Resonant Mixing in Glass Bowl Microbioreactor Investigated by Microparticle Image Velocimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. GB-MBR Microfabrication
2.2. Excitation of Resonant Mixing
2.3. Particle Image Velocimetry
2.4. Laser-Induced Fluorescence (LIF)
2.5. Cultivation
3. Results and Discussion
3.1. Observation of Mixing by Laser-Induced Fluorescence (LIF)
3.2. Convection Obtained by µPIV
3.3. Cultivations of Escherichia coli as Proof of Concept
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wewetzer, S.J.; Kunze, M.; Ladner, T.; Luchterhand, B.; Roth, S.; Rahmen, N.; Kloß, R.; Costa E Silva, A.; Regestein, L.; Büchs, J. Parallel use of shake flask and microtiter plate online measuring devices (RAMOS and BioLector) reduces the number of experiments in laboratory-scale stirred tank bioreactors. J. Biol. Eng. 2015, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Zanzotto, A.; Szita, N.; Boccazzi, P.; Lessard, P.; Sinskey, A.J.; Jensen, K.F. Membrane-aerated microbioreactor for high-throughput bioprocessing. Biotechnol. Bioeng. 2004, 87, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Boccazzi, P.; Zanzotto, A.; Szita, N.; Bhattacharya, S.; Jensen, K.F.; Sinskey, A.J. Gene expression analysis of Escherichia coli grown in miniaturized bioreactor platforms for high-throughput analysis of growth and genomic data. Appl. Microbiol. Biotechnol. 2005, 68, 518–532. [Google Scholar] [CrossRef]
- Hansen, H.G.; Nilsson, C.N.; Lund, A.M.; Kol, S.; Grav, L.M.; Lundqvist, M.; Rockberg, J.; Lee, G.M.; Andersen, M.R.; Kildegaard, H.F. Versatile microscale screening platform for improving recombinant protein productivity in Chinese hamster ovary cells. Sci. Rep. 2015, 5, 18016. [Google Scholar] [CrossRef] [Green Version]
- Krull, R.; Lladó-Maldonado, S.; Lorenz, T.; Büttgenbach, S.; Demming, S. Microbioreactors. In Microsystems forPharmatechnology; Dietzel, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 99–152. ISBN 978-3-319-26918-4. [Google Scholar]
- Peterat, G.; Lladó Maldonado, S.; Edlich, A.; Rasch, D.; Dietzel, A.; Krull, R. Bioreaktionstechnik in mikrofluidischen Reaktoren. Chem. Ing. Tech. 2015, 87, 505–517. [Google Scholar] [CrossRef]
- Demming, S.; Sommer, B.; Llobera, A.; Rasch, D.; Krull, R.; Büttgenbach, S. Disposable parallel poly(dimethylsiloxane) microbioreactor with integrated readout grid for germination screening of Aspergillus ochraceus. Biomicrofluidics 2011, 5, 14104. [Google Scholar] [CrossRef]
- Demming, S.; Peterat, G.; Llobera, A.; Schmolke, H.; Bruns, A.; Kohlstedt, M.; Al-Halhouli, A.; Klages, C.-P.; Krull, R.; Büttgenbach, S. Vertical microbubble column-A photonic lab-on-chip for cultivation and online analysis of yeast cell cultures. Biomicrofluidics 2012, 6, 34106. [Google Scholar] [CrossRef]
- Peterat, G.; Schmolke, H.; Lorenz, T.; Llobera, A.; Rasch, D.; Al-Halhouli, A.T.; Dietzel, A.; Büttgenbach, S.; Klages, C.-P.; Krull, R. Characterization of oxygen transfer in vertical microbubble columns for aerobic biotechnological processes. Biotechnol. Bioeng. 2014, 111, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Lladó Maldonado, S.; Rasch, D.; Kasjanow, A.; Bouwes, D.; Krühne, U.; Krull, R. Multiphase microreactors with intensification of oxygen mass transfer rate and mixing performance for bioprocess development. Biochem. Eng. J. 2018, 139, 57–67. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-s.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Eder, T.; Eder, I.E. 3D Hanging drop culture to establish prostate cancer organoids. Methods Mol. Biol. 2017, 1612, 167–175. [Google Scholar] [CrossRef]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011, 51, e2720. [Google Scholar] [CrossRef] [PubMed]
