Versatile Microfluidic Mixing Platform for High- and Low-Viscosity Liquids via Acoustic and Chemical Microbubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
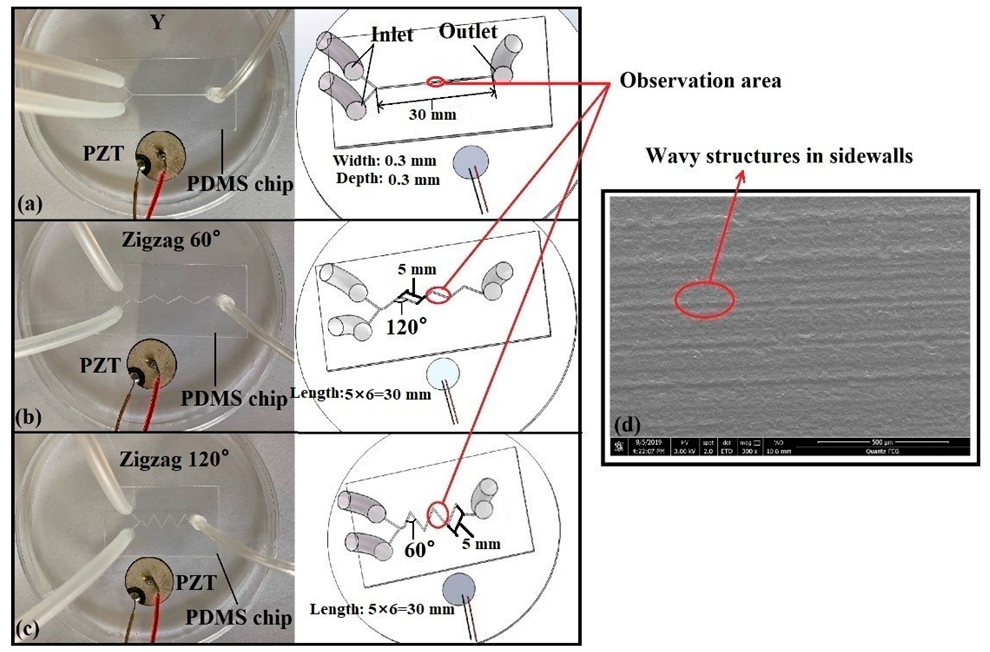
2.2. Fabrication of Microfluidic Chips
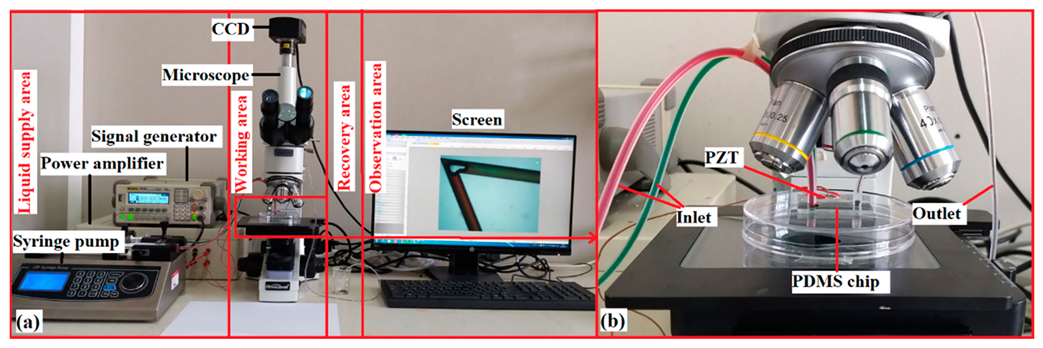
2.3. Experimental Setup
2.4. Mixing Index
3. Results and Discussion
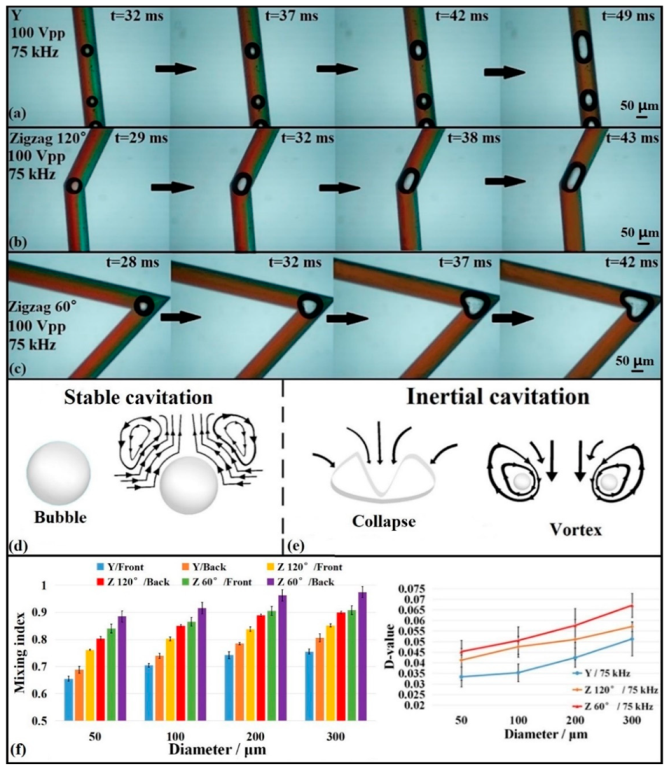
3.1. Comparison of Mixing Efficiency with Different Microbubble Sizes
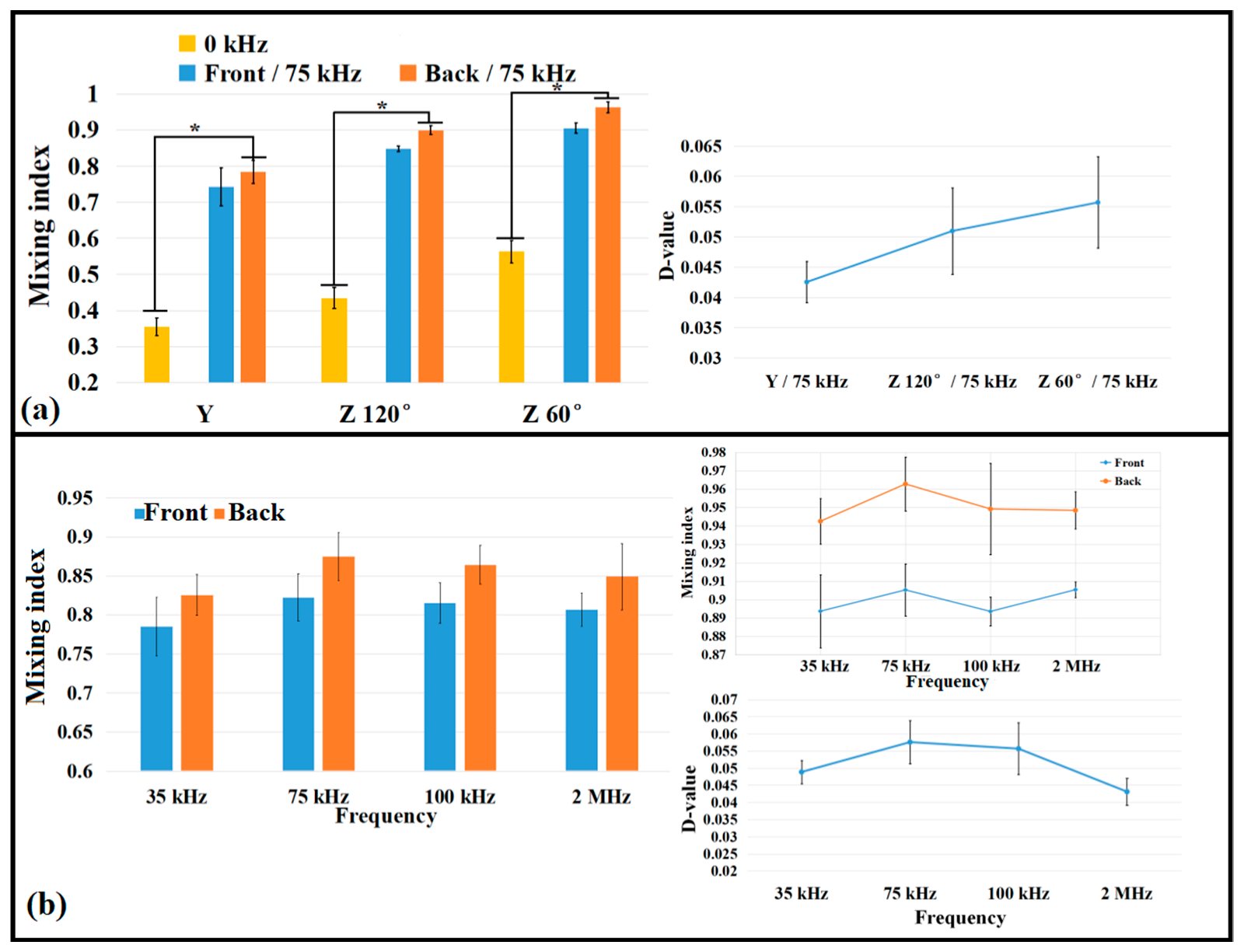
3.2. Comparison of Mixing Efficiency with Different Microchannel Structures
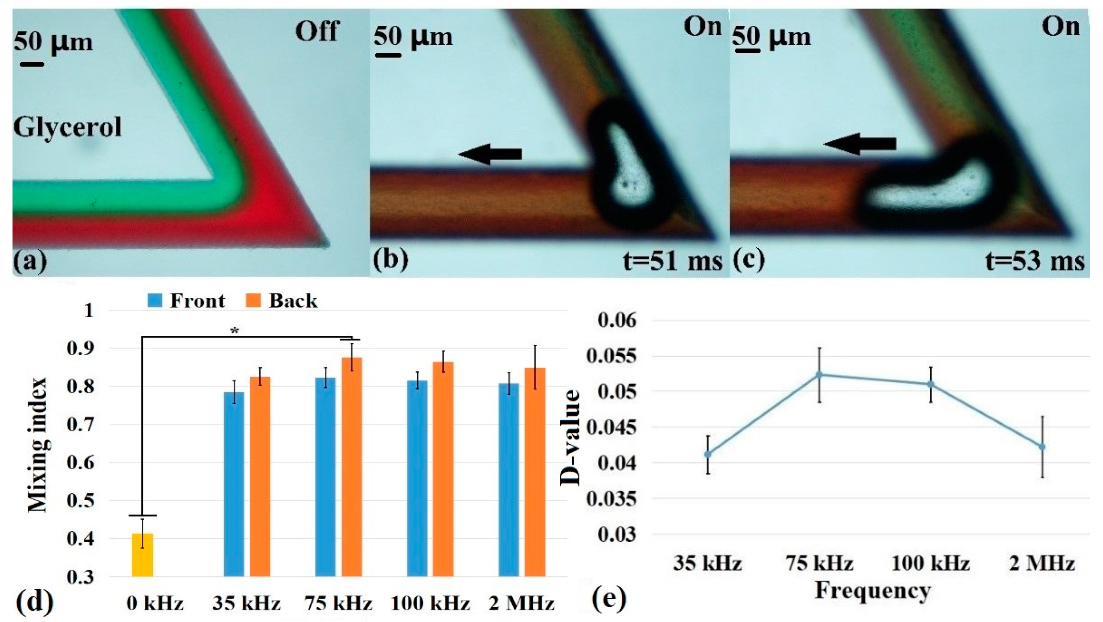
3.3. Comparison of Mixing Effect with Different Fluid Viscosities
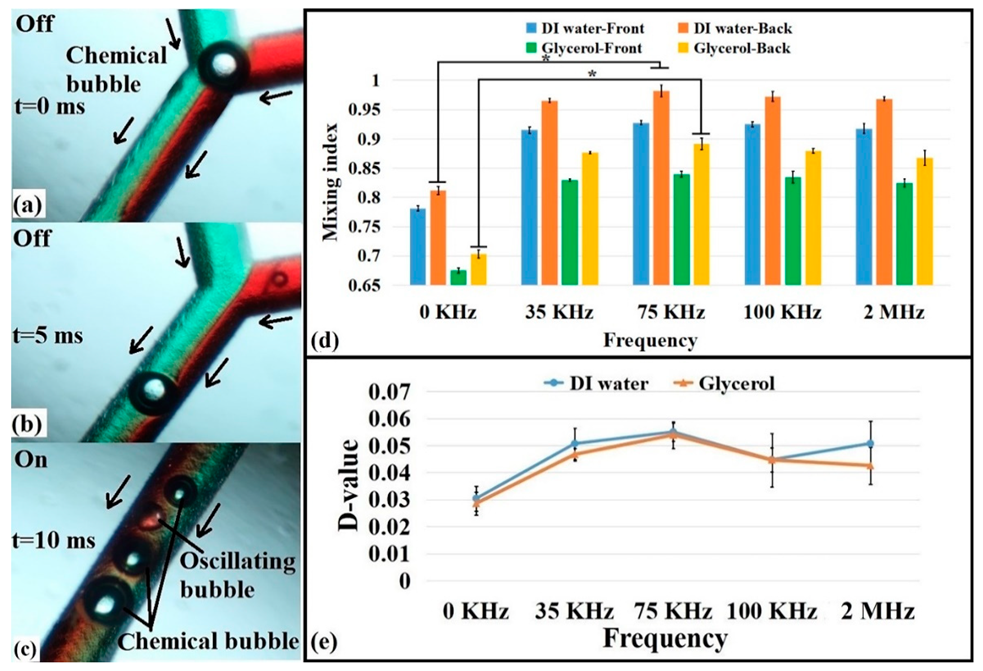
3.4. Mixing Analysis via Chemical Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Yang, T.; Zhang, W.; Ren, C.; Zhang, J.; Zhang, Y.; Cui, T.; Yue, W. Rapid detection of trichlorfon residues by microfluidic paperbased phosphorus-detection chip (μPPC). N. J. Chem. 2019, 43, 7194–7197. [Google Scholar] [CrossRef]
- Maruyama, H.; Otake, T.; Arai, F. Photoprocessible hydrogel microsensor for local environment measurement on a microfluidic chip. IEEE ASME Trans. Mechatron. 2011, 16, 845–852. [Google Scholar] [CrossRef]
- Digiacomo, L.; Palchetti, S.; Giulimondi, F.; Pozzi, D.; Chiozzi, R.Z.; Capriotti, A.L.; Lagna, A.; Caracciolo, G. The biomolecular corona of gold nanoparticles in a controlled microfluidic environment. Lab Chip 2019, 19, 2557–2567. [Google Scholar] [CrossRef]
- Soitu, C.; Feuerborn, A.; Tan, A.N.; Walker, H.; Walsh, P.A.; Castrejón-Pita, A.A.; Cook, P.R.; Walsh, E.J. Microfluidic chambers using fluid walls for cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, E5926–E5933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babic, J.; Griscom, L.; Cramer, J.; Coudreuse, D. An easy-to-build and re-usable microfluidic system for live-cell imaging. BMC Cell Biol. 2018, 19, 8. