Real Time Electronic Feedback for Improved Acoustic Trapping of Micron-Scale Particles
Abstract
:1. Introduction
2. Materials and Methods
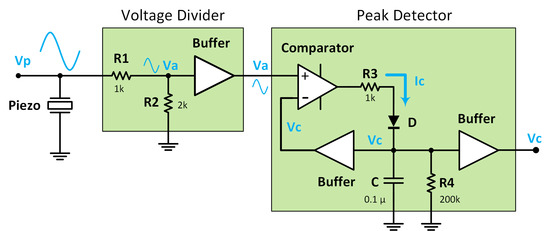
2.1. Instrumentation for Real Time Electronic Feedback
2.2. Chemical Protocol for Cellular Lysis and Mock Samples
3. Results
3.1. Microchip Design for Acoustic Capture of Sperm Cells
3.2. Effect of Increased E-cell Concentration on Acoustic Trapping Efficiency
3.3. Output Voltage Measurements Can Identify Optimal Trapping Frequency
3.4. Shift in Optimal Trapping Frequency with Changes to Fluidic Properties
3.5. Application of Real Time Feedback to a Mock Sexual Assault Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, M.; Chase, R.; DePalma, L. Making Sense of DNA Backlogs, 2012–Myths vs Reality; US Department of Justice, Office of Justice Programs, National Institute of Justice: Washington, DC, USA, 2013; pp. 1–20.
- Strom, K.; Ropero-Miller, J.; Jones, S.; Sikes, N.; Pope, M.; Horstmann, N. The 2007 Survey of Law Enforcement Forensic Evidence Processing. Final Report to the NIJ from Grant 2007F_07165, October 2009. Available online: https://www.ncjrs.gov/pdffiles1/nij/grants/228415.pdf (accessed on 4 September 2019).
- Ritter, N. The road ahead: Unanalyzed Evidence in Sexual Assault Cases. National Institute of Justice Special Report, May 2011. Available online: https://ncjrs.gov/pdffiles1/nij/233279.pdf (accessed on 4 September 2019).
- Hurst, L.; Lothridge, K. 2007 DNA Evidence and Offender Analysis Measurement: DNA Backlogs, Capacity and Funding. Final Report to the NIJ from Grant 2006-MU-BX-K002, January 2010. Available online: https://www.ncjrs.gov/pdffiles1/nij/grants/230328.pdf (accessed on 4 September 2019).
- LaPorte, G.; Waltke, H.; Heurich, C.; Chase, R. DNA Analysis, Capacity Enhancement, and other Forensic Activities; National Institute of Justice Report Forensic Science: Washington, DC, USA, 2018; pp. 1–20.
- Gill, P.; Jefferys, A.; Werrett, D. Forensic application of DNA ‘fingerprints’. Nature 1985, 318, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Vuichard, S.; Borer, U.; Bottinelli, M.; Cossu, C.; Malik, N.; Meier, V.; Gehrig, C.; Sulzer, A.; Morerod, M.; Castella, V. Differential DNA extraction of challenging simulated sexual-assault samples: A Swiss collaborative study. Investig. Genet. 2011, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.; Evander, M.; Horsman-Hall, K.; Nilsson, J.; Laurell, T.; Landers, J. Acoustic differential extraction for forensic analysis of sexual assault evidence. Anal. Chem. 2009, 81, 6089–6095. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Clark, C.; Poe, B.; Lounsbury, J.; Nilsson, J.; Laurell, T.; Landers, J. Isolation of a low number of sperm cells from female DNA in a glass-PDMS-glass microchip via bead-assisted acoustic differential extraction. Anal. Chem. 2019, 91, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Xu, K.; Scott, O.; Hickey, J.; Tsuei, A.; Jackson, K.; Landers, J. Acoustic trapping of sperm cells from mock sexual assault samples. FSI Genet. 2019, 41, 42–49. [Google Scholar] [CrossRef]
- Clark, C.; Xu, K.; Scott, O.; Hickey, J.; Plean, B.; Edwards, C.; Sikorsky, J.; Crouse, C.; Woolf, M.; Landers, J. External evaluation of an acoustic differential extraction prototype in forensic laboratories. FSI Genet. in press.
- Evander, M.; Nilsson, J. Acoustofluidics 20: Applications in acoustic trapping. Lab Chip 2012, 12, 4667–4676. [Google Scholar] [CrossRef]
- Bellastella, G.; Cooper, T.; Battaglia, M.; Strose, A.; Torres, I.; Hellenkemper, B.; Soler, C.; Sinisi, A. Dimensions of human ejaculated spermatozoa in Papanicolaou-stained seminal and swim-up smears obtained from the Integrated Semen Analysis System (ISAS). Asian J. Androl. 2010, 12, 871–879. [Google Scholar] [CrossRef]
- Bruus, H. Acoustofluidics 2: Perturbation theory and ultrasound resonance modes. Lab Chip 2012, 12, 20. [Google Scholar] [CrossRef]
- Bao, M.; Onshage, A.; Iwansson, K. Handbook of Sensors and Actuators; Elsevier Science: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Newton, J.; Schofield, D.; Vlahopoulou, J.; Zhou, Y. Detecting cell lysis using viscosity monitoring in E. coli fermentation to prevent product loss. Am. Inst. Chem. Eng. 2016, 1069–1076. [Google Scholar]
- Dhillon, G.; Brar, S.; Kaur, S.; Verma, M. Rheological studies during submerged citric acid fermentation by Aspergillus niger in stirred fermentor using apple pomace ultrafiltration sludge. Food Bioprocess. Technol. 2013, 6, 1240–1250. [Google Scholar] [CrossRef]
- Perley, C.; Swartz, J.; Cooney, C. Measurement of cell mass concentration with a continuous-flow viscometer. Biotechnol. Bioeng. 1979, 21, 519–523. [Google Scholar] [CrossRef]
- Shimmons, B.; Svrcek, W.; Zajic, J. Cell concentration control by viscosity. Biotechnol. Bioeng. 1976, 18, 1793–1805. [Google Scholar] [CrossRef]
- Atanov, Y.; Berdenikov, A. Relation between fluid viscosity and compressibility. J. Eng. Phys. 1983, 43, 878–879. [Google Scholar] [CrossRef]
- Elert, G. Viscosity. The Physics Hypertextbook. 1998. Available online: https://physics.info/viscosity/ (accessed on 5 June 2019).