- Shilton, R.; Tan, M.K.; Yeo, L.Y.; Friend, J.R. Particle concentration and mixing in microdrops driven by focused surface acoustic waves. J. Appl. Phys. 2008, 104, 14910. [Google Scholar] [CrossRef]
- Shilton, R.J.; Yeo, L.Y.; Friend, J.R. Quantification of surface acoustic wave induced chaotic mixing-flows in microfluidic wells. Sens. Actuators B 2011, 160, 1565–1572. [Google Scholar] [CrossRef]
- Mugele, F.; Staicu, A.; Bakker, R.; van den Ende, D. Capillary Stokes drift: a new driving mechanism for mixing in AC-electrowetting. Lab Chip 2011, 11, 2011–2016. [Google Scholar] [CrossRef]
- Oh, J.M.; Legendre, D.; Mugele, F. Shaken not stirred —On internal flow patterns in oscillating sessile drops. EPL 2012, 98, 34003. [Google Scholar] [CrossRef]
- Stokes, G.G. On the theory of oscillatory waves. Trans. Camb. Philos. Soc. 1847, 441. [Google Scholar]
- Noblin, X.; Buguin, A.; Brochard-Wyart, F. Vibrated sessile drops: transition between pinned and mobile contact line oscillations. Eur. Phys. J. E Soft Matter 2004, 14, 395–404. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Fluid Mechanics. Landau and Lifshitz: Course of Theoretical Physics, Volume 6, 2nd ed.; Elsevier Reference Monographs: Amsterdam, The Netherlands, 2013; ISBN 9781483161044. [Google Scholar]
- Kim, H.; Lim, H.-C. Mode pattern of internal flow in a water droplet on a vibrating hydrophobic surface. J. Phys. Chem. B 2015, 119, 6740–6746. [Google Scholar] [CrossRef]
- Krommenhoek, E.E.; Gardeniers, J.G.E.; Bomer, J.G.; Li, X.; Ottens, M.; van Dedem, G.W.K.; van Leeuwen, M.; van Gulik, W.M.; van der Wielen, L.A.M.; Heijnen, J.J.; et al. Integrated electrochemical sensor array for on-line monitoring of yeast fermentations. Anal. Chem. 2007, 79, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.; Bojko, S.; Bunjes, H.; Dietzel, A. An inert 3D emulsification device for individual precipitation and concentration of amorphous drug nanoparticles. Lab Chip 2018, 18, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Schwerter, M.; Grabner, D.; Hecht, L.; Vierheller, A.; Leester-Schadel, M.; Dietzel, A. Surface-passive pressure sensor by femtosecond laser glass structuring for flip-chip-in-foil integration. J. Microelectromech. Syst. 2016, 25, 517–523. [Google Scholar] [CrossRef]
- Erfle, P.; Riewe, J.; Bunjes, H.; Dietzel, A. Optically monitored segmented flow for controlled ultra-fast mixing and nanoparticle precipitation. Microfluid. Nanofluid. 2017, 21, 1089. [Google Scholar] [CrossRef]
- Cheng, Y.; Sugioka, K.; Masuda, M.; Toyoda, K. 3D microstructuring inside Foturan glass by femtosecond laser. RIKEN Rev. 2003, 50, 101–106. [Google Scholar]
- Williams, J.D.; Schmidt, C.; Serkland, D. Processing advances in transparent Foturan® MEMS. Appl. Phys. A 2010, 99, 777–782. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photonics 2008, 2, 219–225. [Google Scholar] [CrossRef]
- FOTURAN® II Photo-sensitive glass wafer | SCHOTT AG. Available online: https://www.schott.com/advanced_optics/english/products/optical-materials/thin-glass/foturan-2/index.html (accessed on 13 March 2019).
