Cathodically Pretreated AuNPs–BDD Electrode for Detection of Hexavalent Chromium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments, Reagents, and Materials
2.2. Fabrication of BDD Electrode
2.3. Modification of BDD Electrode
2.4. Detection and Analysis of Cr (VI)
3. Results and Discussion
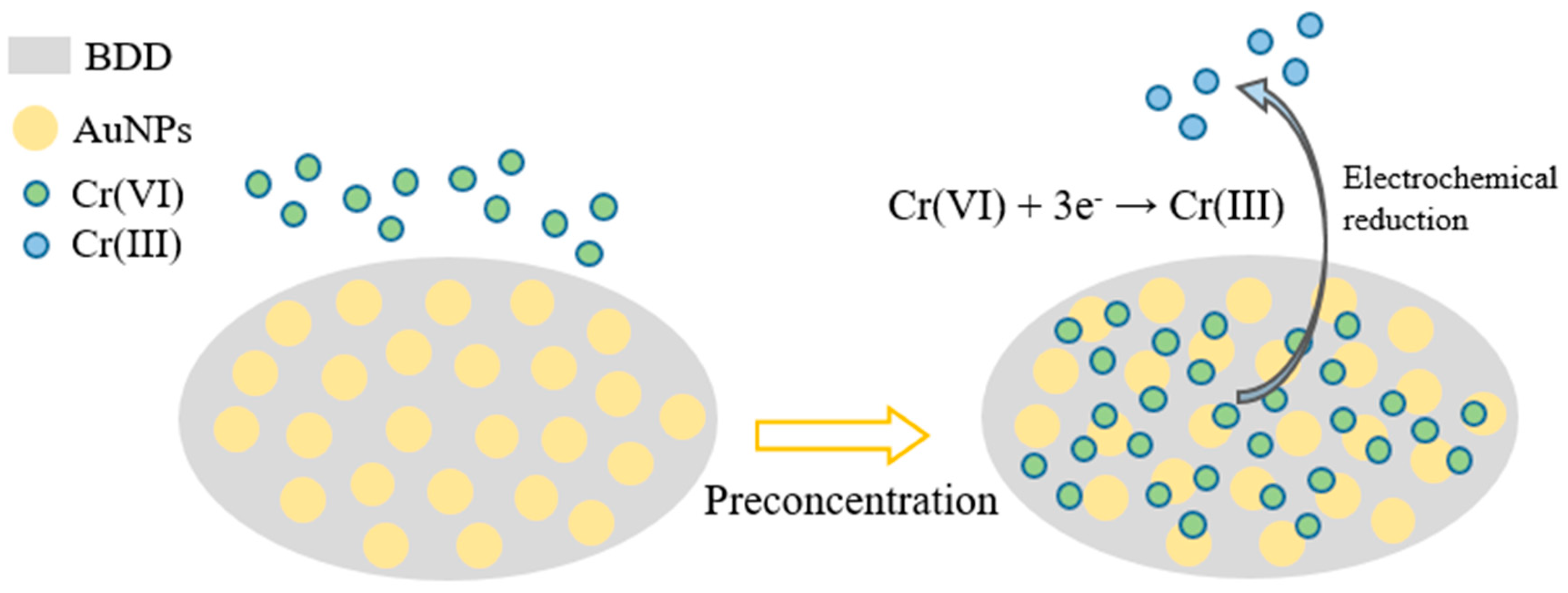
3.1. AuNPs–BDD Electrode Detection Principle
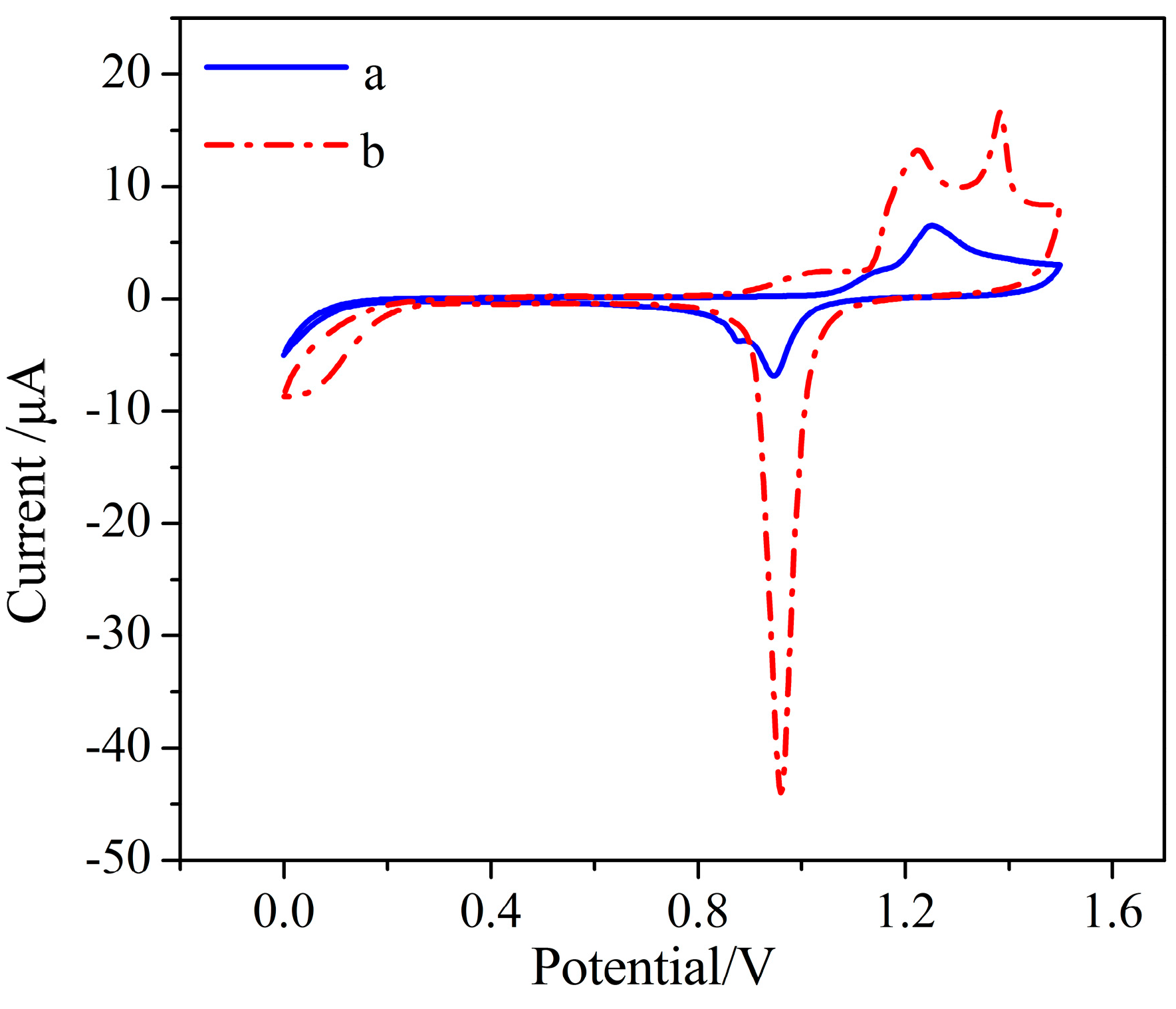
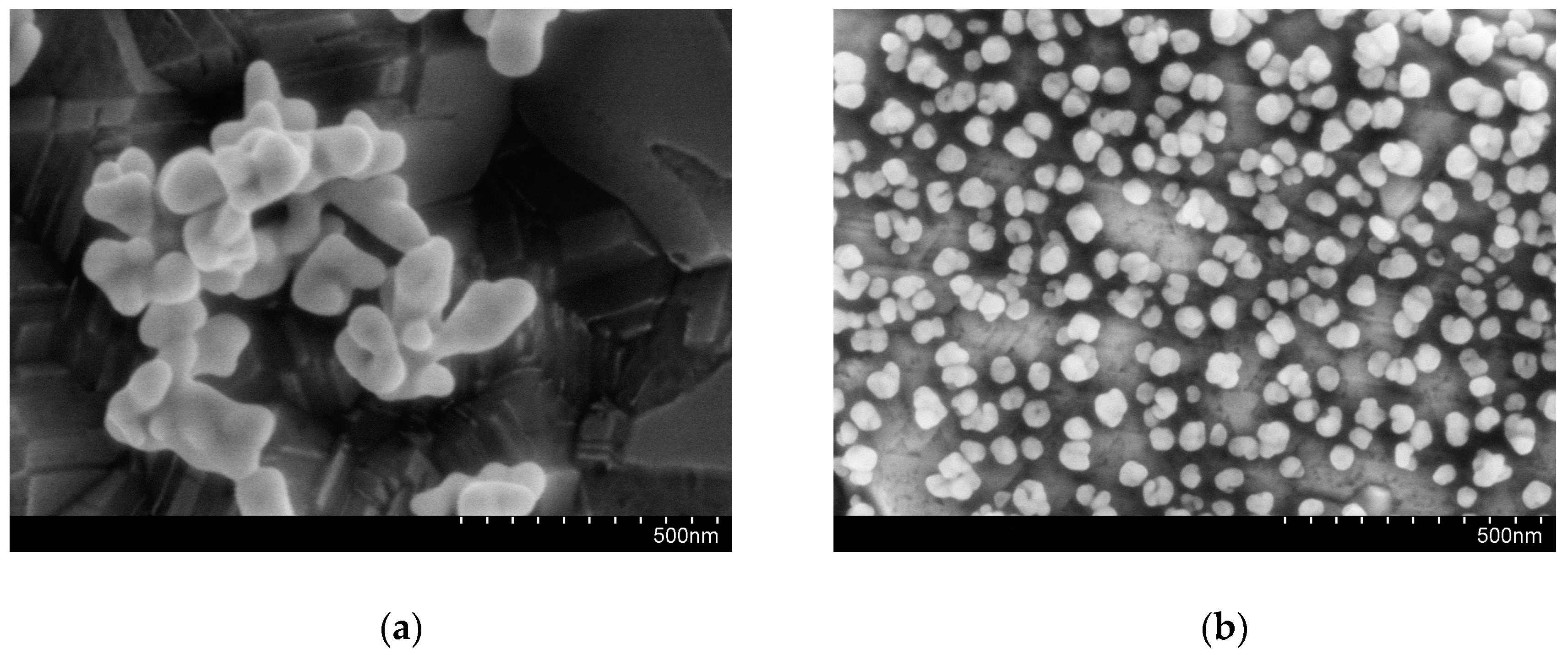
3.2. AuNPs–BDD Electrode Surface Characterization
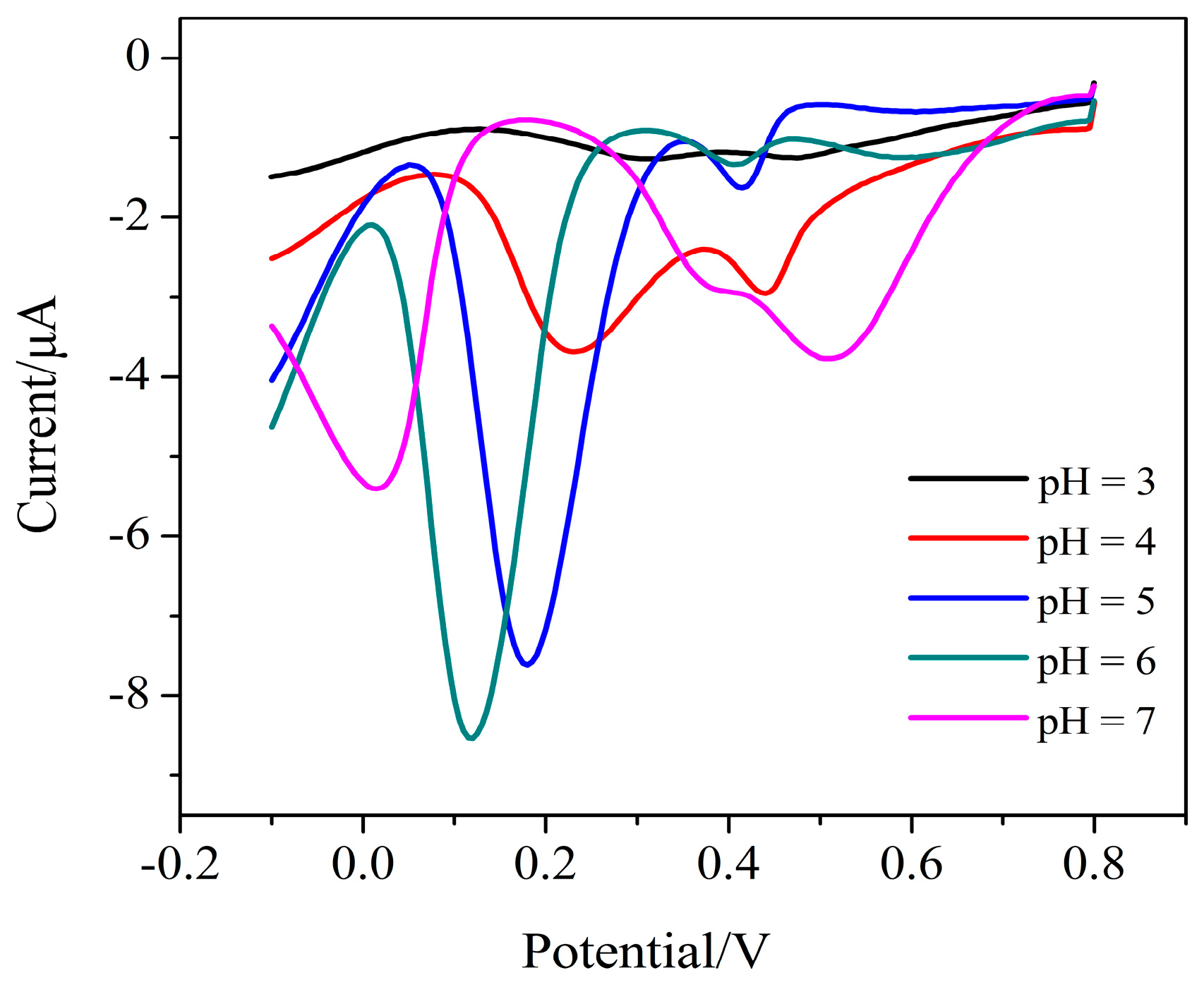
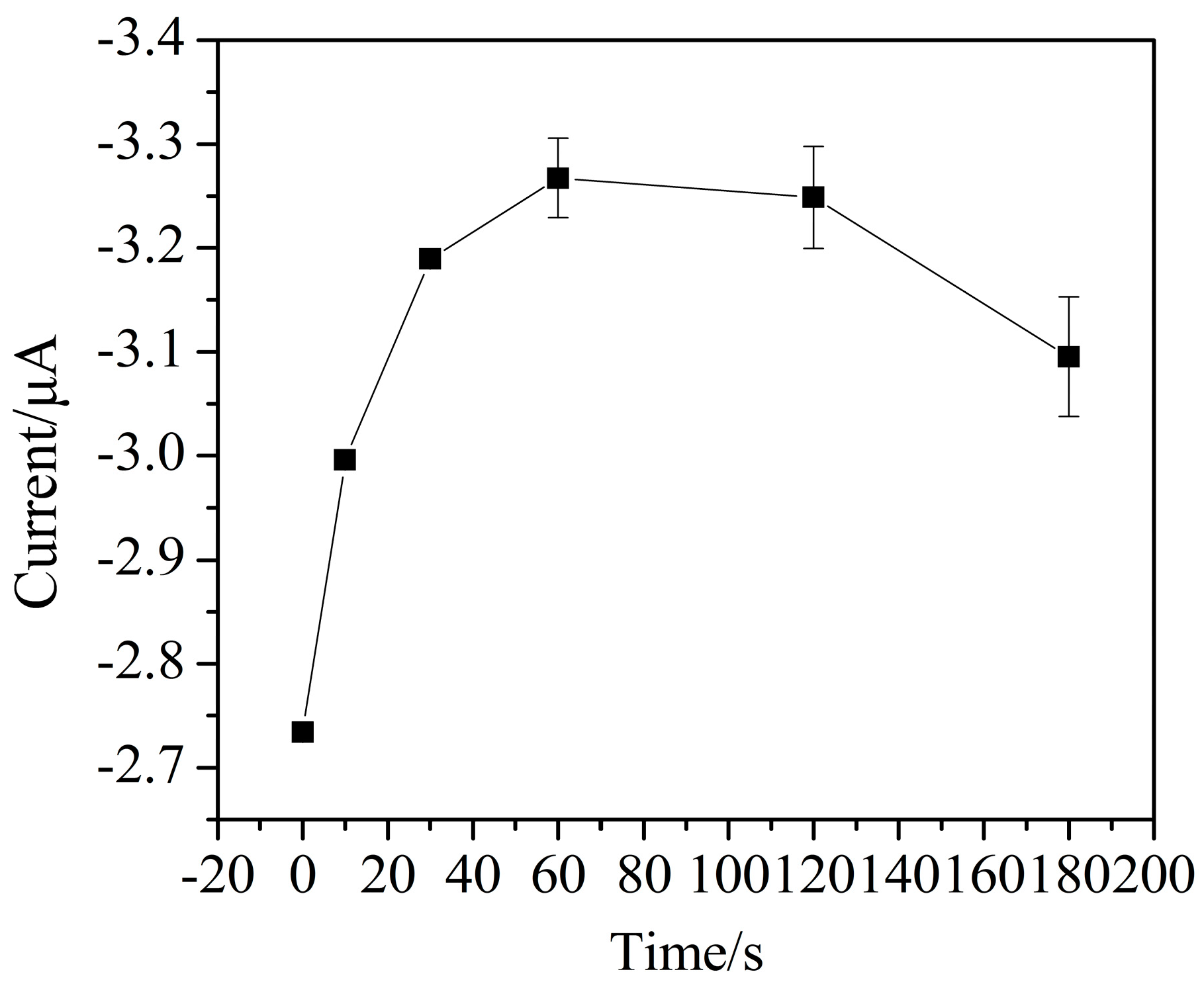
3.3. Parameter Optimization
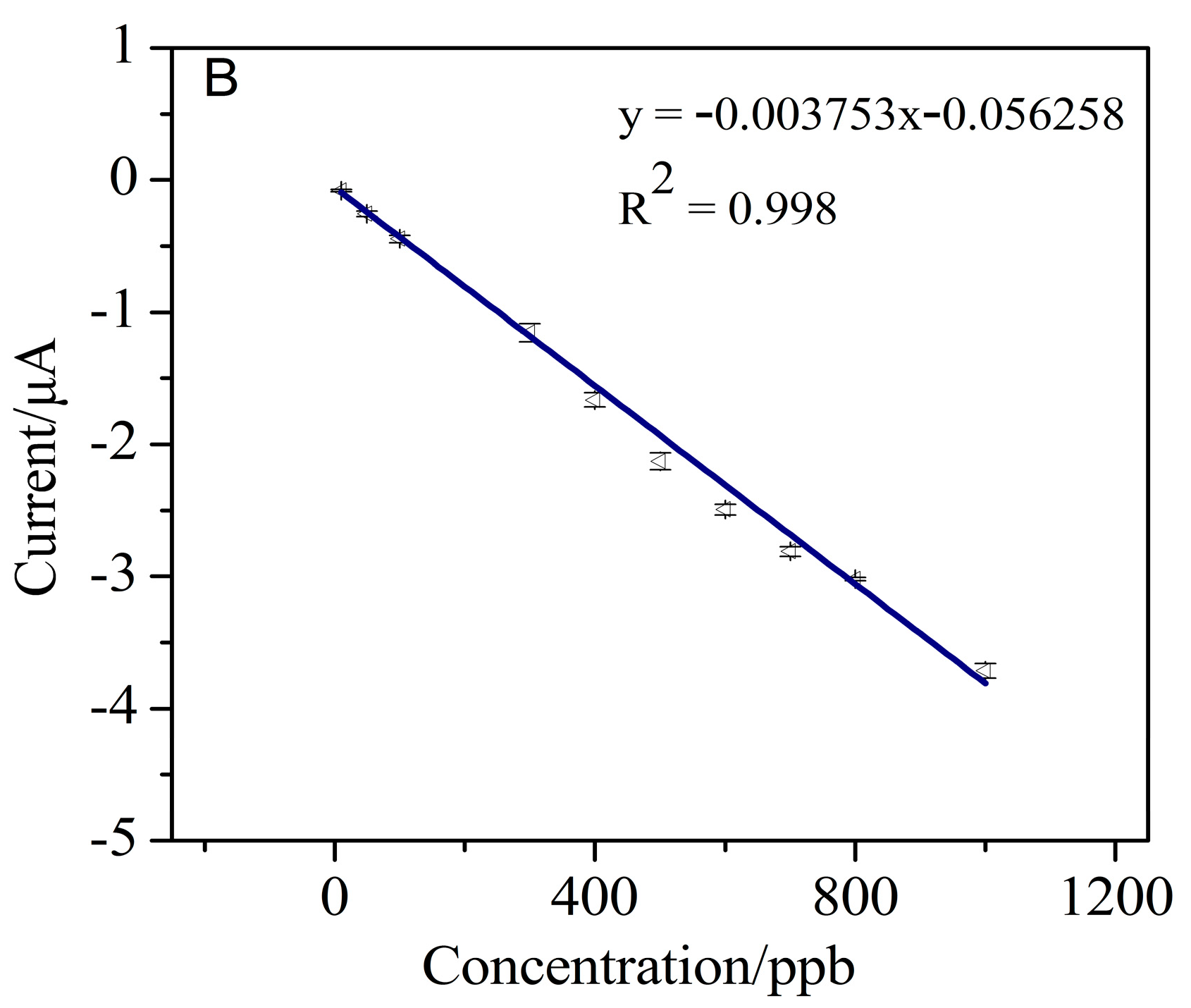
3.4. Detection of Cr (VI)
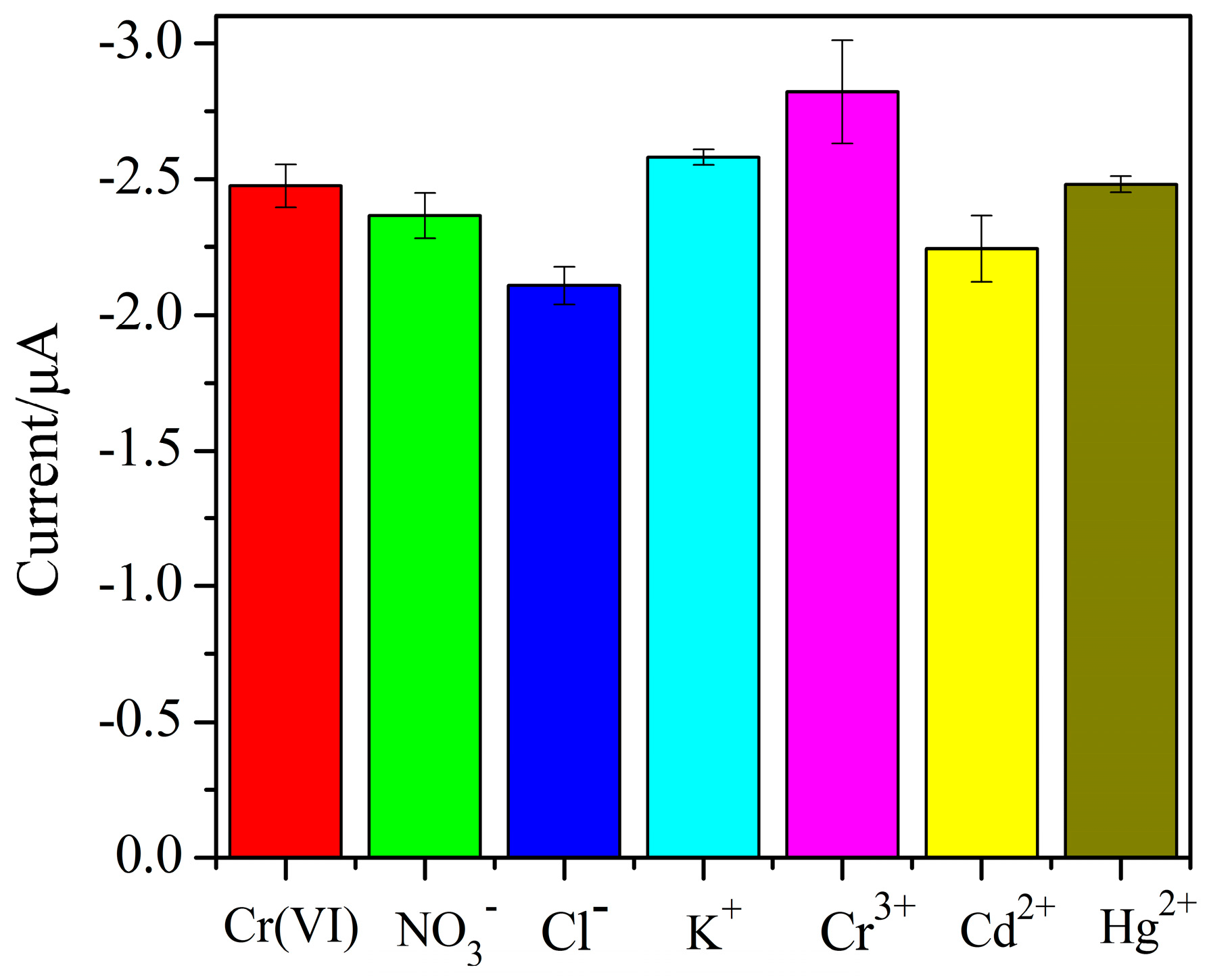
3.5. Selectivity of AuNPs–BDD Electrode
3.6. Real Sample Analysis
3.7. Comparison with Other Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhitkovich, A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(vi). Chem. Res. Toxicol. 2005, 18, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Walter, M. Chromium in human nutrition: A review. J. Nutr. 1993, 123, 626–633. [Google Scholar]
- Williams, C.H.; Iismaa, O.; David, D.J. Determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agric. Sci. 1962, 59, 381–385. [Google Scholar] [CrossRef]
- Sperling, M.; Xu, S.K.; Welz, B. Determination of chromium(iii) and chromium(vi) in water using flow-injection online preconcentration with selective adsorption on activated alumina and flame atomic-absorption spectrometric detection. Anal. Chem. 1992, 64, 3101–3108. [Google Scholar] [CrossRef]
