Flexible Microfluidics: Fundamentals, Recent Developments, and Applications
Abstract
:1. Introduction
2. Materials for Flexible Microfluidics
2.1. Material Properties
2.2. Siloxane Elastomers
2.3. Parylene
2.4. Poly(ethylene terephthalate) (PET)
2.5. Poly(imide) (PI)
2.6. Off-Stoichiometry Thiol-ene
2.7. Others
3. Flexibility and Microfluidic Functions
3.1. Effect of Flexibility on Flow Rate and Pressure Drop
3.2. Effect of Flexibility on Mixing
3.3. Effect of Flexibility on Valving
3.4. Effect of Flexibility on Pumping
3.5. Effect of Flexibility on Separation
4. Recent Applications of Flexible Microfluidics
4.1. Flexible and Wearable Electronics
4.1.1. Mechanical Sensors
4.1.2. Sweat Sensors
4.1.3. Temperature Sensors
4.1.4. Flexible Circuits and Interconnects
4.2. Biology
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019. [Google Scholar]
- Tan, Y.C.; Fisher, J.S.; Lee, A.I.; Cristini, V.; Lee, A.P. Design of microfluidic channel geometries for the control of droplet volume, chemical concentration, and sorting. Lab Chip 2004, 4, 292–298. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13–R39. [Google Scholar] [CrossRef]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35–R64. [Google Scholar] [CrossRef]
- Woias, P.J.S.; Chemical, A.B. Micropumps—Past, progress and future prospects. Sens. Actuators B Chem. 2005, 105, 28–38. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Huang, X.Y.; Chuan, T.K. MEMS-micropumps: A review. J. Fluid. Eng.-T Asme. 2002, 124, 384–392. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2004, 15, R1. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluid. 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Antfolk, M.; Laurell, T. Continuous flow microfluidic separation and processing of rare cells and bioparticles found in blood–A review. Anal. Chim. Acta 2017, 965, 9–35. [Google Scholar] [CrossRef]
- Gunther, A.; Jensen, K.F. Multiphase microfluidics: From flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef]
- Marre, S.; Roig, Y.; Aymonier, C. Supercritical microfluidics: Opportunities in flow-through chemistry and materials science. J. Supercrit. Fluid. 2012, 66, 251–264. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- Dendukuri, D.; Tsoi, K.; Hatton, T.A.; Doyle, P.S. Controlled synthesis of nonspherical microparticles using microfluidics. Langmuir 2005, 21, 2113–2116. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Lu, C.; Verbridge, S.S. Microfluidic Methods for Molecular Biology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Bhagat, A.A.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005, 26, R73–R98. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Ziaie, B.; Baldi, A.; Lei, M.; Gu, Y.; Siegel, R.A. Hard and soft micromachining for BioMEMS: Review of techniques and examples of applications in microfluidics and drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 145–172. [Google Scholar] [CrossRef]
- Gross, P.G.; Kartalov, E.P.; Scherer, A.; Weiner, L.P. Applications of microfluidics for neuronal studies. J. Neurol. Sci. 2007, 252, 135–143. [Google Scholar] [CrossRef]
- Wise, K.D.; Anderson, D.J.; Hetke, J.F.; Kipke, D.R.; Najafi, K. Wireless implantable microsystems: High-density electronic interfaces to the nervous system. Proc. IEEE 2004, 92, 76–97. [Google Scholar] [CrossRef]
- Riahi, R.; Tamayol, A.; Shaegh, S.A.M.; Ghaemmaghami, A.; Dokmeci, M.R.; Khademshosseini, A. Microfluidics for Advanced Drug Delivery Systems. Curr. Opin. Chem. Eng. 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Yeo, J.C.; Yu, J.; Koh, Z.M.; Wang, Z.; Lim, C.T. Wearable tactile sensor based on flexible microfluidics. Lab Chip 2016, 16, 3244–3250. [Google Scholar] [CrossRef]
- Gao, Y.; Ota, H.; Schaler, E.W.; Chen, K.; Zhao, A.; Gao, W.; Fahad, H.M.; Leng, Y.; Zheng, A.; Xiong, F.; et al. Wearable Microfluidic Diaphragm Pressure Sensor for Health and Tactile Touch Monitoring. Adv. Mater. 2017, 29, 1701985. [Google Scholar] [CrossRef]
- Zhang, B.W.; Dong, Q.; Korman, C.E.; Li, Z.Y.; Zaghloul, M.E. Flexible packaging of solid-state integrated circuit chips with elastomeric microfluidics. Sci. Rep. 2013, 3, 1098. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.I.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef]
- Erickson, D.; Li, D.Q. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, P.; Gue, A. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15. [Google Scholar] [CrossRef]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Ludi, H.; Widmer, H.M. Planar Chips Technology for Miniaturization and Integration of Separation Techniques into Monitoring Systems—Capillary Electrophoresis on a Chip. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Rodriguez, I.; Spicar-Mihalic, P.; Kuyper, C.L.; Fiorini, G.S.; Chiu, D.T. Rapid prototyping of glass microchannels. Anal. Chim. Acta 2003, 496, 205–215. [Google Scholar] [CrossRef]
- Tabeling, P. Introduction to Microfluidics; OUP Oxford: Oxford, UK, 2005. [Google Scholar]
- Gravesen, P.; Branebjerg, J.; Jensen, O.S. Microfluidics-a review. J. Micromech. Microeng. 1993, 3, 168. [Google Scholar] [CrossRef]
- Hardy, B.S.; Uechi, K.; Zhen, J.; Pirouz Kavehpour, H. The deformation of flexible PDMS microchannels under a pressure driven flow. Lab Chip 2009, 9, 935–938. [Google Scholar] [CrossRef]
- Raj, A.; Sen, A. Flow-induced deformation of compliant microchannels and its effect on pressure–flow characteristics. Microfluid. Nanofluid. 2016, 20, 31. [Google Scholar] [CrossRef]
- Verma, M.; Kumaran, V. A dynamical instability due to fluid–wall coupling lowers the transition Reynolds number in the flow through a flexible tube. J. Fluid. Mech. 2012, 705, 322–347. [Google Scholar] [CrossRef]
- ChuanáYeo, J.; TeckáLim, C. Emergence of microfluidic wearable technologies. Lab Chip 2016, 16, 4082–4090. [Google Scholar]
- Cheng, S.; Wu, Z. Microfluidic electronics. Lab Chip 2012, 12, 2782–2791. [Google Scholar] [CrossRef]
- Vogt, D.M.; Park, Y.L.; Wood, R.J. Design and Characterization of a Soft Multi-Axis Force Sensor Using Embedded Microfluidic Channels. IEEE Sens. J. 2013, 13, 4056–4064. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Nie, B.; Digiglio, P.; Pan, T. Microflotronics: A Flexible, Transparent, Pressure-Sensitive Microfluidic Film. Adv. Funct. Mater. 2014, 24, 6195–6203. [Google Scholar] [CrossRef]
- Solis-Tinoco, V.; Marquez, S.; Quesada-Lopez, T.; Villarroya, F.; Homs-Corbera, A.; Lechuga, L.M. Building of a flexible microfluidic plasmo-nanomechanical biosensor for live cell analysis. Sens. Actuators B Chem. 2019, 291, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; van den Berg, A. Microfluidic devices for cellomics: A review. Sens. Actuators B Chem. 2003, 92, 315–325. [Google Scholar] [CrossRef]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. An ultra-thin highly flexible microfluidic device for blood oxygenation. Lab Chip 2018, 18, 3780–3789. [Google Scholar] [CrossRef]
