Surface Modification of Silicon Nanowire Based Field Effect Transistors with Stimuli Responsive Polymer Brushes for Biosensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Silicon Nanowire Field Effect Transistors (SiNW-FETs)
2.3. Electrical Measurements
2.4. Real-Time Measurementsof SiNW FETs for Biosensing Applications
2.5. Preparation of Polymer Brushes
2.6. Surface Characterization
2.7. Cell Culture
3. Results
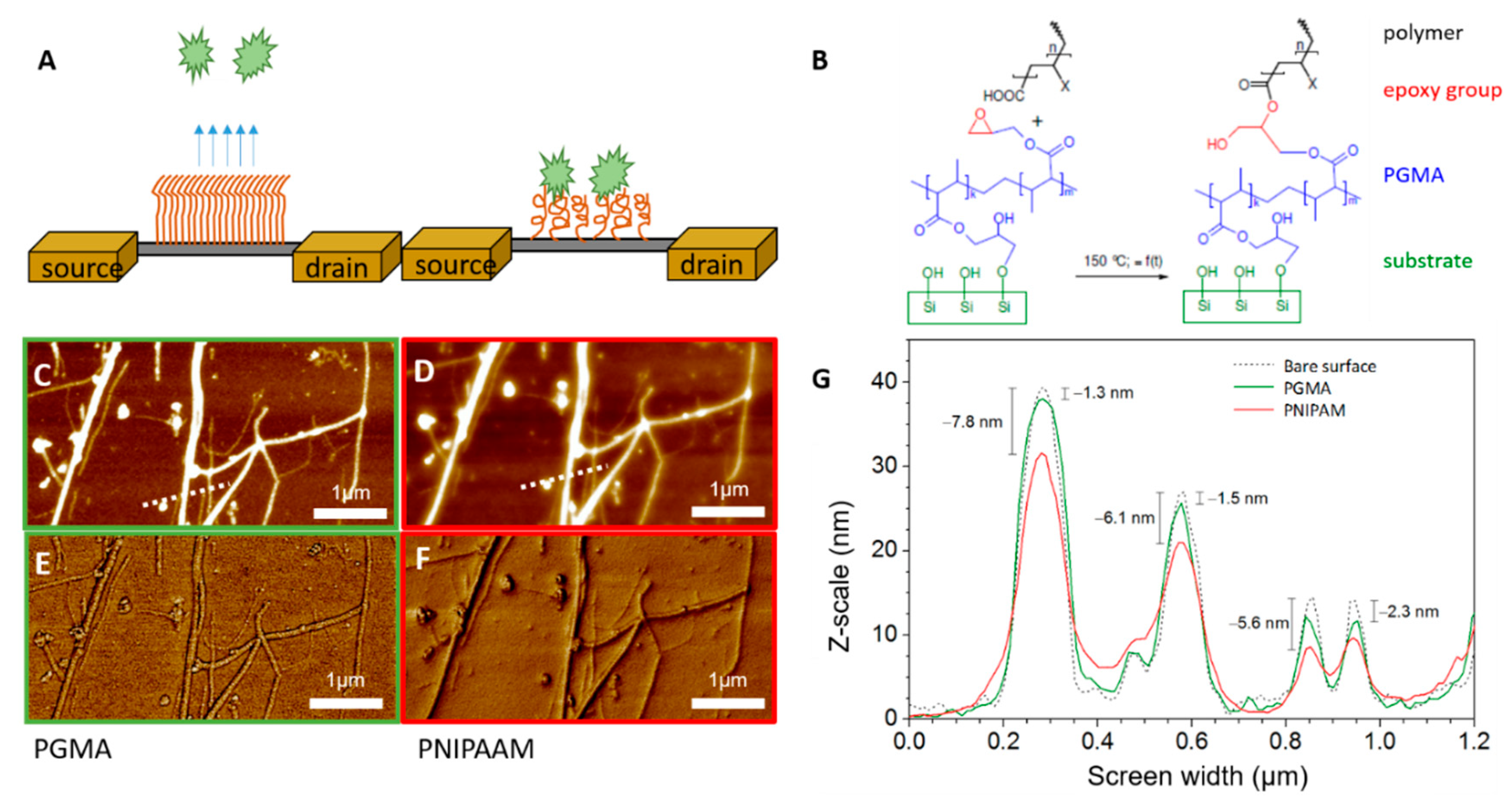
3.1. Sensor Fabrication and Characterization
3.2. Sensor Functionalization with Stimuli-Responsive Polymers
3.3. Phase Transition of Polymer Brushes
3.4. Real-Time Measurements of Stimuli-Responsive Polymer-Modifed SiNW-FETs
3.5. Cell Adsorption on Polymer Brushes
3.6. Electrical Detection of Saos-2 Cells
4. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12, 12–20. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X. Carbon nanomaterials field-effect-transistor-based biosensors. NPG Asia Mater. 2012, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Leyden, M.R.; Messinger, R.J.; Schuman, C.; Sharf, T.; Remcho, V.T.; Squires, T.M.; Minot, E.D. Increasing the detection speed of an all-electronic real-time biosensor. Lab Chip 2012, 12, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Schütt, J.; Ibarlucea, B.; Illing, R.; Zörgiebel, F.M.; Pregl, S.; Nozaki, D.; Weber, W.M.; Mikolajick, T.; Baraban, L.; Cuniberti, G. Compact Nanowire Sensors Probe Microdroplets. Nano Lett. 2016, 16, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Nanowire Field Effect Transistors: Principles and Applications; Kim, D.M.; Jeong, Y.-H. (Eds.) Springer-Verlag: New York, NY, USA, 2014; ISBN 978-1-4614-8123-2. [Google Scholar]
- Li, B.-R.; Chen, C.-W.; Yang, W.-L.; Lin, T.-Y.; Pan, C.-Y.; Chen, Y.-T. Biomolecular recognition with a sensitivity-enhanced nanowire transistor biosensor. Biosens. Bioelectron. 2013, 45, 252–259. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—Field-Effect Transistor Biosensing: Devices and Clinical Applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Monošík, R.; Stred’anský, M.; Šturdík, E. Application of Electrochemical Biosensors in Clinical Diagnosis. J. Clin. Lab. Anal. 2012, 26, 22–34. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irrera, A.; Priolo, F. Ultrasensitive Label- and PCR-Free Genome Detection Based on Cooperative Hybridization of Silicon Nanowires Optical Biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [Green Version]
- Baek, E.; Pregl, S.; Shaygan, M.; Römhildt, L.; Weber, W.M.; Mikolajick, T.; Ryndyk, D.A.; Baraban, L.; Cuniberti, G. Optoelectronic switching of nanowire-based hybrid organic/oxide/semiconductor field-effect transistors. Nano Res. 2015, 8, 1229–1240. [Google Scholar] [CrossRef]