- Sperling, M.; Yin, X.F.; Welz, B. Differential determination of chromium(vi) and total chromium in natural-waters using flow-injection online separation and preconcentration electrothermal atomic-absorption spectrometry. Analyst 1992, 117, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bravo, Y.; Roig-Navarro, A.F.; Lopez, F.J.; Hernandez, F. Multielemental determination of arsenic, selenium and chromium(vi) species in water by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. J. Chromatogr. A 2001, 926, 265–274. [Google Scholar] [CrossRef]
- Zhang, N.; Suleiman, J.S.; He, M.; Hu, B. Chromium(iii)-imprinted silica gel for speciation analysis of chromium in environmental water samples with icp-ms detection. Talanta 2008, 75, 536–543. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakai, T.; Kumagai, H. Simultaneous determination of chromium(iii) and chromium(vi) by ion chromatography with inductively-coupled plasma-mass spectrometry. J. Chromatogr. A 1995, 706, 127–136. [Google Scholar] [CrossRef]
- Beyermann, K.; Rose, H.J.; Christian, R.P. Determination of nanogram amounts of chromium in urine by x-ray fluorescence spectroscopy. Anal. Chim. Acta 1969, 45, 51–55. [Google Scholar] [CrossRef]
- Bidoglio, G.; Gibson, P.N.; Ogorman, M.; Roberts, K.J. X-ray-absorption spectroscopy investigation of surface redox transformations of thallium and chromium on colloidal mineral oxides. Geochim. Cosmochim. Acta 1993, 57, 2389–2394. [Google Scholar] [CrossRef]
