High-Throughput White Blood Cell (Leukocyte) Enrichment from Whole Blood Using Hydrodynamic and Inertial Forces
Abstract
:1. Introduction
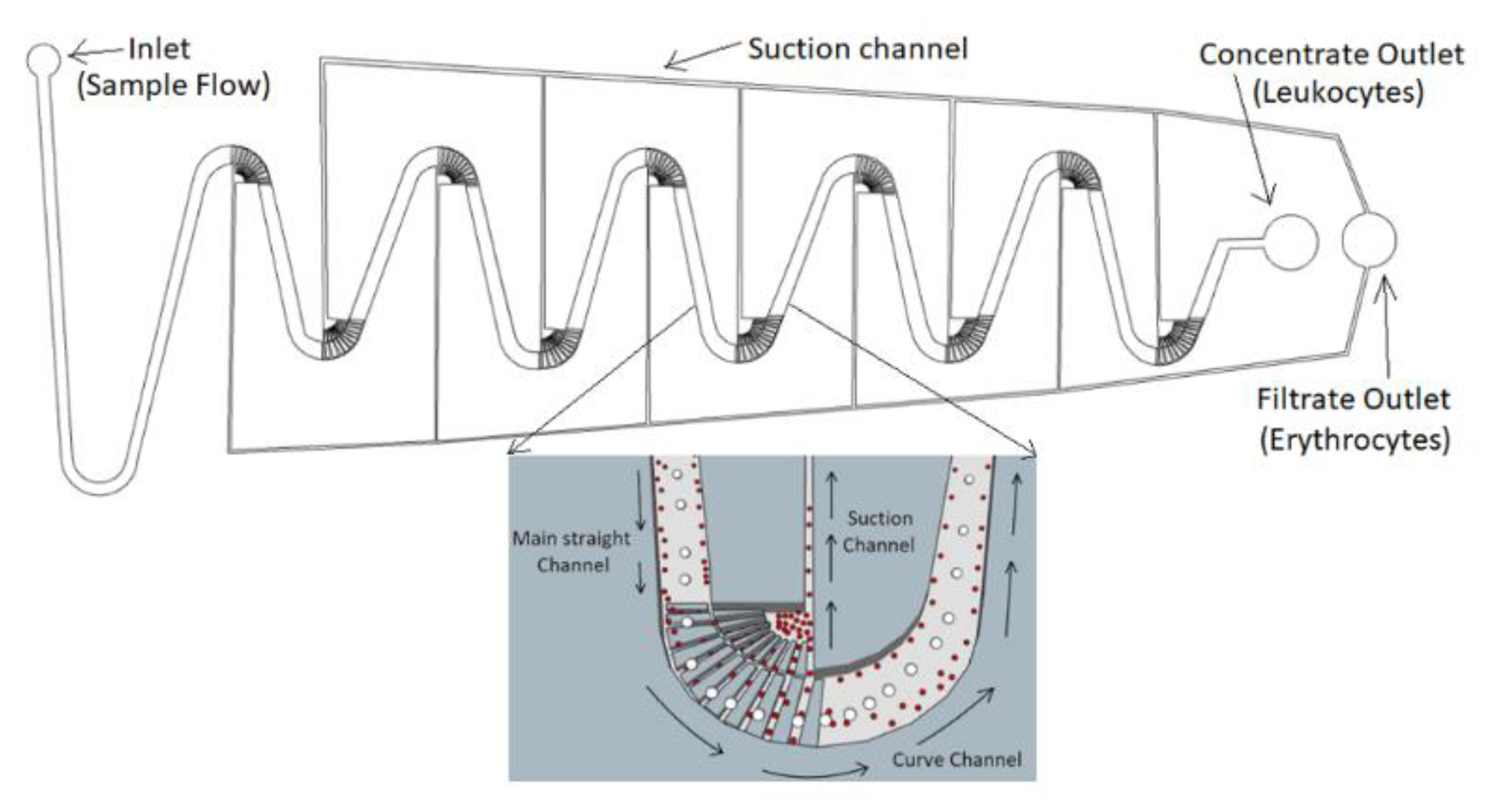
2. Device Design and Working Principle
3. Material and Methods
3.1. Fabrication of Our Separation Microchip
3.2. Sample Preparation
3.3. Experimental Setup
3.4. Recovery Rate
4. Results and Discussion
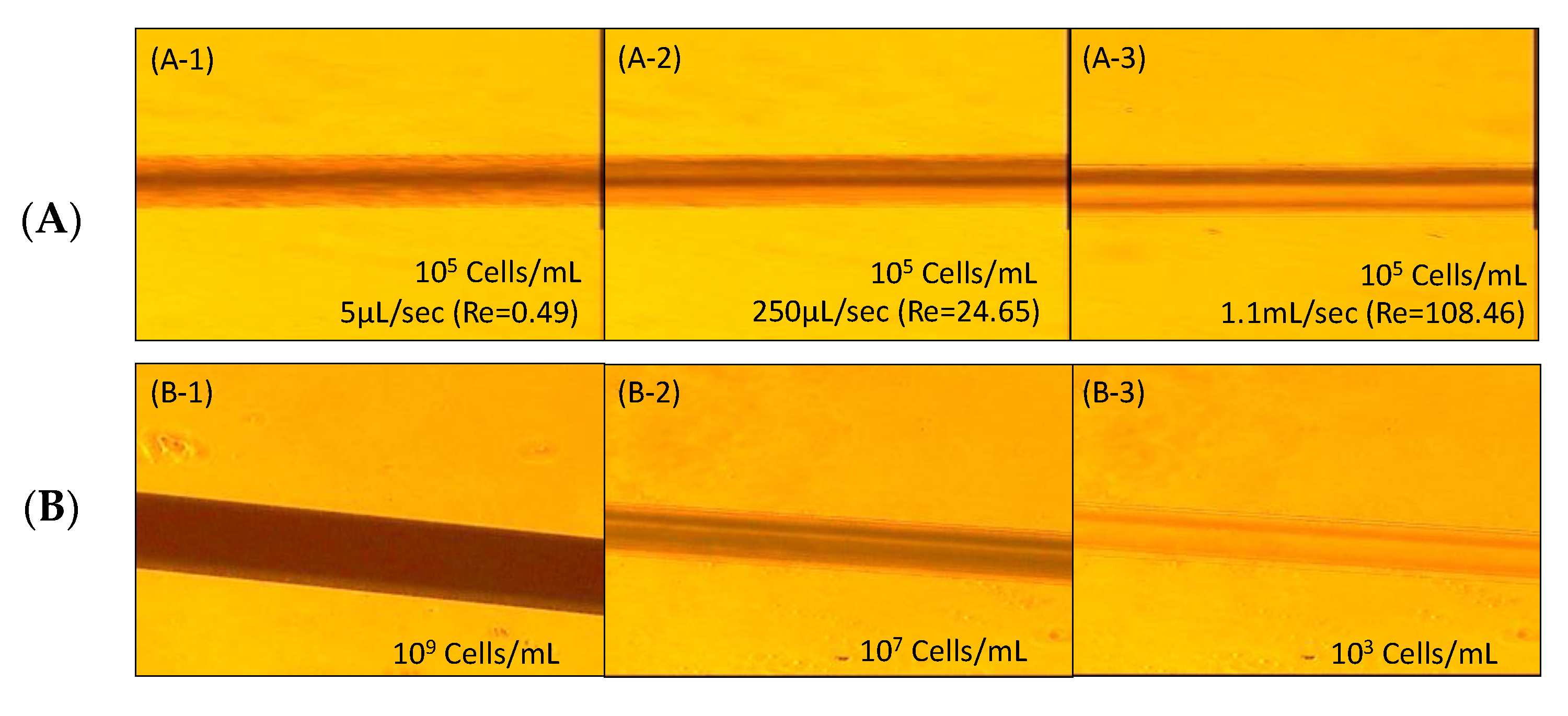
4.1. Inertial Migration Effect
4.2. Separation Efficiency with Hydrodynamic Suction
4.3. Overall Separation Efficiency
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef]
- Bhagat, A.A.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using Dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef]
- Hou, H.W.; Bhagat, A.A.S.; Lee, W.C.; Huang, S.; Han, J.; Lim, C.T. Microfluidic Devices for Blood Fractionation. Micromachines 2011, 2, 319–343. [Google Scholar] [CrossRef] [Green Version]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood cells separation and sorting techniques of passive microfluidic devices: From fabrication to applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Lee, C.-Y.; Tsai, C.-H.; Fu, L.-M. Novel continuous particle sorting in microfluidic chip utilizing cascaded squeeze effect. Microfluid. Nanofluidics 2009, 7, 499–508. [Google Scholar] [CrossRef]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef]
- Morijiri, T.; Sunahiro, S.; Senaha, M.; Yamada, M.; Seki, M. Sedimentation pinched-flow fractionation for size- and density-based particle sorting in microchannels. Microfluid. Nanofluidics 2011, 11, 105–110. [Google Scholar] [CrossRef]
- Lin, B.K.; McFaul, S.M.; Jin, C.; Black, P.C.; Ma, H.S. Highly selective biomechanical separation of cancer cells from leukocytes using microfluidic ratchets and hydrodynamic concentrator. Biomicrofluidics 2013, 7, 034114. [Google Scholar] [CrossRef] [Green Version]
- Takagi, J.; Yamada, M.; Yasuda, M.; Seki, M. Continuous particle separation in a microchannel having asymmetrically arranged multiple branches. Lab Chip 2005, 5, 778–784. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Liu, C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Sollier, E.; Rostaing, H.; Pouteau, P.; Fouillet, Y.; Achard, J.-L. Passive microfluidic devices for plasma extraction from whole human blood. Sens. Actuators B Chem. 2009, 141, 617–624. [Google Scholar] [CrossRef]