- Dong, R.; Liu, Y.; Mou, L.; Deng, J.; Jiang, X. Microfluidics-based biomaterials and biodevices. Adv. Mater. 2019, 31, e1805033. [Google Scholar] [CrossRef]
- Shimizu, A.; Goh, W.H.; Hashimoto, M.; Miura, S.; Onoe, H. ECM-based Stretchable Microfluidic System for in vitro 3D Tissue Culture. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Hou, X.; Zhang, Y.S.; Trujillo-de Santiago, G.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 17016. [Google Scholar] [CrossRef]
- Tsao, C.W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef] [PubMed]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J. Thermosetting Polymers; CRC Press: Boca Raton, FL, USA, 2002; Volume 64. [Google Scholar]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- McKeen, L.W. The Effect of Sterilization on Plastics and Elastomers; William Andrew: Oxford, UK, 2018. [Google Scholar]
- Holden, G. Thermoplastic Elastomers. In Rubber Technology; Morton, M., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 465–481. [Google Scholar]
- Buschow, K.J.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E.J.; Mahajan, S. Encyclopedia of materials. Sci. Technol. 2001, 1, 11. [Google Scholar]
- Clarson, S.J.; Dodgson, K.; Semlyen, J.A. Studies of Cyclic and Linear Poly(Dimethylsiloxanes): 19. Glass-Transition Temperatures and Crystallization Behavior. Polymer 1985, 26, 930–934. [Google Scholar] [CrossRef]
- Lotters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng. 1997, 7, 145–147. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid. Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef]
- Abkarian, M.; Faivre, M.; Horton, R.; Smistrup, K.; Best-Popescu, C.A.; Stone, H.A. Cellular-scale hydrodynamics. Biomed. Mater. 2008, 3, 034011. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Rodrigues, R.O.; Faustino, V.; Pinho, D.; Fernandes, C.S.; Pereira, A.I.; Garcia, V.; Miranda, J.M.; Lima, R. Deformation of Red Blood Cells, Air Bubbles, and Droplets in Microfluidic Devices: Flow Visualizations and Measurements. Micromachines 2018, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [Green Version]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.V.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Ryu, B.H.; Kim, D.E. Development of highly durable and low friction micro-structured PDMS coating based on bio-inspired surface design. Cirp. Ann-Manuf. Technol. 2015, 64, 519–522. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. Rsc Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Abate, A.R.; Lee, D.; Do, T.; Holtze, C.; Weitz, D.A. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip 2008, 8, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectron. Eng. 2009, 86, 1321–1324. [Google Scholar] [CrossRef]
- Yeo, J.C.; Yu, J.; Shang, M.; Loh, K.P.; Lim, C.T. Highly Flexible Graphene Oxide Nanosuspension Liquid-Based Microfluidic Tactile Sensor. Small 2016, 12, 1593–1604. [Google Scholar]
- Kubo, M.; Li, X.; Kim, C.; Hashimoto, M.; Wiley, B.J.; Ham, D.; Whitesides, G.M. Stretchable microfluidic radiofrequency antennas. Adv. Mater. 2010, 22, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, C.; Ding, G.; Yang, Z.; Zhao, X.; Liu, Y.; Tang, Z.; Shao, X.; Yang, S.; Lin, X. A High-Sensitivity Microfluidic Chip Calorimeter for Biochemical Reaction Monitoring Applications. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Jeon, N.L.; Chiu, D.T.; Wargo, C.J.; Wu, H.; Choi, I.S.; Anderson, J.R.; Whitesides, G.M.J.B.M. Microfluidics section: Design and fabrication of integrated passive valves and pumps for flexible polymer 3-dimensional microfluidic systems. Biomed. Microdevices 2002, 4, 117–121. [Google Scholar] [CrossRef]
- Yin, J.Z.; Santos, V.J.; Posner, J.D. Bioinspired flexible microfluidic shear force sensor skin. Sens. Actuators A-Phys. 2017, 264, 289–297. [Google Scholar] [CrossRef]
- Wong, R.D.P.; Posner, J.D.; Santos, V.J. Flexible microfluidic normal force sensor skin for tactile feedback. Sens. Actuators A-Phys. 2012, 179, 62–69. [Google Scholar] [CrossRef]
- Hur, D.; Say, M.G.; Diltemiz, S.E.; Duman, F.; Ersöz, A.; Say, R. 3D Micropatterned All-Flexible Microfluidic Platform for Microwave-Assisted Flow Organic Synthesis. ChemPlusChem 2018, 83, 42–46. [Google Scholar] [CrossRef]
- Kuppusami, S.; Oskouei, R.H. Parylene coatings in medical devices and implants: A review. J. Med. Biol. Eng. 2015, 3, 9–14. [Google Scholar]
- Foley, C.P.; Nishimura, N.; Neeves, K.B.; Schaffer, C.B.; Olbricht, W.L. Flexible microfluidic devices supported by biodegradable insertion scaffolds for convection-enhanced neural drug delivery. Biomed. Microdevices 2009, 11, 915–924. [Google Scholar] [CrossRef]
- Ziegler, D.; Suzuki, T.; Takeuchi, S. Fabrication of flexible neural probes with built-in microfluidic channels by thermal bonding of Parylene. J. Microelectromech. Syst. 2006, 15, 1477–1482. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef]
- Pellinen, D.S.; Moon, T.; Vetter, R.; Miriani, R.; Kipke, D.R. Multifunctional flexible parylene-based intracortical microelectrodes. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006. [Google Scholar]
- Seo, S.; Kim, J.; Choi, Y.-H.; You, J.B.; Im, S.G.; Lee, W. Parylene based thin-film microfluidic lens array fabricated by iCVD nano-adhesive bonding. Polymer 2019, 181, 121763. [Google Scholar] [CrossRef]
- Jung, B.J.; Kim, J.; Kim, J.A.; Jang, H.; Seo, S.; Lee, W. PDMS-Parylene Hybrid, Flexible Microfluidics for Real-Time Modulation of 3D Helical Inertial Microfluidics. Micromachines 2018, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Zhao, Y.; Lin, S.; Wang, B.; Yeung, C.; Cheng, X.; Wang, Z.; Cai, T.; Yu, W.; King, K.; et al. A rapid and low-cost fabrication and integration scheme to render 3D microfluidic architectures for wearable biofluid sampling, manipulation, and sensing. Lab Chip 2019, 19, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Haque, R.; Joho, M.O.; Bionaz, A.; van der Wal, P.; Briand, D. 3D-Integration of Printed Electrochemical Sensors in Pet Microfluidics for Biochemical Sensing. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Metz, S.; Holzer, R.; Renaud, P. Polyimide-based microfluidic devices. Lab Chip 2001, 1, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Polyimide microfluidic devices with integrated nanoporous filtration areas manufactured by micromachining and ion track technology. J. Micromech. Microeng. 2003, 14, 324.