- Luke, C.L. Determination of trace elements in inorganic and organic materials by X-ray fluorescence spectroscopy. Anal. Chim. Acta 1968, 41, 237–250. [Google Scholar] [CrossRef]
- Qin, Y.-W.; Meng, W.; Zheng, B.-H.; Su, Y.-B. Heavy metal pollution in tidal zones of bohai bay using the dated sediment cores. J. Environ. Sci. 2006, 18, 610–615. [Google Scholar]
- Jin, W.; Yan, K. Recent advances in electrochemical detection of toxic cr(vi). RSC Adv. 2015, 5, 37440–37450. [Google Scholar] [CrossRef]
- Mardegan, A.; Cettolin, M.; Kamath, R.; Vascotto, V.; Stortini, A.M.; Ugo, P.; Scopece, P.; Madou, M.; Moretto, L.M. Speciation of trace levels of chromium with bismuth modified pyrolyzed photoresist carbon electrodes. Electroanalysis 2015, 27, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Tyszczuk-Rotko, K.; Madejska, K.; Domanska, K. Ultrasensitive hexavalent chromium determination at bismuth film electrode prepared with mediator. Talanta 2018, 182, 62–68. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhang, W.; Zhou, S.; Xue, Z.L.; Xu, L.; Gu, Y.; Miao, Y. Improved bi film wrapped single walled carbon nanotubes for ultrasensitive electrochemical detection of trace cr(vi). Electrochim. Acta 2013, 113, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Kachoosangi, R.T.; Compton, R.G. Voltammetric determination of chromium(vi) using a gold film modified carbon composite electrode. Sens. Actuators B Chem. 2013, 178, 555–562. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Highly sensitive and selective electrochemical detection of sub-ppb level chromium(vi) using nano-sized gold particle. Talanta 2008, 76, 161–165. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Huang, X.; Shi, J.; Zou, X.; Li, Z.; Cui, X. Adsorptive stripping voltammetry determination of hexavalent chromium by a pyridine functionalized gold nanoparticles/three-dimensional graphene electrode. Microchem. J. 2019, 149, 104022. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Yubero, F.; Espinos, J.P.; Gonzalez-Elipe, A.R.; Lambert, R.M. Synthesis, characterization and performance of robust poison resistant ultrathin film yttria stabilized zirconia nickel anodes for application in solid electrolyte fuel cells. J. Power Sources 2016, 324, 679–686. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Yubero, F.; Gonzalez-Elipe, A.R.; Lambert, R.M. Microstructural engineering and use of efficient poison resistant au-doped ni-gdc ultrathin anodes in methane-fed solid oxide fuel cells. Int. J. Hydrog. Energy 2018, 43, 885–893. [Google Scholar] [CrossRef]
- Parra-Barranco, J.; Garcia-Garcia, F.J.; Rico, V.; Borras, A.; Lopez-Santos, C.; Frutos, F.; Barranco, A.; Gonzalez-Elipe, A.R. Anisotropic in-plane conductivity and dichroic gold plasmon resonance in plasma-assisted ito thin films e-beam-evaporated at oblique angles. ACS Appl. Mater. Interfaces 2015, 7, 10993–11001. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, R.G.; Muthoosamy, K.; Zhou, M.; Ashokkumar, M.; Huang, N.M.; Manickam, S. Sonochemical and sustainable synthesis of graphene-gold (g-au) nanocomposites for enzymeless and selective electrochemical detection of nitric oxide. Biosens. Bioelectron. 2017, 87, 622–629. [Google Scholar]
