Using pH-Activable Carbon Nanoparticles as Cell Imaging Probes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNPs
2.3. Characterizations
2.4. Cell Studies In Vitro
2.4.1. Cell Culture
2.4.2. In Vitro Cytotoxicity
2.5. Cellular Imaging
3. Results and Discussion
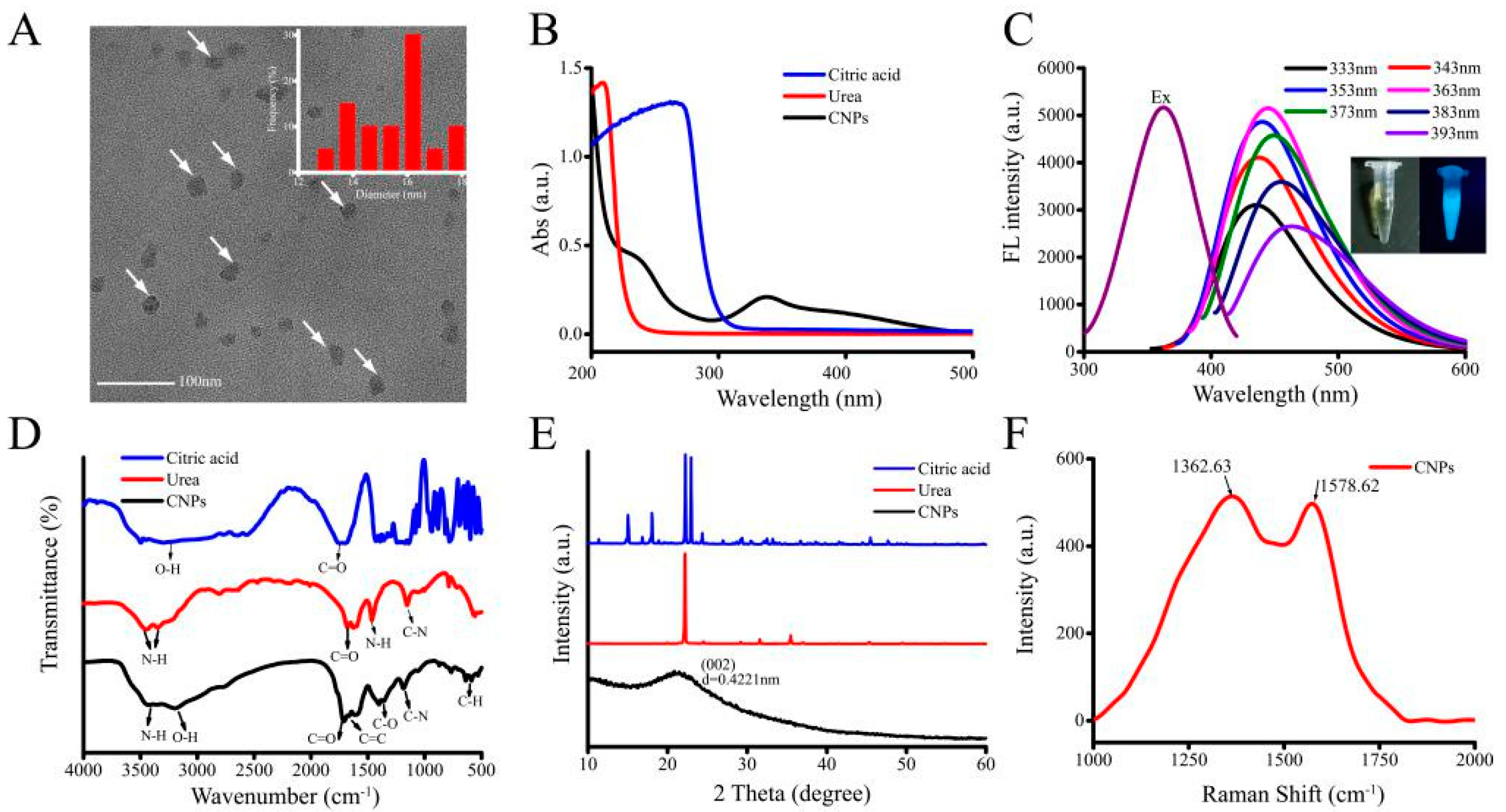
3.1. Characterization of CNPs
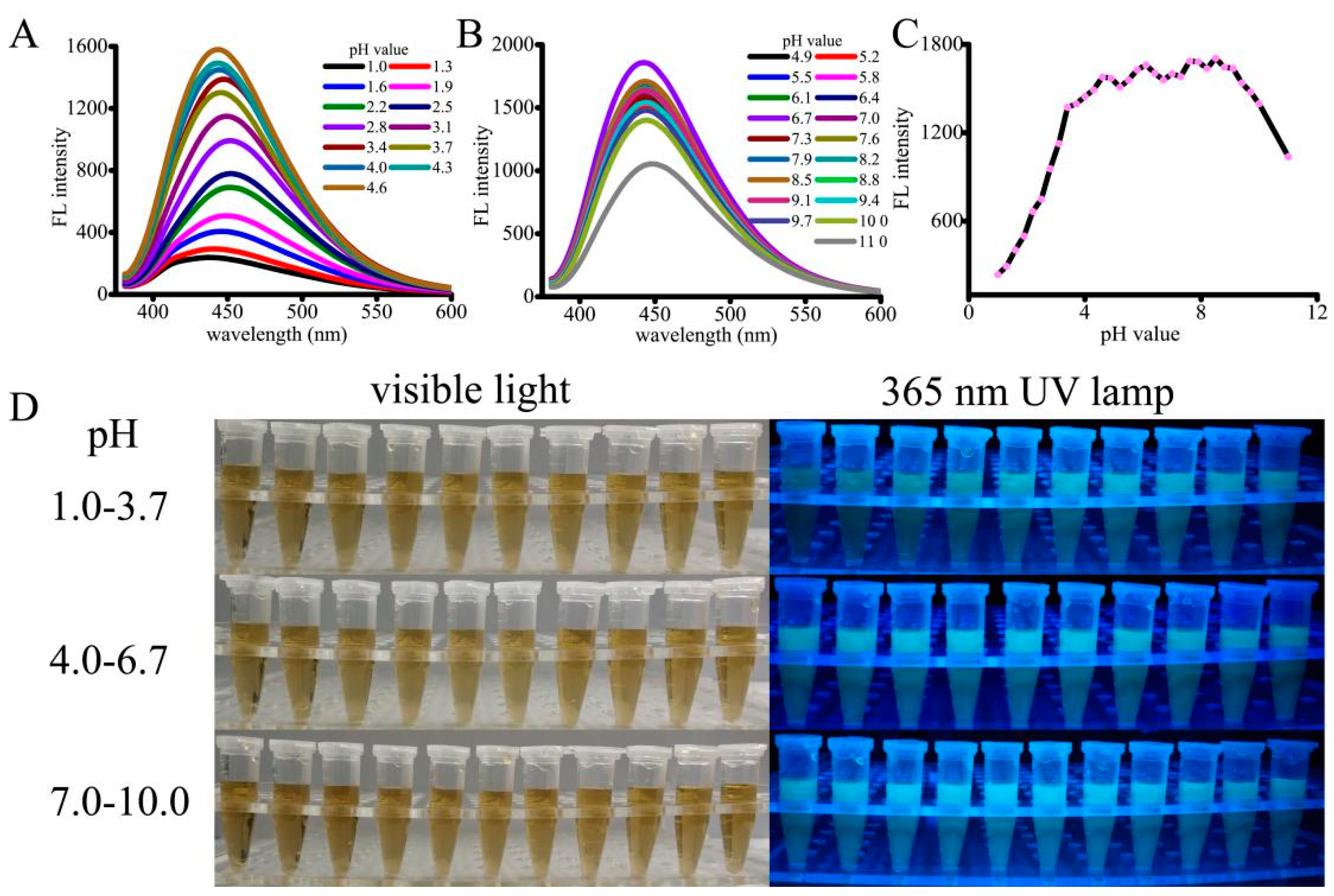
3.2. pH-Sensitive Fluorescence Intensity of the CNPs
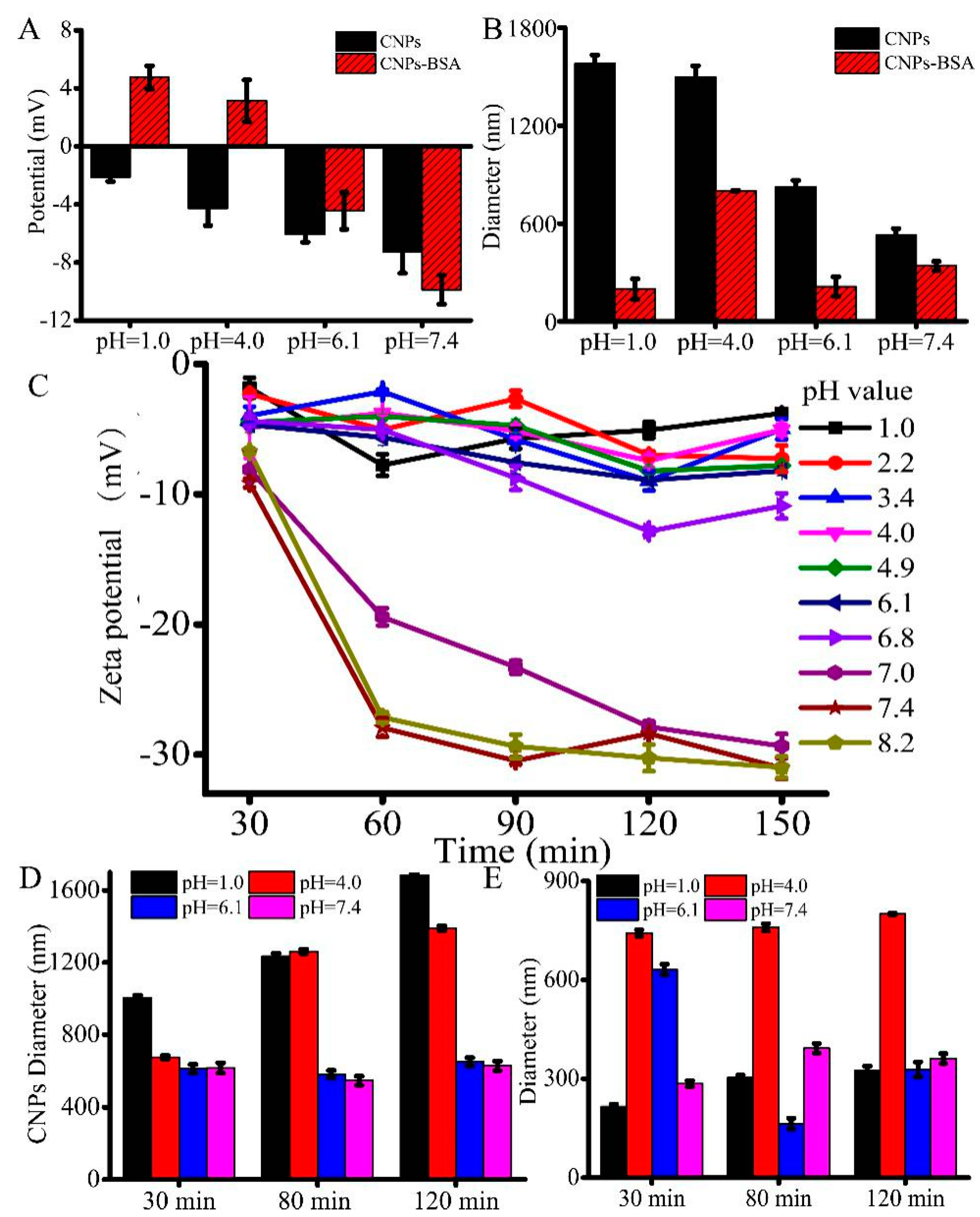
3.3. pH-Responsive Changes in the Surface Charge as Well as the Diameter of CNPs
3.4. Cytotoxicity of CNPs
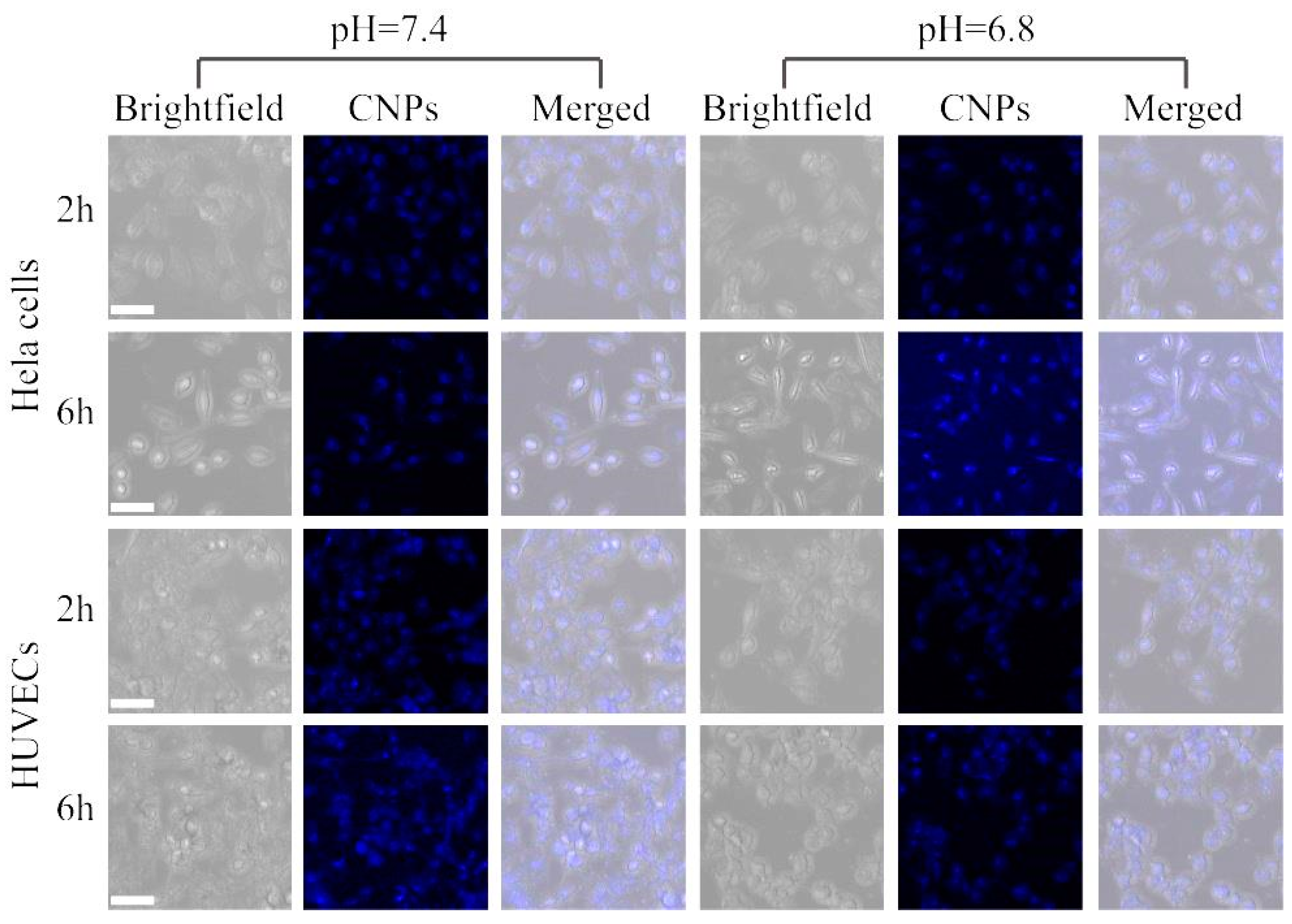
3.5. Cell Imaging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, J.-T.; Ko, K.-P.; Shi, H.; Ren, W.X.; Verwilst, P.; Koo, S.; Lee, J.Y.; Chi, S.-G.; Kim, J.S. PLK1-Targeted fluorescent tumor imaging with high signal-to-background ratio. ACS Sens. 2017, 2, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Hanaoka, K.; Takayanagi, T.; Toki, Y.; Egawa, T.; Kamiya, M.; Komatsu, T.; Ueno, T.; Terai, T.; Yoshida, K.; et al. Analysis of chemical equilibrium of silicon-substituted fluorescein and its application to develop a scaffold for red fluorescent probes. Anal. Chem. 2015, 87, 9061–9069. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.-A.; Eskes, E.; Wilms, T.; Ludovico, P.; Winderickx, J. pH homeostasis links the nutrient sensing PKA/TORC1/Sch9 menage-a-trois to stress tolerance and longevity. Microb. Cell 2018, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, N.; Khosrowabadi, E.; Hassinen, A.; Harrus, D.; Glumoff, T.; Kietzmann, T.; Kellokumpu, S. Abnormal Golgi pH homeostasis in cancer cells impairs apical targeting of carcinoembryonic antigen by inhibiting its glycosyl-phosphatidylinositol anchor-mediated association with lipid rafts. Antioxid. Redox Sign. 2017, 30, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2008, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Bhunia, S.K.; Dalal, C.; Jana, N.R. Red fluorescent carbon nanoparticle-based cell imaging probe. ACS Appl. Mater. Inter. 2016, 8, 9305–9313. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Angsantikul, P.; Liu, W.; Esteban-Fernández de Ávila, B.; Thamphiwatana, S.; Xu, M.; Sandraz, E.; Wang, X.; Delezuk, J.; Gao, W.; et al. Micromotors spontaneously neutralize gastric acid for pH-responsive payload release. Angew. Chem. Int. Ed. 2017, 56, 2156–2161. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Riederer, B.; Nikolovska, K.; Singh, A.K.; Mäkelä, K.A.; Seidler, A.; Liu, Y.; Gros, G.; Bartels, H.; et al. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol. 2018, 222, 12923. [Google Scholar] [CrossRef]
- Liu, X.; Su, Y.; Tian, H.; Yang, L.; Zhang, H.; Song, X.; Foley, J.W. Ratiometric fluorescent probe for lysosomal pH measurement and imaging in living cells using single-wavelength excitation. Anal. Chem. 2017, 89, 7038–7045. [Google Scholar] [CrossRef]
- Lin, X.; Su, J.; Lin, H.; Sun, X.; Liu, B.; Kankala, R.K.; Zhou, S.F. Luminescent carbon nanodots based aptasensors for rapid detection of kanamycin residue. Talanta 2019, 202, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 2018, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Yazdani, M.; Lohse, S.E.; Bakhtiary, Z.; Serpooshan, V.; Ghavami, M.; Asadian, M.; Mashaghi, S.; Dreaden, E.C.; Mashaghi, A.; et al. Nanoparticle surface functionality dictates cellular and systemic toxicity. Chem. Mater. 2017, 29, 6578–6595. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Mohammadi, S.; Salimi, A. Current advances of carbon dots based biosensors for tumor marker detection, cancer cells analysis and bioimaging. TrAC Trends. Anal. Chem. 2019, 115, 83–99. [Google Scholar] [CrossRef]