- Metz, S.; Jiguet, S.; Bertsch, A.; Renaud, P. Polyimide and SU-8 microfluidic devices manufactured by heat-depolymerizable sacrificial material technique. Lab Chip 2004, 4, 114–120. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A. Skin-worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Metz, S.; Trautmann, C.; Bertsch, A.; Renaud, P. Flexible microchannels with integrated nanoporous membranes for filtration and separation of molecules and particles. In Proceedings of the Technical Digest. MEMS 2002 IEEE International Conference. Fifteenth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 02CH37266), Las Vegas, NV, USA, 24 January 2002. [Google Scholar]
- Metz, S.; Bertsch, A.; Bertrand, D.; Renaud, P. Flexible polyimide probes with microelectrodes and embedded microfluidic channels for simultaneous drug delivery and multi-channel monitoring of bioelectric activity. Biosens. Bioelectron. 2004, 19, 1309–1318. [Google Scholar] [CrossRef]
- Phan, H.P.; Zhong, Y.; Nguyen, T.K.; Park, Y.; Dinh, T.; Song, E.; Vadivelu, R.K.; Masud, M.K.; Li, J.; Shiddiky, M.J.A.; et al. Long-Lived, Transferred Crystalline Silicon Carbide Nanomembranes for Implantable Flexible Electronics. ACS Nano 2019, 13, 11572–11581. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Pfreundt, A.; Svendsen, W.E.; Dimaki, M. Fabrication of polyimide based microfluidic channels for biosensor devices. J. Micromech. Microeng. 2015, 25, 035022. [Google Scholar] [CrossRef] [Green Version]
- Wego, A.; Pagel, L. A self-filling micropump based on PCB technology. Sens. Actuators A-Phys 2001, 88, 220–226. [Google Scholar] [CrossRef]
- Boden, R.; Lehto, M.; Simu, U.; Thornell, G.; Hjort, K.; Schweitz, J.A. A polymeric paraffin actuated high-pressure micropump. Sens. Actuators A-Phys 2006, 127, 88–93. [Google Scholar] [CrossRef]
- Komatsuzaki, H.; Suzuki, K.; Liu, Y.W.; Kosugi, T.; Ikoma, R.; Youn, S.W.; Takahashi, M.; Maeda, R.; Nishioka, Y. Flexible Polyimide Micropump Fabricated Using Hot Embossing. Jpn. J. Appl. Phys. 2011, 50. [Google Scholar] [CrossRef]
- Min, K.I.; Lee, T.H.; Park, C.P.; Wu, Z.Y.; Girault, H.H.; Ryu, I.; Fukuyama, T.; Mukai, Y.; Kim, D.P. Monolithic and flexible polyimide film microreactors for organic microchemical applications fabricated by laser ablation. Angew. Chem. Int. Ed. Engl. 2010, 49, 7063–7067. [Google Scholar] [CrossRef] [PubMed]
- Carlborg, C.F.; Haraldsson, T.; Öberg, K.; Malkoch, M.; van der Wijngaart, W. Beyond PDMS: Off-stoichiometry thiol–ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 2011, 11, 3136–3147. [Google Scholar] [CrossRef] [Green Version]
- Saharil, F.; Carlborg, C.F.; Haraldsson, T.; Van der Wijngaart, W. Biocompatible “click” wafer bonding for microfluidic devices. Lab Chip 2012, 12, 3032–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, B.T.; Reddy, S.; Davis, R.H.; Bowman, C.N.J.S.; Chemical, A.B. Tailorable low modulus, reversibly deformable elastomeric thiol–ene materials for microfluidic applications. Sens. Actuators B Chem. 2007, 120, 473–480. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhou, Y.L.; Wang, D.H.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef]