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Geng, C.; Ren, H.; Yu, X.; Liu, B. Microfluidic chip for cancer cell detection and diagnosis. J. Mech. Med. Biol. 2018, 18, 183001. [Google Scholar] [CrossRef]
- Fraas, R.; Hübner, J.F.; Diehm, J.; Faas, R.; Hausmann, R.; Franzreb, M. A Compartmented microfluidic reactor for protein modification via solid-phase reactions—Semi-automated examination of two PEGylation routes. Biotechnol. Bioprocess. Eng. 2019, 24, 382–394. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, H.-Y.; Wu, C.-C.; Wu, C.-W.; Chang, K.-P.; Yu, J.-S. BRAF protein immunoprecipitation, elution, and digestion from cell extract using a microfluidic mixer for mutant BRAF protein quantification by mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 1085–1094. [Google Scholar] [CrossRef]
- Sinkala, E.; Sollier-Christen, E.; Renier, C.; Rosas-Canyelles, E.; Che, J.; Heirich, K.; Duncombe, T.A.; Vlassakis, J.; Yamauchi, K.A.; Huang, H.; et al. Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat. Commun. 2017, 8, 14622. [Google Scholar] [CrossRef] [Green Version]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758. [Google Scholar] [CrossRef] [Green Version]
- Thom, J.; Lvey, L.; Eikelboom, J. Normal plasma mixing studies in the laboratory diagnosis of lupus anticoagulant. J. Thromb. Haemost. 2003, 1, 2689–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Todd, N.W.; Liu, Z.; Zhan, M.; Fang, H.; Peng, H.; Alattar, M.; Deepak, J.; Stas, S.A.; Jiang, F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Hillier, C.; Komenda, P.; de Faria, R.L.; Santos, S.; Levin, D.; Zhang, M.; Lin, F. An all-on-chip method for testing neutrophil chemotaxis induced by fMLP and COPD patient’s sputum. Technology 2016, 4, 104–109. [Google Scholar] [CrossRef]