- Ivandini, T.A.; Wicaksono, W.P.; Saepudin, E.; Rismetov, B.; Einaga, Y. Anodic stripping voltammetry of gold nanoparticles at boron-doped diamond electrodes and its application in immunochromatographic strip tests. Talanta 2015, 134, 136–143. [Google Scholar] [CrossRef]
- Chaiyo, S.; Chailapakul, O.; Siangproh, W. Highly sensitive determination of mercury using copper enhancer by diamond electrode coupled with sequential injection-anodic stripping voltammetry. Anal. Chim. Acta 2014, 852, 55–62. [Google Scholar] [CrossRef]
- Gao, C.-Y.; Tong, J.-H.; Bian, C.; Sun, J.-Z.; Li, Y.; Wang, J.-F.; Gong, S.; Hui, Y.; Xu, Y.-H.; Wang, X.-Q.; et al. Determination of trace zn(ii), cd(ii) and pb(ii) metal ions using in-situ bismuth-modified boron doped diamond electrode. Chin. J. Anal. Chem. 2018, 46, 217–224. [Google Scholar]
- Zhang, T.; Li, C.; Mao, B.; An, Y. Determination of cd2+ by ultrasound-assisted square wave anodic stripping voltammetry with a boron-doped diamond electrode. Ionics 2015, 21, 1761–1769. [Google Scholar] [CrossRef]
- Chooto, P.; Wararatananurak, P.; Innuphat, C. Determination of trace levels of pb(ii) in tap water by anodic stripping voltammetry with boron-doped diamond electrode. Scienceasia 2010, 36, 150–156. [Google Scholar] [CrossRef]