- Tran, D.P.; Pham, T.T.T.; Wolfrum, B.; Offenhäusser, A.; Thierry, B. CMOS-Compatible Silicon Nanowire Field-Effect Transistor Biosensor: Technology Development toward Commercialization. Materials 2018, 11, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, D.-Y.; Pregl, S.; Park, S.J.; Baraban, L.; Cuniberti, G.; Mikolajick, T.; Weber, W.M. Scaling and Graphical Transport-Map Analysis of Ambipolar Schottky-Barrier Thin-Film Transistors Based on a Parallel Array of Si Nanowires. Nano Lett. 2015, 15, 4578–4584. [Google Scholar] [CrossRef] [PubMed]
- Panes-Ruiz, L.A.; Shaygan, M.; Fu, Y.; Liu, Y.; Khavrus, V.; Oswald, S.; Gemming, T.; Baraban, L.; Bezugly, V.; Cuniberti, G. Toward Highly Sensitive and Energy Efficient Ammonia Gas Detection with Modified Single-Walled Carbon Nanotubes at Room Temperature. ACS Sens. 2018, 3, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS₂ field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors - Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Raymo, F.M.; Yildiz, I. Luminescent chemosensors based on semiconductor quantum dots. Phys. Chem. Chem. Phys. 2007, 9, 2036–2043. [Google Scholar] [CrossRef]
- Leyden, M.R.; Schuman, C.; Sharf, T.; Kevek, J.; Remcho, V.T.; Minot, E.D. Fabrication and characterization of carbon nanotube field-effect transistor biosensors. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7779, 77790. [Google Scholar]
- Maehashi, K.; Matsumoto, K. Label-Free Electrical Detection Using Carbon Nanotube-Based Biosensors. Sensors 2009, 9, 5368–5378. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Karni, T.; Qing, Q.; Li, Q.; Fang, Y.; Lieber, C.M. Graphene and Nanowire Transistors for Cellular Interfaces and Electrical Recording. Nano Lett. 2010, 10, 1098–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarlucea, B.; Rim, T.; Baek, C.K.; de Visser, J.A.G.M.; Baraban, L.; Cuniberti, G. Nanowire sensors monitor bacterial growth kinetics and response to antibiotics. Lab Chip 2017, 17, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
- Ibarlucea, B.; Römhildt, L.; Zörgiebel, F.; Pregl, S.; Vahdatzadeh, M.; Weber, W.M.; Mikolajick, T.; Opitz, J.; Baraban, L.; Cuniberti, G. Gating Hysteresis as an Indicator for Silicon Nanowire FET Biosensors. Appl. Sci. 2018, 8, 950. [Google Scholar] [CrossRef] [Green Version]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst Limit in Dual-Gated Si-Nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.; Rastogi, A.; Ober, C. Polymer brushes for electrochemical biosensors. Soft Matter 2011, 7, 297–302. [Google Scholar] [CrossRef]
- Kuroki, H.; Tokarev, I.; Minko, S. Responsive Surfaces for Life Science Applications. Annu. Rev. Mater. Res. 2012, 42, 343–372. [Google Scholar] [CrossRef]