- Prabhakar, N.; Khan, M.H.; Peurla, M.; Chang, H.-C.; Hänninen, P.E.; Rosenholm, J.M. Intracellular trafficking of fluorescent nanodiamonds and regulation of their cellular toxicity. ACS Omega 2017, 2, 2689–2693. [Google Scholar] [CrossRef]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Bacchetta, R.; Santo, N.; Valenti, I.; Maggioni, D.; Longhi, M.; Tremolada, P. Comparative toxicity of three differently shaped carbon nanomaterials on Daphnia magna: Does a shape effect exist? Nanotoxicology 2018, 12, 201–223. [Google Scholar] [CrossRef]
- Demaurex, N. pH homeostasis of cellular organelles. News Physiol. Sci. 2002, 17, 1–5. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, T.; Li, Y.; Huang, G.; White, M.A.; Gao, J. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv. Drug Deliver. Rev. 2017, 113, 87–96. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
- Lin, X.; Su, J.; Lin, H.; Zhou, S.-F.; Sun, X.; Liu, B.; Zeng, M. Carbon nanoparticles with oligonucleotide probes for a label-free sensitive antibiotic residues detection based on competitive analysis. Sci. Rep. 2019, 9, 3489. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Donkor, D.A.; Tang, X.S. Tube length and cell type-dependent cellular responses to ultra-short single-walled carbon nanotube. Biomaterials 2014, 35, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Almendro, V.; Marusyk, A.; Polyak, K. Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, X.X.; Lin, L.Y.; Feng, Q.; Kitamori, T.; Lin, J.-M.; Sun, J.S. Integrated microfluidic platform with multiple functions to probe tumor–endothelial cell interaction. Anal. Chem. 2017, 89, 10037–10044. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Su, J.; Kankala, R.K.; Zeng, M.; Zhou, S.-F.; Lin, X. Using pH-Activable Carbon Nanoparticles as Cell Imaging Probes. Micromachines 2019, 10, 568. https://doi.org/10.3390/mi10090568
Lin H, Su J, Kankala RK, Zeng M, Zhou S-F, Lin X. Using pH-Activable Carbon Nanoparticles as Cell Imaging Probes. Micromachines. 2019; 10(9):568. https://doi.org/10.3390/mi10090568
Chicago/Turabian StyleLin, Honggui, Jianlong Su, Ranjith Kumar Kankala, Mingrong Zeng, Shu-Feng Zhou, and Xuexia Lin. 2019. "Using pH-Activable Carbon Nanoparticles as Cell Imaging Probes" Micromachines 10, no. 9: 568. https://doi.org/10.3390/mi10090568