- Yang, S.; Guo, F.; Kiraly, B.; Lu, M.; Leong, K.W.; Huang, T.J. Microfluidic synthesis of multifunctional Janus particles for biomedical applications. Lab Chip 2012, 12, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Schabas, G.; Yusuf, H.; Moffitt, M.G.; Sinton, D. Controlled self-assembly of quantum dots and block copolymers in a microfluidic device. Langmuir ACS J. Surf. Colloids 2008, 24, 637–643. [Google Scholar] [CrossRef]
- Gao, D.; Jin, F.; Zhou, M.; Jiang, Y. Recent advances in single cell manipulation and biochemical analysis on microfluidics. Analyst 2019, 144, 766–781. [Google Scholar] [CrossRef]
- Lam, R.H.; Cui, X.; Guo, W.; Thorsen, T. High-throughput dental biofilm growth analysis for multiparametric microenvironmental biochemical conditions using microfluidics. Lab Chip 2016, 16, 1652–1662. [Google Scholar] [CrossRef]
- Mao, X.; Huang, T.J. Microfluidic diagnostics for the developing world. Lab Chip 2012, 12, 1412–1416. [Google Scholar] [CrossRef]
- Mao, X.; Waldeisen, J.R.; Huang, T.J. “Microfluidic drifting”—Implementing three-dimensional hydrodynamic focusing with a single-layer planar microfluidic device. Lab Chip 2007, 7, 1260–1262. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Huang, X. Mixing in microchannels based on hydrodynamic focusing and time-interleaved segmentation: Modelling and experiment. Lab Chip 2005, 5, 1320–1326. [Google Scholar] [CrossRef]