- Alvankarian, J.; Bahadorimehr, A.; Majlis, B.Y. A pillar-based microfilter for isolation of white blood cells on elastomeric substrate. Biomicrofluidics 2013, 7, 14102. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, W.; Liu, G.; Lu, W.; Fu, J. Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes. Lab Chip 2014, 14, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-Y.; Huang, C.-K.; Lu, Y.-W. Enhancement of microfluidic particle separation using cross-flow filters with hydrodynamic focusing. Biomicrofluidics 2016, 10, 011906. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, K.C.; Pan, Y.C.; Lee, T.P.; Hsiung, L.C.; Lin, C.M.; Wo, A.M. Separation and detection of rare cells in a microfluidic disk via negative selection. Lab Chip 2011, 11, 474–483. [Google Scholar] [CrossRef]
- Hur, S.C.; Mach, A.J.; di Carlo, D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Kasper, S.; Papautsky, I. Enhanced size-dependent trapping of particles using microvortices. Microfluid. Nanofluidics 2013, 15, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Hahn, Y.; Hong, D.; Kang, J.; Choi, S. A reconfigurable microfluidics platform for microparticle separation and fluid mixing. Micromachines 2016, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Park, J.-K. Continuous hydrophoretic separation and sizing of microparticles using slanted obstacles in a microchannel. Lab Chip 2007, 7, 890–897. [Google Scholar] [CrossRef]
- Di Carlo, D.; Edd, J.F.; Irimia, D.; Tompkins, R.G.; Toner, M. Equilibrium separation and filtration of particles using differential inertial focusing. Anal. Chem. 2008, 80, 2204–2211. [Google Scholar] [CrossRef]
- Ramachandraiah, H.; Ardabili, S.; Faridi, A.M.; Gantelius, J.; Kowalewski, J.M.; Mårtensson, G.; Russom, A. Dean flow-coupled inertial focusing in curved channels. Biomicrofluidics 2014, 8, 034117. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, M.; Liu, C.; Zhang, Y.; Liu, D.; Liu, W.; Jiang, X. Double spiral microchannel for label-free tumor cell separation and enrichment. Lab Chip 2012, 12, 3952–3960. [Google Scholar] [CrossRef] [Green Version]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.S.; Chaudhuri, P.K.; Lim, C.T. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Yoon, H.J.; Stella, P.; Nagrath, S. Cascaded spiral microfluidic device for deterministic and high purity continuous separation of circulating tumor cells. Biomicrofluidics 2014, 8, 064117. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.M.; Zubajlo, R.E.; Smela, E.; White, I.M. High-throughput particle separation and concentration using spiral inertial filtration. Biomicrofluidics 2014, 8, 024105. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, P.; Cox, R.G. The lateral migration of spherical particles sedimenting in a stagnant bounded fluid. J. Fluid Mech. 2006, 80, 561–591. [Google Scholar] [CrossRef]