- Honório, G.G.; Azevedo, G.C.; Matos, M.A.C.; De Oliveira, M.A.L.; Matos, R.C. Use of boron-doped diamond electrode pre-treated cathodically for the determination of trace metals in honey by differential pulse voltammetry. Food Control 2014, 36, 42–48. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, N.; Yang, B.; Wang, C.; Zhuang, H.; Tian, Q.; Zhai, Z.; Liu, L.; Jiang, X. Hybrid diamond/graphite films as electrodes for anodic stripping voltammetry of trace ag+ and cu2+. Sens. Actuators B Chem. 2016, 231, 194–202. [Google Scholar] [CrossRef]
- McLaughlin, M.H.S.; Pakpour-Tabrizi, A.C.; Jackman, R.B. Diamond electrodes for high sensitivity mercury detection in the aquatic environment: Influence of surface preparation and gold nanoparticle activity. Electroanalysis 2019, 31, 1775–1782. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Brocenschi, R.F.; Medeiros, R.A.; Fatibello-Filho, O.; Rocha-Filho, R.C. Analytical applications of electrochemically pretreated boron--doped diamond electrodes. ChemElectroChem 2020, 7, 1291–1311. [Google Scholar] [CrossRef]
- Pires Eisele, A.P.; Clausen, D.N.; Teixeira Tarley, C.R.; Dall’Antonia, L.H.; Sartori, E.R. Simultaneous square-wave voltammetric determination of paracetamol, caffeine and orphenadrine in pharmaceutical formulations using a cathodically pretreated boron-doped diamond electrode. Electroanalysis 2013, 25, 1734–1741. [Google Scholar] [CrossRef]
- El-Deab, M.S.; Arihara, K.; Ohsaka, T. Fabrication of au(111)-like polycrystalline gold electrodes and their applications to oxygen reduction. J. Electrochem. Soc. 2004, 151, E213–E218. [Google Scholar] [CrossRef]