- Schonfeld, F.; Hessel, F.; Hofmann, C. An optimised split-and-recombine micro-mixer with uniform ‘chaotic’ mixing. Lab Chip 2004, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, S.H.; Kwon, T.H.; Ahn, C.H. A serpentine laminating micromixer combining splitting/recombination and advection. Lab Chip 2005, 5, 739–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.M.; Wang, Z.P.; Koh, Y.K.; May, K.T. A microfluidic mixer with self-excited ‘turbulent’ fluid motion for wide viscosity ratio applications. Lab Chip 2010, 10, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Leung, W.W. Flow and mixing in rotating zigzag microchannel. Chem. Eng. J. 2013, 215–216, 561–578. [Google Scholar] [CrossRef]
- Tsai, J.H.; Lin, L. Active microfluidic mixer and gas bubble filter driven by thermal bubble micropump. Sens. Actuators A Phys. 2002, 97–98, 665–671. [Google Scholar] [CrossRef]
- Glasgow, I.; Aubry, N. Enhancement of microfluidic mixing using time pulsing. Lab Chip 2003, 3, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hellman, A.N.; Rau, K.R.; Yoon, H.H.; Bae, S.; Palmer, J.F.; Phillips, K.S. Laser-induced mixing in microfluidic channels. Anal. Chem. 2007, 79, 4484–4492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bau, H.H.; Zhong, J.; Yi, M. A minute magneto hydro dynamic (MHD) mixer. Sens. Actuators B Chem. 2001, 79, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Yang, R.-J. Electrokinetic mixing in microfluidic systems. Microfluid. Nanofluid. 2007, 3, 501–525. [Google Scholar] [CrossRef]
- Phan, H.V.; Coskun, M.B.; Sesen, M.; Pandraud, G.; Neild, A.; Alan, T. Vibrating membrane with discontinuities for rapid and efficient microfluidic mixing. Lab Chip 2015, 15, 4206–4216. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Yang, C. Mixing enhancement for high viscous fluids in a microfluidic chamber. Lab Chip 2011, 11, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Goto, H.; Matsumoto, M.; Maeda, R. Active micromixer for microfluidic systems using lead-zirconate-titanate (PZT)-generated ultrasonic vibration. Electrophoresis 2000, 21, 116–119. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Shi, J.; Juluri, B.K.; Huang, T.J. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 2009, 9, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Yang, J.; Pindera, M.Z.; Athavale, M.; Grodzinski, P. Bubble-induced acoustic micromixing. Lab Chip 2002, 2, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef]
- Ozcelik, A.; Ahmed, D.; Xie, Y.; Nama, N.; Qu, Z.; Nawaz, A.A.; Huang, T.J. An acoustofluidic micromixer via bubble inception and cavitation from microchannel sidewalls. Anal. Chem. 2014, 86, 5083–5088. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Meijer, H.E.H.; Singh, M.K.; Kang, T.G.; den Toonder, J.M.J.; Anderson, P.D. Passive and active mixing in microfluidic devices. Macromol. Symp. 2009, 279, 201–209. [Google Scholar] [CrossRef]
- Kunti, G.; Bhattacharya, A.; Chakraborty, S. Rapid mixing with high-throughput in a semi-active semi-passive micromixer. Electrophoresis 2017, 38, 1310–1317. [Google Scholar] [CrossRef]
- Depuru Mohan, N.K.; Greenblatt, D.; Nayeri, C.N.; Paschereit, C.O.; Panchapakesan, N.R. Vortex-enhanced mixing through active and passive flow control methods. Exp. Fluids 2015, 56, 51. [Google Scholar] [CrossRef]
- Orbay, S.; Ozcelik, A.; Lata, J.; Kaynak, M.; Xu, M.; Huang, T.J. Mixing high-viscosity fluids via acoustically driven bubbles. J. Micromech. Microeng. 2017, 27, 015008. [Google Scholar] [CrossRef] [PubMed]
- Enert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82. [Google Scholar]
- Zhang, X.-S.; Chu, S.-G.; Peter, N.; Zhang, H.-X. Fabrication of tunable wetting PDMS membrane by nanostructuring and plasma treatment. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013. [Google Scholar]