- Frey, L.J.; Vorländer, D.; Rasch, D.; Ostsieker, H.; Müller, B.; Schulze, M.; Schenkendorf, R.; Mayr, T.; Grosch, J.-H.; Krull, R. Novel electrodynamic oscillation technique enables enhanced mass transfer and mixing for cultivation in micro-bioreactor. Biotechnol. Prog. 2019. accepted. [Google Scholar] [CrossRef]
- Tipler, P.A.; Mosca, G. Schwingungen. In Physik: Für Wissenschaftler und Ingenieure, 7th ed.; Tipler, P.A., Mosca, G., Wagner, J., Kommer, C., Eds.; Springer Spektrum: Berlin, Germany, 2015; pp. 413–454. ISBN 978-3-642-54165-0. [Google Scholar]
- Calluaud, D.; David, L. Stereoscopic particle image velocimetry measurements of the flow around a surface-mounted block. Exp. Fluids 2004, 36, 53–61. [Google Scholar] [CrossRef]
- Meinhart, C.D.; Wereley, S.T.; Santiago, J.G. A PIV algorithm for estimating time-averaged velocity fields. J. Fluids Eng. 2000, 122, 285. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [PubMed]
- Kensy, F.; Zang, E.; Faulhammer, C.; Tan, R.-K.; Büchs, J. Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates. Microb. Cell Fact. 2009, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Samorski, M.; Müller-Newen, G.; Büchs, J. Quasi-continuous combined scattered light and fluorescence measurements: a novel measurement technique for shaken microtiter plates. Biotechnol. Bioeng. 2005, 92, 61–68. [Google Scholar] [CrossRef]
- Kottmeier, K.; Weber, J.; Müller, C.; Bley, T.; Büchs, J. Asymmetric division of Hansenula polymorpha reflected by a drop of light scatter intensity measured in batch microtiter plate cultivations at phosphate limitation. Biotechnol. Bioeng. 2009, 104, 554–561. [Google Scholar] [CrossRef]
- Soolaman, D.M.; Yu, H.-Z. Water microdroplets on molecularly tailored surfaces: correlation between wetting hysteresis and evaporation mode switching. J. Phys. Chem. B 2005, 109, 17967–17973. [Google Scholar] [CrossRef] [PubMed]