- Chen, T.; Ferris, R.; Zhang, J.; Ducker, R.; Zauscher, S. Stimulus-responsive polymer brushes on surfaces: Transduction mechanisms and applications. Prog. Polym. Sci. 2010, 35, 94–112. [Google Scholar] [CrossRef]
- Cole, M.A.; Voelcker, N.H.; Thissen, H.; Griesser, H.J. Stimuli-responsive interfaces and systems for the control of protein-surface and cell-surface interactions. Biomaterials 2009, 30, 1827–1850. [Google Scholar] [CrossRef]
- Curreli, M.; Zhang, R.; Ishikawa, F.N.; Chang, H.-K.; Cote, R.J.; Zhou, C.; Thompson, M.E. Real-Time, Label-Free Detection of Biological Entities Using Nanowire-Based FETs. IEEE Trans. Nanotechnol. 2008, 7, 651–667. [Google Scholar] [CrossRef]
- Todd, S.J.; Scurr, D.J.; Gough, J.E.; Alexander, M.R.; Ulijn, R.V. Enzyme-Activated RGD Ligands on Functionalized Poly(ethylene glycol) Monolayers: Surface Analysis and Cellular Response. Langmuir 2009, 25, 7533–7539. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, I.; Minko, S.; Fendler, J.H.; Hutter, E. Nanosensors based on responsive polymer brushes and gold nanoparticle enhanced transmission surface plasmon resonance spectroscopy. J. Am. Chem. Soc. 2004, 126, 15950–15951. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Sheparovych, R.; Katz, E.; Minko, S. Chemical Gating with Nanostructured Responsive Polymer Brushes: Mixed Brush versus Homopolymer Brush. ACS Nano 2008, 2, 41–52. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Lee, H.-H.; Liedberg, B.; Aastrup, T.; Nicholls, I.A. Protein-resistant hyperbranched polyethyleneimine brush surfaces. J. Colloid Interface Sci. 2013, 396, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, I.; Tokareva, I.; Minko, S. Optical nanosensor platform operating in near-physiological pH range via polymer-brush-mediated plasmon coupling. ACS Appl. Mater. Interfaces 2011, 3, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef]
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, S.; Nakamura, M.; Park, J.-W.; Aizawa, H.; Yamada, K.; Hirata, M. Evaluation of a high-affinity QCM immunosensor using antibody fragmentation and 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer. Biosens. Bioelectron. 2004, 20, 1134–1139. [Google Scholar] [CrossRef]
- Chen, T.; Chang, D.P.; Liu, T.; Desikan, R.; Datar, R.; Thundat, T.; Berger, R.; Zauscher, S. Glucose-responsive polymer brushes for microcantilever sensing. J. Mater. Chem. 2010, 20, 3391–3395. [Google Scholar] [CrossRef] [Green Version]
- Tam, T.K.; Ornatska, M.; Pita, M.; Minko, S.; Katz, E. Polymer Brush-Modified Electrode with Switchable and Tunable Redox Activity for Bioelectronic Applications. J. Phys. Chem. C 2008, 112, 8438–8445. [Google Scholar] [CrossRef]