- Korolczuk, M. Application of pulsed potential accumulation for minimization of interferences from surfactants in voltammetric determination of traces of cr(vi). Electroanalysis 2000, 12, 837–840. [Google Scholar] [CrossRef]
- Welch, C.M.; Nekrassova, O.; Compton, R.G. Reduction of hexavalent chromium at solid electrodes in acidic media: Reaction mechanism and analytical applications. Talanta 2005, 65, 74–80. [Google Scholar] [CrossRef]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Influence of the gold nanoparticles electrodeposition method on hg(ii) trace electrochemical detection. Electrochim. Acta 2012, 73, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Tu, J.; Gan, Y.; Liang, T.; Wan, H.; Wang, P. A miniaturized electrochemical system for high sensitive determination of chromium(vi) by screen-printed carbon electrode with gold nanoparticles modification. Sens. Actuators B Chem. 2018, 272, 582–588. [Google Scholar] [CrossRef]
- Sari, T.K.; Jin, J.; Zein, R.; Munaf, E. Anodic stripping voltammetry for the determination of trace cr(vi) with graphite/styrene-acrylonitrile copolymer composite electrodes. Anal. Sci. 2017, 33, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, Y.; Zhao, Q.; Hou, L.; Han, Z. Polyoxometalate-based crystalline catalytic materials for efficient electrochemical detection of cr(vi). Sens. Actuators B Chem. 2020, 305, 127469. [Google Scholar] [CrossRef]
- Breslin, C.B.; Branagan, D.; Garry, L.M. Electrochemical detection of cr(vi) with carbon nanotubes decorated with gold nanoparticles. J. Appl. Electrochem. 2019, 49, 195–205. [Google Scholar] [CrossRef]