- Chang, W.Y.; Chu, C.H.; Lin, Y.C. A flexible piezoelectric sensor for microfluidic applications using polyvinylidene fluoride. IEEE Sens. J. 2008, 8, 495–500. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.M.; Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 µm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 11301–1130113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.M.; Sinton, D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Lim, S. Stretchable Complementary Split Ring Resonator (CSRR)-Based Radio Frequency (RF) Sensor for Strain Direction and Level Detection. Sensors 2016, 16, 1667. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Zhang, S.; Hjort, K.; Hilborn, J.; Wu, Z. PDMS-based elastomer tuned soft, stretchable, and sticky for epidermal electronics. Adv. Mater. 2016, 28, 5830–5836. [Google Scholar] [CrossRef]
- Noh, H.S.; Moon, K.S.; Cannon, A.; Hesketh, P.J.; Wong, C.P. Wafer bonding using microwave heating of parylene intermediate layers. J. Micromech. Microeng. 2004, 14, 625–631. [Google Scholar] [CrossRef]
- Kim, J.H.; You, J.B.; Im, S.G.; Lee, W.H. Molding and bonding of thin film parylene for flexible microfluidics. In Proceedings of the InterPACK/ICNMM 2015, Gyeongju, Korea, 25–29 October 2015. [Google Scholar]
- Foulds, I.G.; Conchouso, D.; Castro, D. Microfluidic Device for High-Volume Production and Processing of Monodisperse Emulsions. WO Patent 2015068045A3, 2015. [Google Scholar]
- Derix, J.; Gerlach, G.; Wetzel, S.; Perike, S.; Funk, R.H. Porous polyethylene terephthalate membranes in microfluidic applications. Phys. Status Solidi. A 2009, 206, 442–448. [Google Scholar] [CrossRef]
- Raj, A.; Suthanthiraraj, P.P.A.; Sen, A.K. Pressure-driven flow through PDMS-based flexible microchannels and their applications in microfluidics. Microfluid. Nanofluid. 2018, 22, 128. [Google Scholar] [CrossRef]
- Wang, X.; Christov, I.C. Theory of the flow-induced deformation of shallow compliant microchannels with thick walls. Proc. Royal. Soc. Lond. 2019, 475. [Google Scholar] [CrossRef] [Green Version]
- Ozsun, O.; Yakhot, V.; Ekinci, K.L. Non-invasive measurement of the pressure distribution in a deformable micro-channel. J. Fluid. Mech. 2013, 734. [Google Scholar] [CrossRef] [Green Version]
- Gervais, T.; El-Ali, J.; Gunther, A.; Jensen, K.F. Flow-induced deformation of shallow microfluidic channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Christov, I.C.; Cognet, V.; Shidhore, T.C.; Stone, H.A. Flow rate-pressure drop relation for deformable shallow microfluidic channels. J. Fluid. Mech. 2018, 841, 267–286. [Google Scholar] [CrossRef] [Green Version]
- Adzima, B.J.; Velankar, S.S. Pressure drops for droplet flows in microfluidic channels. J. Micromech. Microeng. 2006, 16, 1504–1510. [Google Scholar] [CrossRef]
- Cheung, P.; Toda-Peters, K.; Shen, A.Q. In situ pressure measurement within deformable rectangular polydimethylsiloxane microfluidic devices. Biomicrofluidics 2012, 6, 026501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, M.K.; DasGupta, S.; Chakraborty, S. Hydrodynamics in deformable microchannels. Microfluid. Nanofluid. 2017, 21, 70. [Google Scholar] [CrossRef]
- Shidhore, T.C.; Christov, I.C. Static response of deformable microchannels: A comparative modelling study. J. Phys. Condens. Matter. 2018, 30, 054002. [Google Scholar] [CrossRef] [Green Version]
- Ottino, J.M.; Wiggins, S. Introduction: Mixing in microfluidics. Phil. Trans. R. Soc. Lond. A 2004, 2004, 923–935. [Google Scholar] [CrossRef]
- Srinivas, S.S.; Kumaran, V. Effect of viscoelasticity on the soft-wall transition and turbulence in a microchannel. J. Fluid. Mech. 2017, 812, 1076–1118. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, J.; DasGupta, S.; Chakraborty, S. Flow-induced deformation in a microchannel with a non-Newtonian fluid. Biomicrofluidics 2018, 12, 034116. [Google Scholar] [CrossRef]