- Chernova, A.A.; Kopysov, S.P.; Tonkov, L.E. Simulation of a liquid drop on a vibrating hydrophobic surface. IOP Conf. Ser. Mater. Sci. Eng. 2016, 158, 12026. [Google Scholar] [CrossRef] [Green Version]
- Mampallil, D.; van den Ende, D.; Mugele, F. Controlling flow patterns in oscillating sessile drops by breaking azimuthal symmetry. Appl. Phys. Lett. 2011, 99, 154102. [Google Scholar] [CrossRef]
- Rühs, P.A.; Böni, L.; Fuller, G.G.; Inglis, R.F.; Fischer, P. In-situ quantification of the interfacial rheological response of bacterial biofilms to environmental stimuli. PLOS ONE 2013, 8, e78524. [Google Scholar] [CrossRef]






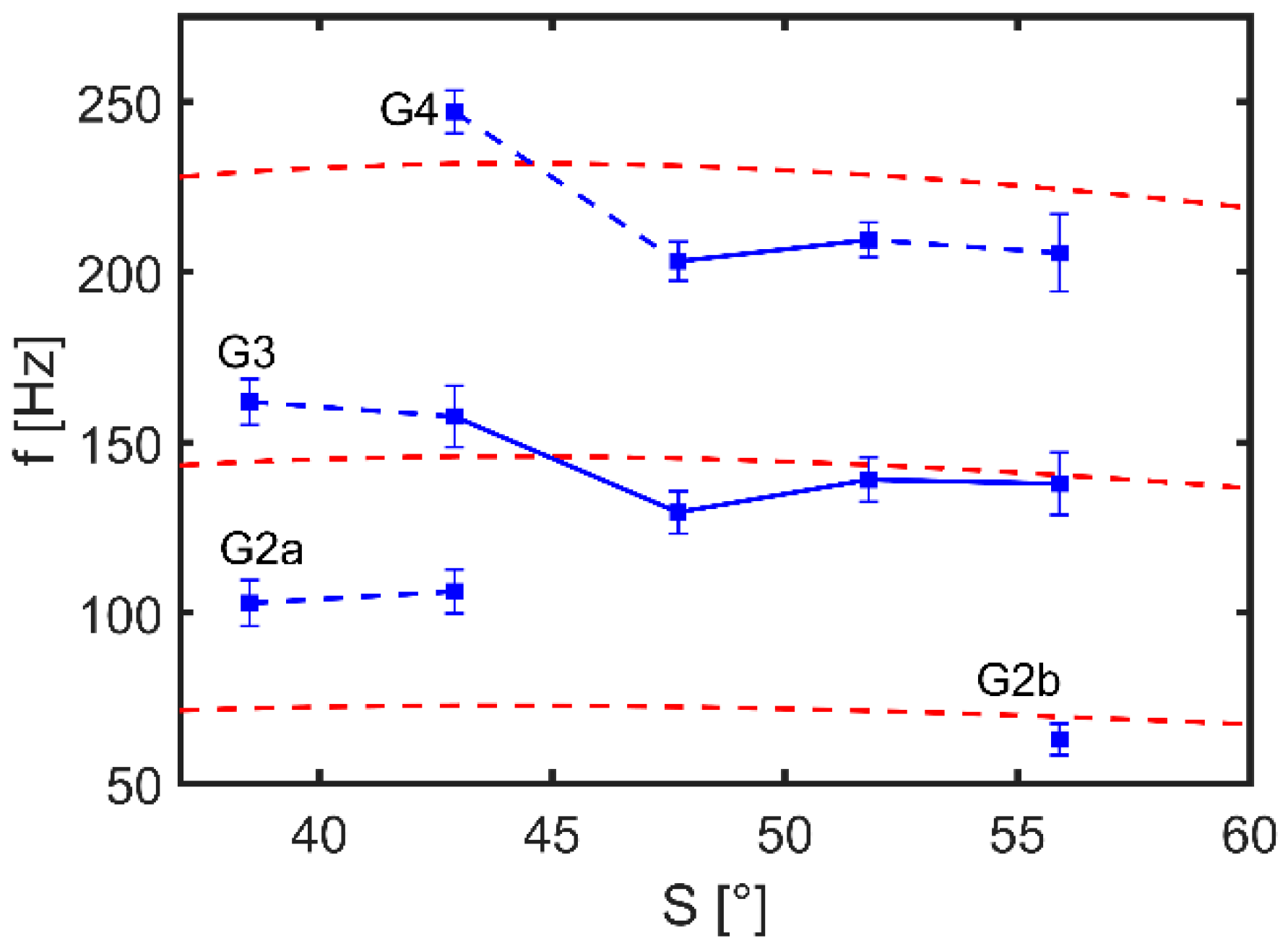




| Deviation (%) | ||
|---|---|---|
| 40 | 38.5 | 3.8 |
| 45 | 42.9 | 4.7 |
| 50 | 47.7 | 4.6 |
| 55 | 51.8 | 5.8 |
| 60 | 55.9 | 6.8 |
| 2 | 0.58 | 0.31 | |
| 3 | 0.76 | 0.55 | |
| 4 | 0.95 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinen, S.; Frey, L.J.; Krull, R.; Dietzel, A. Resonant Mixing in Glass Bowl Microbioreactor Investigated by Microparticle Image Velocimetry. Micromachines 2019, 10, 284. https://doi.org/10.3390/mi10050284
Meinen S, Frey LJ, Krull R, Dietzel A. Resonant Mixing in Glass Bowl Microbioreactor Investigated by Microparticle Image Velocimetry. Micromachines. 2019; 10(5):284. https://doi.org/10.3390/mi10050284
Chicago/Turabian StyleMeinen, Sven, Lasse Jannis Frey, Rainer Krull, and Andreas Dietzel. 2019. "Resonant Mixing in Glass Bowl Microbioreactor Investigated by Microparticle Image Velocimetry" Micromachines 10, no. 5: 284. https://doi.org/10.3390/mi10050284
APA StyleMeinen, S., Frey, L. J., Krull, R., & Dietzel, A. (2019). Resonant Mixing in Glass Bowl Microbioreactor Investigated by Microparticle Image Velocimetry. Micromachines, 10(5), 284. https://doi.org/10.3390/mi10050284