- Amir, L.; Tam, T.K.; Pita, M.; Meijler, M.M.; Alfonta, L.; Katz, E. Biofuel Cell Controlled by Enzyme Logic Systems. J. Am. Chem. Soc. 2009, 131, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lu, X.; Hu, J.; Li, J. Reversible Immobilization and Direct Electron Transfer of Cytochrome c on a pH-Sensitive Polymer Interface. Chem. A Eur. J. 2007, 13, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, S.H.; Kim, E.; Kim, J.-D.; An, S.-Y.; Lim, H.S.; Lee, H.H.; Kim, D.H.; Ryu, D.Y.; Lee, D.R.; et al. Polymer Brush As a Facile Dielectric Surface Treatment for High-Performance, Stable, Soluble Acene-Based Transistors. Chem. Mater. 2010, 22, 5377–5382. [Google Scholar] [CrossRef]
- Hee Park, S.; Sung Lee, H.; Kim, J.-D.W.; Breiby, D.; Kim, E.; Don Park, Y.; Yeol Ryu, D.; Ryeol Lee, D.; Ho Cho, J. A polymer brush organic interlayer improves the overlying pentacene nanostructure and organic field-effect transistor performance. J. Mater. Chem. 2011, 21, 15580–15586. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, J.H.; Ahn, J.; Lim, J.Y.; Kim, E.; Im, S. 2D MoSe2 Transistor with Polymer-Brush/Channel Interface. Adv. Mater. Interfaces 2018, 5, 1800812. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Giussi, J.M.; Knoll, W.; Azzaroni, O. Reversible Switching of the Dirac Point in Graphene Field-Effect Transistors Functionalized with Responsive Polymer Brushes. Langmuir 2019, 35, 8038–8044. [Google Scholar] [CrossRef]
- Liu, S.; Jamali, S.; Liu, Q.; Maia, J.; Baek, J.-B.; Jiang, N.; Xu, M.; Dai, L. Conformational Transitions of Polymer Brushes for Reversibly Switching Graphene Transistors. Macromolecules 2016, 49. [Google Scholar] [CrossRef]
- Joh, D.Y.; McGuire, F.; Abedini-Nassab, R.; Andrews, J.B.; Achar, R.K.; Zimmers, Z.; Mozhdehi, D.; Blair, R.; Albarghouthi, F.; Oles, W.; et al. Poly(oligo(ethylene glycol) methyl ether methacrylate) Brushes on High-κ Metal Oxide Dielectric Surfaces for Bioelectrical Environments. ACS Appl. Mater. Interfaces 2017, 9, 5522–5529. [Google Scholar] [CrossRef]
- Hwang, D.-H.; Nomura, A.; Kim, J.; Kim, J.-H.; Cho, H.; Lee, C.; Ohno, K.; Tsujii, Y. Synthesis and characterization of polystyrene brushes for organic thin film transistors. J. Nanosci. Nanotechnol. 2012, 12, 4137–4141. [Google Scholar] [CrossRef]
- Qu, Z.; Xu, H.; Gu, H. Synthesis and Biomedical Applications of Poly((meth)acrylic acid) Brushes. ACS Appl. Mater. Interfaces 2015, 7, 14537–14551. [Google Scholar] [CrossRef]
- Ku, G.M.; Kim, J.W.; Jang, Y.; Kim, S.; Lim, K.; Lee, W.H. Interfacial Polymer Brush Layer for DNA Sensors Based on Graphene Transistors. Fibers Polym. 2018, 19, 2483–2488. [Google Scholar] [CrossRef]