| Electrolytes | Reduction Peak Current (μA) |
|---|---|
| Nitric acid | - |
| Hydrochloric acid | - |
| Phosphate buffer | −3.14 |
| Sodium acetate buffer | −8.74 |
| Heavy Metal | Added (μg/L) | Found (μg/L) | Recovery (%) |
|---|---|---|---|
| Cr (VI) | 0 | 0 | |
| 300 | 316.8 ± 23.4 | 105.6 | |
| 500 | 509.9 ± 19.7 | 102.0 | |
| 900 | 880.5 ± 10.9 | 97.8 |
| Electrode | Method | Linear Range (ppb) | Limit of Detection (ppb) | Reference |
|---|---|---|---|---|
| Graphite/styrene–acrylonitrile copolymer composite electrode | SWASV | 0–150 | 4.5 | [40] |
| Polyoxometalate-based electrode | amperometry | 25–18,900 | 2.7 | [41] |
| AuNP–carbon nanotubes | amperometry | 40–11,500 | 36 | [42] |
| AuNPs–BDD | CSV | 10–1000 | 1.19 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xiong, C.; Gao, C.; Li, Y.; Bian, C.; Xia, S. Cathodically Pretreated AuNPs–BDD Electrode for Detection of Hexavalent Chromium. Micromachines 2020, 11, 1095. https://doi.org/10.3390/mi11121095
Xu Y, Xiong C, Gao C, Li Y, Bian C, Xia S. Cathodically Pretreated AuNPs–BDD Electrode for Detection of Hexavalent Chromium. Micromachines. 2020; 11(12):1095. https://doi.org/10.3390/mi11121095
Chicago/Turabian StyleXu, Yuhao, Chenyu Xiong, Chengyao Gao, Yang Li, Chao Bian, and Shanhong Xia. 2020. "Cathodically Pretreated AuNPs–BDD Electrode for Detection of Hexavalent Chromium" Micromachines 11, no. 12: 1095. https://doi.org/10.3390/mi11121095