- Yang, S.; Lee, S.S.; Ahn, S.W.; Kang, K.; Shim, W.; Lee, G.; Kim, J.M. Deformability-selective particle entrainment and separation in a rectangular microchannel using medium viscoelasticity. Soft Matter 2012, 8, 5011–5019. [Google Scholar] [CrossRef]
- Chung, A.J. A Minireview on inertial microfluidics fundamentals: Inertial particle focusing and secondary flow. BioChip J. 2019, 13, 53–63. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in microchannels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef]
- Chen, Y.-L. Inertia- and deformation-driven migration of a soft particle in confined shear and Poiseuille flow. RSC Adv. 2014, 4, 17908–17916. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Sluyter, R.; Li, W.; Alici, G.; Nguyen, N.T. Inertial particle separation by differential equilibrium positions in a symmetrical serpentine micro-channel. Sci. Rep. 2014, 4, 4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.H.; Ha, J.B.; Bahk, Y.K.; Arakawa, T.; Shoji, S.; Go, J.S. Size-selective separation of micro beads by utilizing secondary flow in a curved rectangular microchannel. Lab Chip 2009, 9, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Graells, T.; Walldén, M.; Hjort, K. Inertial focusing with sub-micron resolution for separation of bacteria. Lab Chip 2019, 19, 1257–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, H.; Sollier, E.; Weaver, W.M.; di Carlo, D. Intrinsic particle-induced lateral transport in microchannels. Proc. Natl. Acad. Sci. USA 2012, 109, 11593–11598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Hu, G. High-throughput particle manipulation based on hydrodynamic effects in microchannels. Micromachines 2017, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, W.; Alici, G. Inertial microfluidics: Mechanisms and applications. In Advanced Mechatronics and MEMS Devices II; Springer: Berlin/Heidelberg, Germany, 2017; pp. 563–593. [Google Scholar]
- Lee, D.J.; Brenner, H.; Youn, J.R.; Song, Y.S. Multiplex particle focusing via hydrodynamic force in viscoelastic fluids. Sci. Rep. 2013, 3, 3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Microchannel Dimension (µm) | Obstacle in Curve Channel | Sample Solution (Whole Blood) | Flow Rate (µL/sec) | |
|---|---|---|---|---|
| Chip 1 | W × H = 200 × 58 R = 200 | NO (shown in Figure 3A) | 4.3 × 108 cells/mL | 5, 20, 250, 1100 |
| Chip 2 | W × H = 200 × 85 R = 100 | YES (shown in Figure 3B) | 4.3 × 109 cells/mL | 5, 20, 100, 1100 |