- Patne, R.; Giribabu, D.; Shankar, V. Consistent formulations for stability of fluid flow through deformable channels and tubes. J. Fluid. Mech. 2017, 827, 31–66. [Google Scholar] [CrossRef]
- Srinivas, S.S.; Kumaran, V. Transitions to different kinds of turbulence in a channel with soft walls. J. Fluid. Mech. 2017, 822, 267–306. [Google Scholar] [CrossRef]
- Kumaran, V.; Bandaru, P. Ultra-fast microfluidic mixing by soft-wall turbulence. Chem. Eng. Sci. 2016, 149, 156–168. [Google Scholar] [CrossRef]
- Verma, M.K.S.; Kumaran, V. A multifold reduction in the transition Reynolds number, and ultra-fast mixing, in a micro-channel due to a dynamical instability induced by a soft wall. J. Fluid. Mech. 2013, 727, 407–455. [Google Scholar] [CrossRef]
- Verma, M.K.S.; Kumaran, V. Effect of ultra-fast mixing in a microchannel due to a soft wall on the room temperature synthesis of gold nanoparticles. Sadhana-Acad. Proc. Eng. Sci. 2015, 40, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Terray, A.; Oakey, J.; Marr, D.W.M. Microfluidic control using colloidal devices. Science 2002, 296, 1841–1844. [Google Scholar] [CrossRef]
- Baek, J.Y.; Park, J.Y.; Ju, J.I.; Lee, T.S.; Lee, S.H. A pneumatically controllable flexible and polymeric microfluidic valve fabricated via in situ development. J. Micromech. Microeng. 2005, 15, 1015–1020. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.P.; Tavakol, B.; Froehlicher, G.; Stone, H.A. Control and manipulation of microfluidic flow via elastic deformations. Soft Matter 2013, 9, 7049–7053. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D. Elasticity and Stability of Shape Changing Structures. Curr. Opin. Colloid. Interface Sci. 2019, 40, 118–137. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.; Moulton, D.E.; Vella, D. Passive Control of Viscous Flow via Elastic Snap-Through. Phys. Rev. Lett. 2017, 119, 144502. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.; Moulton, D.E.; Vella, D. Dynamics of viscoelastic snap-through. J. Mech. Phys. Solids 2019, 124, 781–813. [Google Scholar] [CrossRef]
- Inamdar, T.C. Unsteady Fluid-Structure Interactions in Soft-Walled Microchannels; Purdue University: Lafayette, IN, USA, 2018. [Google Scholar]
- Gomez, M. Ghosts and Bottlenecks in Elastic Snap-Through; University of Oxford: Oxford, UK, 2018. [Google Scholar]
- Mohith, S.; Karanth, P.N.; Kulkarni, S.M. Recent trends in mechanical micropumps and their applications: A review. Mechatronics 2019, 60, 34–55. [Google Scholar] [CrossRef]
- Tavakol, B.; Bozlar, M.; Punckt, C.; Froehlicher, G.; Stone, H.A.; Aksay, I.A.; Holmes, D.P. Buckling of dielectric elastomeric plates for soft, electrically active microfluidic pumps. Soft Matter 2014, 10, 4789–4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.H.; Guan, E.G.; Li, P.X.; Wang, F.J.; Liang, C.M.; Zhao, Y.Z. Deflection behavior of a piezo-driven flexible actuator for vacuum micropumps. Sens. Actuators A-Phys 2017, 267, 30–41. [Google Scholar] [CrossRef]
- Bengtsson, K.; Robinson, N.D. A large-area, all-plastic, flexible electroosmotic pump. Microfluid. Nanofluid. 2017, 21, 178. [Google Scholar] [CrossRef] [Green Version]
- Guevara-Pantoja, P.E.; Jimenez-Valdes, R.J.; Garcia-Cordero, J.L.; Caballero-Robledo, G.A. Pressure-actuated monolithic acrylic microfluidic valves and pumps. Lab Chip 2018, 18, 662–669. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, D.; Spinks, G.M.; Innis, P.C.; Megill, W.M.; Wallace, G.G. TITAN: A conducting polymer based microfluidic pump. Smart Mater. Struct. 2005, 14, 1511–1516. [Google Scholar] [CrossRef]