- Doinikov, A. Acoustic radiation forces: Classical theory and recent advances. Recent Res. Devel Acoust. 2003, 1, 39–67. [Google Scholar]
- Sepehrirahnama, S.; Chau, F.; Lim, K. Effects of viscosity and acoustic streaming on the interparticle radiation force between rigid spheres in a standing wave. Phys. Rev. 2016, 93, 023307. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, B.; Evander, M.; Wahlstrom, J.; Nilsson, J. Frequency tracking in acoustic trapping for improved performance stability and system surveillance. Lab Chip 2014, 14, 1005–1013. [Google Scholar] [CrossRef]
- Dual, J.; Hahn, P.; Leibacher, I.; Moller, D.; Schwarz, T. Acoustofluidics 6: Experimental characterization of ultrasonic particle manipulation devices. Lab Chip 2012, 12, 852–862. [Google Scholar] [CrossRef]
- Hawkes, J.; Coakley, W. A continuous flow ultrasonic cell-filtering method. Enzyme Microb. Technol. 1996, 19, 57–62. [Google Scholar] [CrossRef]
- Kwiatkowski, C.; Marston, P. Resonator frequency shift due to ultrasonically induced microparticle migration in an aqueous suspension: Observations and model for the maximum frequency shift. J. Acoust. Soc. Am. 1998, 103, 3290–3300. [Google Scholar] [CrossRef]
- McLucas, J. Wideband peak detector operates over wide input-frequency range. EDN Des. Ideas. 2007. Available online: https://www.edn.com/design/analog/4324882/Wideband-peak-detector-operates-over-wide-input-frequency-range (accessed on 28 April 2019).
- Uchino, K. Piezoelectric ceramics for transducers. In Ultrasonic Transducers; Woodhead Publishing Series in Electronic and Optical Materials: Sawston, UK; Cambridge, UK, 2012; pp. 70–116. [Google Scholar]
- Millikan, R.; Grover, M. Elements of electricity; American Technical Society: Chicago, IL, USA, 1917; p. 54. [Google Scholar]
- Aroom, K.; Harting, M.; Cox, C.; Radharkrishnan, R.; Smith, C.; Gill, B. Bioimpedance analysis: A guide to simple design and implementation. J. Surg. Res. 2009, 153, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2006, 36, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Oncescu, V.; O’Dell, D.; Erickson, D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 2013, 13, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Awofeso, O.; Choi, S.; Jung, Y.; Bae, E. Colorimetric analysis of saliva–alcohol test strips by smartphone-based instruments using machine-learning algorithms. Appl. Opt. 2017, 56, 84–92. [Google Scholar] [CrossRef]
- Soldat, D.J.; Barak, P.; Lepore, B.J. Microscale colorimetric analysis using a desktop scanner and automated digital image analysis. J. Chem. Educ. 2009, 86, 617. [Google Scholar] [CrossRef]
- Cabaret, F.; Bonnot, S.; Fradette, L.; Tanguy, P.A. Mixing time analysis using colorimetric methods and image processing. Ind. Eng. Chem. Res. 2007, 46, 5032–5042. [Google Scholar] [CrossRef]
- Capitan-Vallvey, L.F.; Lopez-Ruiz, N.; Martinez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef]
- Krauss, S.T.; Holt, V.C.; Landers, J.P. Simple reagent storage in polyester-paper hybrid microdevices for colorimetric detection. Sens. Actuators B Chem. 2017, 246, 740–747. [Google Scholar] [CrossRef]
- Barthel, K.U. 3D-data representation with ImageJ, In Proceedings of the ImageJ Conference, Luxembourg, 18–19 May 2006.
- Rasband, W.S. ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, 2011. Available online: http://imagej.nih.gov/ij/ (accessed on 6 October 2019).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, C.P.; Farmehini, V.; Spiers, L.; Woolf, M.S.; Swami, N.S.; Landers, J.P. Real Time Electronic Feedback for Improved Acoustic Trapping of Micron-Scale Particles. Micromachines 2019, 10, 489. https://doi.org/10.3390/mi10070489
Clark CP, Farmehini V, Spiers L, Woolf MS, Swami NS, Landers JP. Real Time Electronic Feedback for Improved Acoustic Trapping of Micron-Scale Particles. Micromachines. 2019; 10(7):489. https://doi.org/10.3390/mi10070489
Chicago/Turabian StyleClark, Charles P., Vahid Farmehini, Liam Spiers, M. Shane Woolf, Nathan S. Swami, and James P. Landers. 2019. "Real Time Electronic Feedback for Improved Acoustic Trapping of Micron-Scale Particles" Micromachines 10, no. 7: 489. https://doi.org/10.3390/mi10070489
APA StyleClark, C. P., Farmehini, V., Spiers, L., Woolf, M. S., Swami, N. S., & Landers, J. P. (2019). Real Time Electronic Feedback for Improved Acoustic Trapping of Micron-Scale Particles. Micromachines, 10(7), 489. https://doi.org/10.3390/mi10070489