| Channel Type | Mechanism | Particles/Cell Size (D = diameter in μm) | Channel Dimension (μm) Width (W) × Height (H) | Flow Rate (or Throughput) | Recovery Rate, or Capture Efficiency (η) | Ref |
|---|---|---|---|---|---|---|
| Channel with filters | Hydrodynamic focusing, filtration | PC3 cells in whole bloods. | W = 100, H = 30 Wgap = 4 (filter) | 10 μL/min | 94.5% (PC3 cells) | [14] |
| Trapping reservoir | Inertial/ Trapping by vortices | MCF7 cells; HeLa cells | W = 50 H = 70 m | 7.5 × 106 cells/sec | η = 10% (HeLa) η = 23% (MCF7) | [16] |
| Asymmetric serpentine | Differential inertial focusing | Polystyrene beads (D = 3.1, 9.0) | W = 100~650 H = 50 | ~1.0 mL/min | ~56% (3.1 μm particle, two tiers) | [20] |
| Double spiral | Dean flow | MCF-7, Hela in whole blood | W = 300 H = 50 | 3.33 × 107 cells/min | 88.5% | [22] |
| Slanted spiral | Dean flow | T24, MCF-7, MDA-MB-231 in blood | W = 600; H = 80 /130 (inner/outer) | 1700 μL/min. | η = 80% (T24) 85% (MCF-7) 87% (MDA-MB-231). | [23] |
| Symmetrical serpentine | Inertial | polystyrene beads (D = 3, 10; 5, 13) | W = 200 H = 42 | 600 μL/min | >97% (large particles); >92% (small particles) | [31] |
| Serpentine with Suction channel | Inertial, Dean flow and suction | RBC, WBC (D = 8, 15). | W = 200 H = 58 | 1 × 108 cells/sec, or 250 μL/sec | 93.2% | Our Work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombodorj, B.; Tseng, H.C.; Chang, H.-Y.; Lu, Y.-W.; Tumurpurev, N.; Lee, C.-W.; Ganbat, B.; Wu, R.-G.; Tseng, F.-G. High-Throughput White Blood Cell (Leukocyte) Enrichment from Whole Blood Using Hydrodynamic and Inertial Forces. Micromachines 2020, 11, 275. https://doi.org/10.3390/mi11030275
Lombodorj B, Tseng HC, Chang H-Y, Lu Y-W, Tumurpurev N, Lee C-W, Ganbat B, Wu R-G, Tseng F-G. High-Throughput White Blood Cell (Leukocyte) Enrichment from Whole Blood Using Hydrodynamic and Inertial Forces. Micromachines. 2020; 11(3):275. https://doi.org/10.3390/mi11030275
Chicago/Turabian StyleLombodorj, Batzorig, Horas Cendana Tseng, Hwan-You Chang, Yen-Wen Lu, Namnan Tumurpurev, Chun-Wei Lee, Batdemberel Ganbat, Ren-Guei Wu, and Fan-Gang Tseng. 2020. "High-Throughput White Blood Cell (Leukocyte) Enrichment from Whole Blood Using Hydrodynamic and Inertial Forces" Micromachines 11, no. 3: 275. https://doi.org/10.3390/mi11030275
APA StyleLombodorj, B., Tseng, H. C., Chang, H.-Y., Lu, Y.-W., Tumurpurev, N., Lee, C.-W., Ganbat, B., Wu, R.-G., & Tseng, F.-G. (2020). High-Throughput White Blood Cell (Leukocyte) Enrichment from Whole Blood Using Hydrodynamic and Inertial Forces. Micromachines, 11(3), 275. https://doi.org/10.3390/mi11030275