- Ma, T.; Sun, S.X.; Li, B.Q.; Chu, J.R. Piezoelectric peristaltic micropump integrated on a microfluidic chip. Sens. Actuators A-Phys 2019, 292, 90–96. [Google Scholar] [CrossRef]
- Iverson, B.D.; Garimella, S.V. Recent advances in microscale pumping technologies: A review and evaluation. Microfluid. Nanofluid. 2008, 5, 145–174. [Google Scholar] [CrossRef]
- Jeong, O.C.; Park, S.W.; Yang, S.S.; Pak, J.J. Fabrication of a peristaltic PDMS micropump. Sens. Actuators A-Phys. 2005, 123–124, 453–458. [Google Scholar] [CrossRef]
- Xiang, J.W.; Cai, Z.L.; Zhang, Y.; Wang, W.J. A micro-cam actuated linear peristaltic pump for microfluidic applications. Sens. Actuators A-Phys 2016, 251, 20–25. [Google Scholar] [CrossRef]
- Shutko, A.V.; Gorbunov, V.S.; Guria, K.G.; Agladze, K.I. Biocontractile microfluidic channels for peristaltic pumping. Biomed. Microdevices 2017, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, U.; Cetin, B.; Yildirim, E. A novel zero-dead-volume sample loading interface for microfluidic devices: Flexible hydraulic reservoir (FHR). J. Micromech. Microeng. 2018, 28, 097001. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Gao, Y.; Wu, M.; Zhou, R.; Chung, D.; Caraveo, G.; Xu, J. Acoustofluidic stick-and-play micropump built on foil for single-cell trapping. Lab Chip 2019, 19, 3045–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.B.; Zhang, J.; Yan, S.; Yuan, D.; Du, H.P.; Alici, G.; Li, W.H. High-throughput sheathless and three-dimensional microparticle focusing using a microchannel with arc-shaped groove arrays. Sci. Rep. 2017, 7, 41153. [Google Scholar] [CrossRef] [Green Version]
- Lenshof, A.; Laurell, T. Continuous separation of cells and particles in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1203–1217. [Google Scholar] [CrossRef]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2018, 144, 87–113. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Pan, C.; Yuan, D.; Alici, G.; Du, H.P.; Zhu, Y.G.; Li, W.H. An integrated dielectrophoresis-active hydrophoretic microchip for continuous particle filtration and separation. J. Micromech. Microeng. 2015, 25, 084010. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Zhang, J.; Yuan, Y.; Lovrecz, G.; Alici, G.; Du, H.P.; Zhu, Y.G.; Li, W.H. A hybrid dielectrophoretic and hydrophoretic microchip for particle sorting using integrated prefocusing and sorting steps. Electrophoresis 2015, 36, 284–291. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Ebrahimi Warkiani, M.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yuan, D.; Zhao, Q.B.; Yan, S.; Tang, S.Y.; Tan, S.H.; Guo, J.H.; Xia, H.M.; Nguyen, N.T.; Li, W.H. Tunable particle separation in a hybrid dielectrophoresis (DEP)-inertial microfluidic device. Sens. Actuators B Chem. 2018, 267, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.J. A Minireview on Inertial Microfluidics Fundamentals: Inertial Particle Focusing and Secondary Flow. Biochip J. 2019, 13, 53–63. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Kong, F.; Yeo, J.C.; Yu, L.; Sonam, S.; Dao, M.; Gong, X.; Lim, C.T. Soft tubular microfluidics for 2D and 3D applications. Proc. Natl. Acad. Sci. USA 2017, 114, 10590–10595. [Google Scholar] [CrossRef] [Green Version]
- Hahn, Y.K.; Hong, D.; Kang, J.H.; Choi, S. A reconfigurable microfluidics platform for microparticle separation and fluid mixing. Micromachines 2016, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic gallium-indium (EGaIn): A liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Asghari, M.; Serhatlioglu, M.; Saritas, R.; Guler, M.T.; Elbuken, C. Tape’n Roll Inertial Microfluidics. Sens. Actuators A-Phys. 2019, 111630. [Google Scholar] [CrossRef]