- Raimbault, V.; Rebiere, D.; Dejous, C.; Guirardel, M.; Conedera, V. Acoustic Love wave platform with PDMS microflfluidic chip. Sens. Actuators A Phys. 2008, 142, 160–165. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Feng, X.; Liu, B.-F. A rapid microfluidic mixer for high-viscosity fluids to track ultrafast early folding kinetics of G-quadruplex under molecular crowding conditions. Anal. Chem. 2012, 84, 9025–9032. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Xu, J. On the quantification of mixing in microfluidics. J. Lab. Autom. 2014, 19, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Zwaan, E.; Le Gac, S.; Tsuji, K.; Ohl, C.D. Controlled cavitation in microfluidic systems. Phys. Rev. Lett. 2007, 98, 254501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versluis, M. How snapping shrimp snap: Through cavitating bubbles. Science 2000, 289, 2114–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Tsai, C.-H.D.; Lin, X.-Y. Experimental study on microfluidic mixing with different zigzag angles. Micromachines 2019, 10, 583. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Sun, B. Versatile Microfluidic Mixing Platform for High- and Low-Viscosity Liquids via Acoustic and Chemical Microbubbles. Micromachines 2019, 10, 854. https://doi.org/10.3390/mi10120854
Guan Y, Sun B. Versatile Microfluidic Mixing Platform for High- and Low-Viscosity Liquids via Acoustic and Chemical Microbubbles. Micromachines. 2019; 10(12):854. https://doi.org/10.3390/mi10120854
Chicago/Turabian StyleGuan, Yanfang, and Baichuan Sun. 2019. "Versatile Microfluidic Mixing Platform for High- and Low-Viscosity Liquids via Acoustic and Chemical Microbubbles" Micromachines 10, no. 12: 854. https://doi.org/10.3390/mi10120854




