rheology on the molecular and cellular events underlying arterial thrombosis. J. Mol. Med. 2006, 84, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Casa, L.D.C.; Deaton, D.H.; Ku, D.N. Role of high shear rate in thrombosis. J. Vasc. Surg. 2015, 61, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Padro, T.; Badimon, L. Atherosclerosis and thrombosis: insights from large animal models. J. Biomed. Biotechnol. 2011, 2011, 907575. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid. Redox. Sign. 2015, 22, 760–771. [Google Scholar] [CrossRef]
- Suo, J.; Ferrara, D.E.; Dan, S.; Guldberg, R.E.; Taylor, W.R.; Giddens, D.P. Hemodynamic shear stresses in mouse aortas. Arterioscl. Throm. Vas. 2007, 27, 346–351. [Google Scholar] [CrossRef]
- Daugherty, A. Daugherty, mouse models of atherosclerosis. Am. J. Med. Sci. 2002, 323, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097. [Google Scholar] [CrossRef] [PubMed]
- Van, K.R.; Cosemans, J.M.; Heemskerk, J.W. Measurement of whole blood thrombus formation using parallel-plate flow chambers—A practical guide. Platelets 2012, 23, 229–242. [Google Scholar]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.W.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach-Teschl, C.; López, J.A. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Nat. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Westein, E.; Meer, A.D.V.D.; Kuijpers, M.J.E.; Frimat, J.P.; Berg, A.V.D.; Heemskerk, J.W.M. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Nat. Acad. Sci. USA 2013, 110, 1357. [Google Scholar] [CrossRef]
- Nesbitt, W.S.; Westein, E.; Tovar-Lopez, F.J.; Tolouei, E.; Mitchell, A.; Fu, J.; Carberry, J.; Fouras, A.; Jackson, S.P. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 2009, 15, 665–673. [Google Scholar] [CrossRef]
- Westein, E.; De, W.S.; Lamers, M.; Cosemans, J.M.; Heemskerk, J.W. Monitoring in vitro thrombus formation with novel microfluidic devices. Platelets 2012, 23, 501–509. [Google Scholar] [CrossRef]
- Zhu, S.; Herbig, B.A.; Li, R.; Colace, T.V.; Muthard, R.W.; Neeves, K.B.; Diamond, S.L. In microfluidico: Recreating in vivo hemodynamics using miniaturized devices. Biorheology 2015, 52, 303. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A.J. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. Clin. Invest. 2012, 122, 408. [Google Scholar] [CrossRef]
- Costa, P.F.; Albers, H.J.; Jea, L.; Hht, M.; Van, d.H.L.; Passier, R.; Van, d.B.A.; Malda, J.; Ad, V.D.M. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Sarvepalli, D.P.; Schmidtke DWNollert, M.U. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Ann. Biomed. Eng. 2009, 37, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ku, D.N.; Forest, C.R. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 2012, 12, 1355–1362. [Google Scholar] [CrossRef]
- Estrada, R.; Giridharan, G.A.; Nguyen, M.D.; Roussel, T.J.; Sethu, P. Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal. Chem. 2011, 83, 3170–3177. [Google Scholar] [CrossRef]
- Tortora, G.J.; Derrickson, B.H. The cardiovascular system: Blood vessels and hemodynamics. In Principles of Anatomy and Physiology, 13th ed; Tortora, G.J., Derrickson, B.H., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Marieb, E.N.; Hoehn, K. The cardiovascular system: Blood vessels. In Human Anatomy and Physiology, 9th ed.; Marieb, E.N., Hoehn, K., Eds.; Pearson Education: London, UK, 2013. [Google Scholar]
- Mejia, J.; Mongrain, R.; Bertrand, O.F. Accurate prediction of wall shear stress in a stented artery: Newtonian versus non-newtonian models. J. Biomech. Eng. 2011, 133, 074501. [Google Scholar] [CrossRef]
- Shahidi, M. Thrombosis and von Willebrand factor. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2016. [Google Scholar]
- Dhanesha, N.; Prakash, P.; Doddapattar, P.; Khanna, I.; Pollpeter, M.J.; Nayak, M.K.; Staber, J.M.; Chauhan, A.K. Endothelial cell-derived VWF is the major determinant that mediates VWF-dependent acute ischemic stroke by promoting post-ischemic thrombo-inflammation. Arterioscl. Throm. Vas. 2016, 36, 1829. [Google Scholar] [CrossRef]
- Doddapattar, P.; Dhanesha, N.; Chorawala, M.R.; Tinsman, C.; Jain, M.; Nayak, M.K.; Staber, J.M.; Chauhan, A.K. Endothelial cell-derived von willebrand factor, but not platelet-derived, promotes atherosclerosis in apolipoprotein e-deficient mice. Arterioscler. Throm. Vas. Biol. 2018, 38, 520–528. [Google Scholar] [CrossRef]
- Bark, D.L.; Ku, D.N. Platelet transport rates and binding kinetics at high shear over a thrombus. Biophys. J. 2013, 105, 502–511. [Google Scholar] [CrossRef] [Green Version]








| Region | Spindle-Shaped Cell Number | Total Cell Number | Spindle-Shaped Cell Percentage |
|---|---|---|---|
| A | 38 | 177 | 21.46% |
| B | 58 | 173 | 33.53% |
| C | 56 | 181 | 30.94% |
| D | 21 | 226 | 9.29% |
| Case | Widest Diameter (μm) | Deformation Length (μm) | Narrowest Diameter (μm) | Percentage of Stenosis | Flow Rate | Widest Diameter (μm) |
|---|---|---|---|---|---|---|
| 1 | 318 | 2970 | 125 | 84.5% | 3.50 × 10−3 | 14,000 |
| 2 | 576 | 3620 | 330 | 67.2% | 1.00 × 10−3 | 800 |
| 3 | 820 | 3910 | 390 | 77.4% | 0.53 × 10−3 | 520 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, J.; Wan, W.; Chen, C.; Wang, X.; Zhao, P.; Hou, Y.; Tian, H.; Wang, J.; Nandakumar, K.; et al. Engineering a Bi-Conical Microchip as Vascular Stenosis Model. Micromachines 2019, 10, 790. https://doi.org/10.3390/mi10110790
Li Y, Wang J, Wan W, Chen C, Wang X, Zhao P, Hou Y, Tian H, Wang J, Nandakumar K, et al. Engineering a Bi-Conical Microchip as Vascular Stenosis Model. Micromachines. 2019; 10(11):790. https://doi.org/10.3390/mi10110790
Chicago/Turabian StyleLi, Yan, Jianchun Wang, Wei Wan, Chengmin Chen, Xueying Wang, Pei Zhao, Yanjin Hou, Hanmei Tian, Jianmei Wang, Krishnaswamy Nandakumar, and et al. 2019. "Engineering a Bi-Conical Microchip as Vascular Stenosis Model" Micromachines 10, no. 11: 790. https://doi.org/10.3390/mi10110790