- Yeo, J.C.; Kenry; Yu, J.H.; Loh, K.P.; Wang, Z.P.; Lim, C.T. Triple-State Liquid-Based Microfluidic Tactile Sensor with High Flexibility, Durability, and Sensitivity. ACS Sens. 2016, 1, 543–551. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef]
- Jung, T.; Yang, S. Highly stable liquid metal-based pressure sensor integrated with a microfluidic channel. Sensors 2015, 15, 11823–11835. [Google Scholar] [CrossRef] [Green Version]
- Alfadhel, A.; Ouyang, J.; Mahajan, C.G.; Forouzandeh, F.; Cormier, D.; Borkholder, D.A. Inkjet printed polyethylene glycol as a fugitive ink for the fabrication of flexible microfluidic systems. Mater. Des. 2018, 150, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Lee, K.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 2018, 14, e1802876. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Chen, K.; Lin, Y.; Kiriya, D.; Shiraki, H.; Yu, Z.; Ha, T.; Javey, A. Highly deformable liquid-state heterojunction sensors. Nat. Commun. 2014, 5, 5032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Gao, M.; Zhang, L.J.; Deng, Z.S.; Gui, L. A Handy Flexible Micro-Thermocouple Using Low-Melting-Point Metal Alloys. Sensors 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.G.; Chang, S.T. Microfluidic capacitive sensors with ionic liquid electrodes and CNT/PDMS nanocomposites for simultaneous sensing of pressure and temperature. J. Mater. Chem. C 2017, 5, 1910–1919. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.H.; Yang, H.B.; Zhen, X.; Ma, L.F.; Williams, D.; Sun, X.D.; Lang, M.F. Highly transparent and flexible circuits through patterning silver nanowires into microfluidic channels. Chem. Commun. 2018, 54, 4923–4926. [Google Scholar] [CrossRef]
- Fallahi, H.; Azizi, H.; Ghasemi, I.; Karrabi, M. Preparation and properties of electrically conductive, flexible and transparent silver nanowire/poly (lactic acid) nanocomposites. Org. Electron. 2017, 44, 74–84. [Google Scholar] [CrossRef]
- Satoungar, M.T.; Azizi, H.; Fattahi, S.; Mehrizi, M.K.; Fallahi, H. Effect of Different Mediated Agents on Morphology and Crystallinity of Synthesized Silver Nanowires Prepared by Polyol Process. J. Nanomater. 2016, 2016, 5. [Google Scholar] [CrossRef]
- Azizi, H.; Fallahi, H.; Ghasemi, I.; Karrabi, M.; Nazemian, M. Silane Modification of Carbon Nanotubes and Preparation of Silane Cross-Linked LLDPE/MWCNT Nanocomposites. In Proceedings of the 11th International Seminar on Polymer Science and Technology, Tehran, Iran, 6–9 October 2014. [Google Scholar]
- Wu, F.; Chen, S.; Chen, B.; Wang, M.; Min, L.; Alvarenga, J.; Ju, J.; Khademhosseini, A.; Yao, Y.; Zhang, Y.S.; et al. Bioinspired Universal Flexible Elastomer-Based Microchannels. Small 2018, 14, e1702170. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Sanchez, B.N.; Silva, S.F.; Pinho, D.; Vega, E.J.; Lima, R. Generation of micro-sized PDMS particles by a flow focusing technique for biomicrofluidics applications. Biomicrofluidics 2016, 10, 014122. [Google Scholar] [CrossRef] [Green Version]
- Pinho, D.; Muñoz-Sánchez, B.; Anes, C.; Vega, E.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 103399. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.R.; Peri, S.S.S.; Sabane, V.P.; Mansur, N.; Gao, J.X.; Nguyen, K.T.; Weidanz, J.A.; Iqbal, S.M.; Abhyankar, V.V. One-step fabrication of flexible nanotextured PDMS as a substrate for selective cell capture. Biomed. Phys. Eng. Express 2018, 4, 025015. [Google Scholar] [CrossRef] [Green Version]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Biomaterials. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-W.; McCall, J.G.; Zhang, Y.; Huang, Y.; Bruchas, M.; Rogers, J.A. Soft microfluidic neural probes for wireless drug delivery in freely behaving mice. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015. [Google Scholar]
- Sim, J.Y.; Haney, M.P.; Park, S.I.; McCall, J.G.; Jeong, J.W. Microfluidic neural probes: In vivo tools for advancing neuroscience. Lab Chip 2017, 17, 1406–1435. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Zhang, Y.; Qu, G.; Wei, S.; Liu, Z.; Kong, T. Ultrastretchable and Wireless Bioelectronics Based on All-Hydrogel Microfluidics. Adv. Mater. 2019, 31, e1902783. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yu, Y.; Wang, H.; Zhang, H.; Zhang, X.; Zhao, Y. Conductive Polymer Hydrogel Microfibers from Multiflow Microfluidics. Small 2019, 15, e1805162. [Google Scholar] [CrossRef]

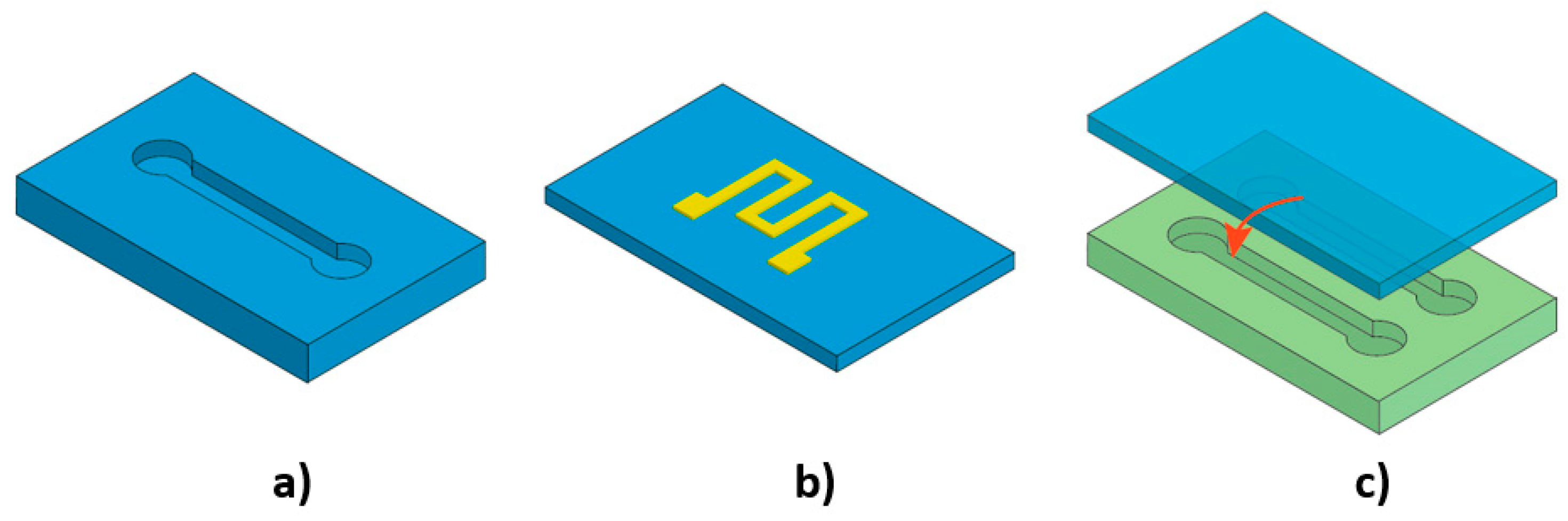
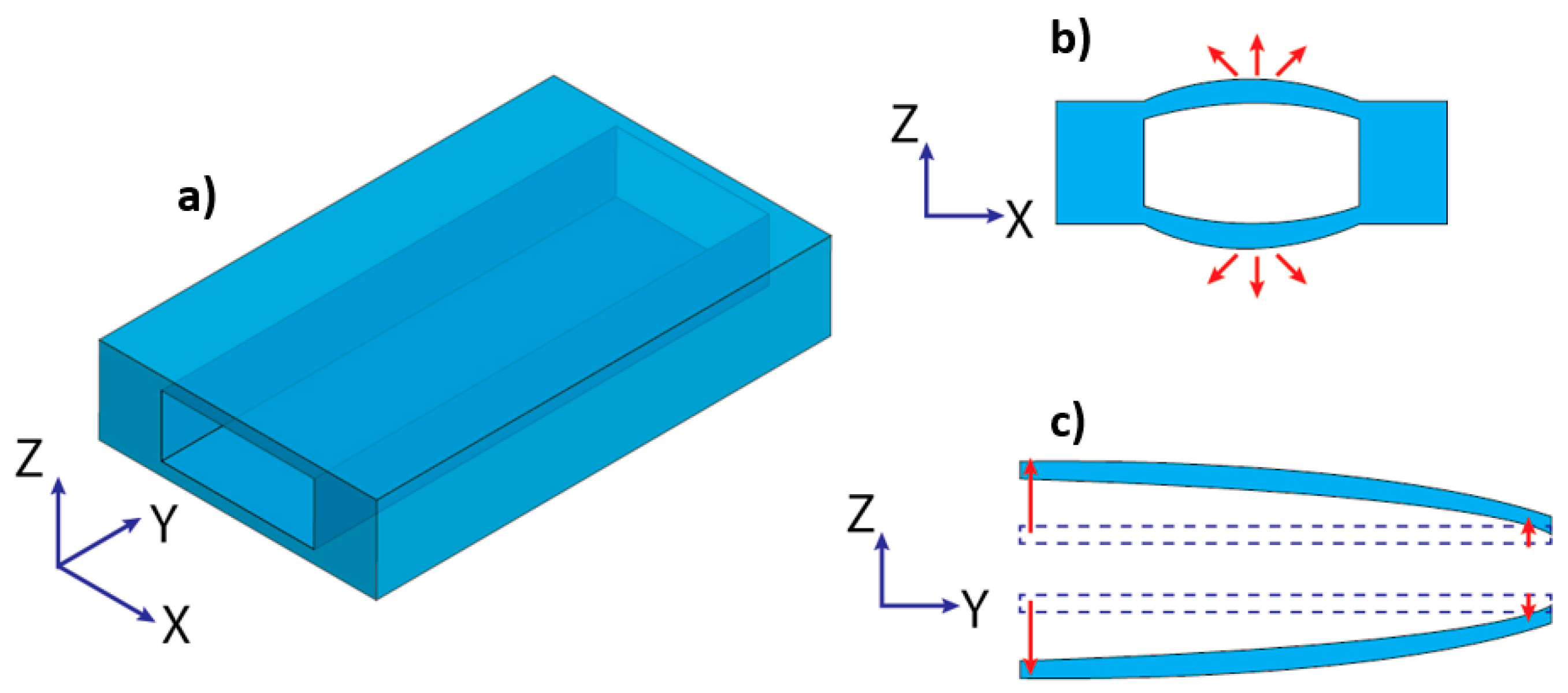

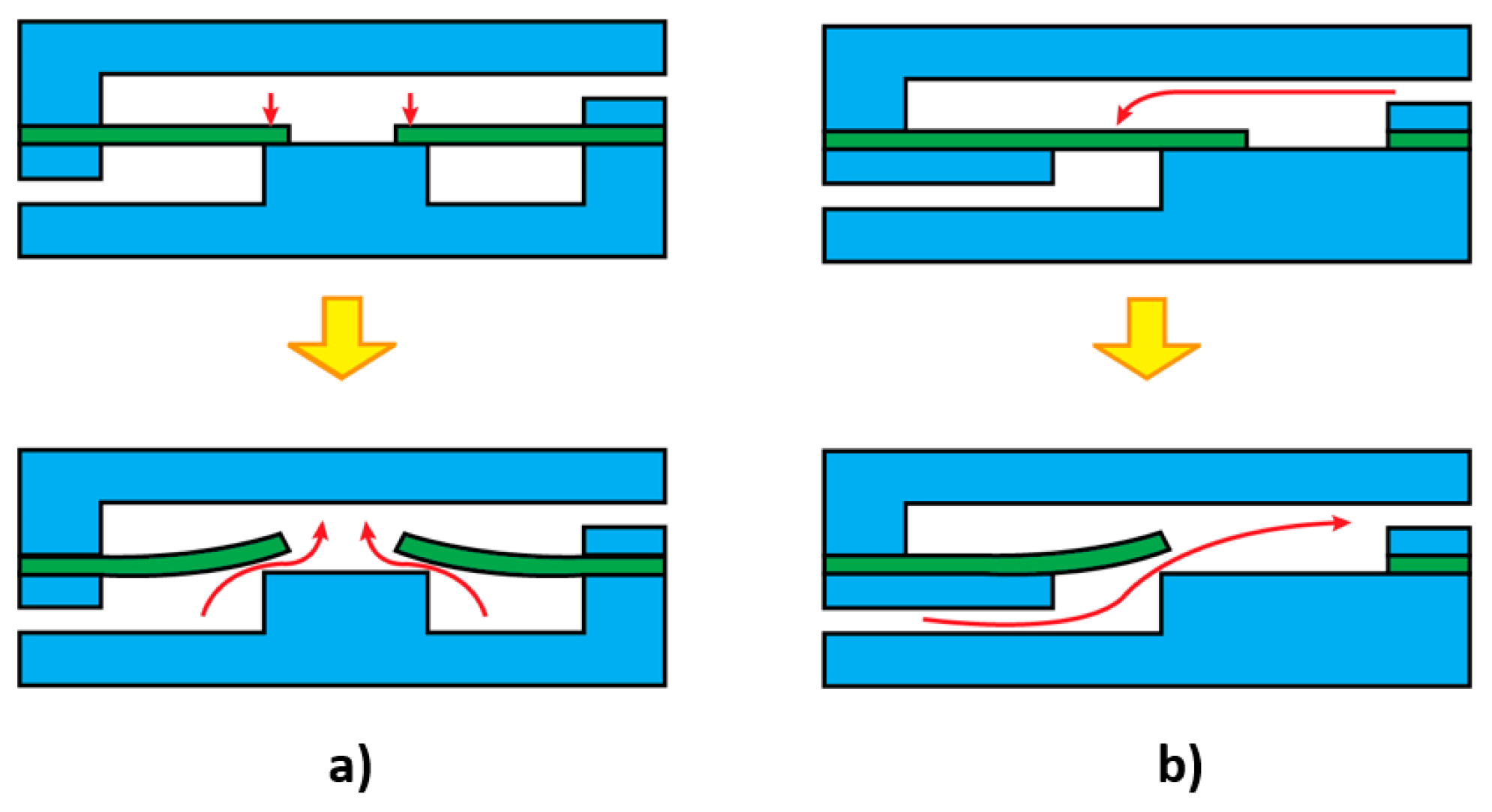



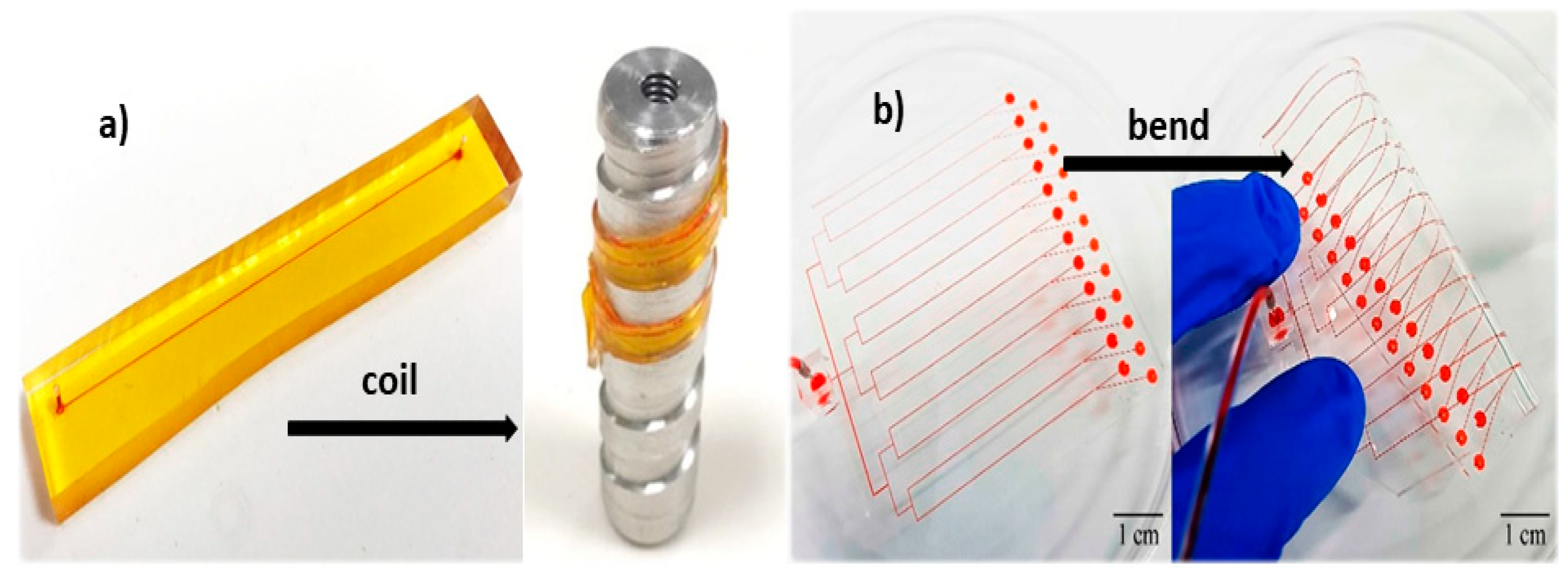

| Materials | Tg 1 (°C) | Young’s Modulus | Advantages | Disadvantages | Fabrication Techniques | References |
|---|---|---|---|---|---|---|
| PDMS | −125 | <1000 kPa | low Tg, low shear and Youngs modulus, high optical transparency, durability, gas permeability | hydrophobic nature, incompatibility with solvents | soft lithography, plasma-enhanced bonding | [63,69,70,73,74] |
| Ecoflex | NA | 40 kPa | low Young’s modulus, highly flexible, high tear strength, and large elongation | non-transparent, high viscosity, incompatible with plasma bonding | soft lithography | [27,77,78,114,115] |
| Parylene | <90 | 2.7–4 GPa | biocompatibility, low water absorption, transparent, solvent resistance, surface conformality | costly, complicated fabrication, hard to handle, low adhesion to substrates | vapor deposition bonding | [84,85,86,87,88,89,90,116,117] |
| PI | 300–400 | 2.5 GPa | biocompatibility, high thermal stability, good sealing properties, chemical inertness | opaque, moisture absorption | lamination, sacrificial layer techniques, wet/dry etching, hot embossing | [93,94,95,96,97,98,103,104,116] |
| OSTE | 25–70 | 0.25–2 GPa | scalable commercial production possibility, low polymerization shrinkage stress, direct lamination and bonding | very high OS ratios can lead to residual monomers that may affect cells and proteins | soft lithography | [105,106,108,118] |
| PET | 69–78 | 2–2.7 GPa | good gas and moisture barrier properties, chemically inert, recyclable | poor chemical resistance, needs surface treatment for bonding due to the low plasma bonding strength | moulding by hot embossing, thermal bonding | [27,91,92,119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. https://doi.org/10.3390/mi10120830
Fallahi H, Zhang J, Phan H-P, Nguyen N-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines. 2019; 10(12):830. https://doi.org/10.3390/mi10120830
Chicago/Turabian StyleFallahi, Hedieh, Jun Zhang, Hoang-Phuong Phan, and Nam-Trung Nguyen. 2019. "Flexible Microfluidics: Fundamentals, Recent Developments, and Applications" Micromachines 10, no. 12: 830. https://doi.org/10.3390/mi10120830
APA StyleFallahi, H., Zhang, J., Phan, H.-P., & Nguyen, N.-T. (2019). Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines, 10(12), 830. https://doi.org/10.3390/mi10120830