- Koenig, M.; Bittrich, E.; König, U.; Rajeev, B.L.; Müller, M.; Eichhorn, K.-J.; Thomas, S.; Stamm, M.; Uhlmann, P. Adsorption of enzymes to stimuli-responsive polymer brushes: Influence of brush conformation on adsorbed amount and biocatalytic activity. Colloids Surf. B Biointerfaces 2016, 146, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Ferrand-Drake del Castillo, G.; Koenig, M.; Müller, M.; Eichhorn, K.-J.; Stamm, M.; Uhlmann, P.; Dahlin, A. Enzyme Immobilization in Polyelectrolyte Brushes: High Loading and Enhanced Activity Compared to Monolayers. Langmuir 2019, 35, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Huet, G.L.; Conard, T.; Demoustier-Champagne, S.; Du Prez, F.E.; Landoulsi, J.; Dupont-Gillain, C.C. Design of Mixed PEO/PAA Brushes with Switchable Properties Toward Protein Adsorption. Biomacromolecules 2013, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Nakayama, Y.; Matsuda, T. In vivo evaluation of poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as an in situ-formable scaffold. J. Artif. Organs 2005, 7, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shen, L.; Wu, C. LLS and FTIR Studies on the Hysteresis in Association and Dissociation of Poly(N-isopropylacrylamide) Chains in Water. Macromolecules 2006, 39, 2325–2329. [Google Scholar] [CrossRef]
- Pregl, S.; Weber, W.M.; Nozaki, D.; Kunstmann, J.; Baraban, L.; Opitz, J.; Mikolajick, T.; Cuniberti, G. Parallel arrays of Schottky barrier nanowire field effect transistors: Nanoscopic effects for macroscopic current output. Nano Res. 2013, 6, 381–388. [Google Scholar] [CrossRef]
- Fan, Z.; Ho, J.C.; Jacobson, Z.A.; Yerushalmi, R.; Alley, R.L.; Razavi, H.; Javey, A. Wafer-Scale Assembly of Highly Ordered Semiconductor Nanowire Arrays by Contact Printing. Nano Lett. 2008, 8, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cui, Y.; Huynh, L.; Barrelet, C.J.; Bell, D.C.; Lieber, C.M. Controlled Growth and Structures of Molecular-Scale Silicon Nanowires. Nano Lett. 2004, 4, 433–436. [Google Scholar] [CrossRef]
- Skucha, K.; Fan, Z.; Jeon, K.; Javey, A.; Boser, B. Palladium/silicon nanowire Schottky barrier-based hydrogen sensors. Sens. Actuators B Chem. 2010, 145, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold Regime has the Optimal Sensitivity for Nanowire FET Biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, M.; Ohmi, T.; Hasegawa, E.; Kawakami, M.; Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 1990, 68, 1272–1281. [Google Scholar] [CrossRef]
- Bittrich, E.; Kuntzsch, M.; Eichhorn, K.-J.; Uhlmann, P. Complex pH- and temperature-sensitive swelling behavior of mixed polymer brushes. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1606–1615. [Google Scholar] [CrossRef]
- Zörgiebel, F.M.; Pregl, S.; Römhildt, L.; Opitz, J.; Weber, W.; Mikolajick, T.; Baraban, L.; Cuniberti, G. Schottky barrier-based silicon nanowire pH sensor with live sensitivity control. Nano Res. 2014, 7, 263–271. [Google Scholar] [CrossRef]
- Gong, P.; Wu, T.; Genzer, J.; Szleifer, I. Behavior of Surface-Anchored Poly(acrylic acid) Brushes with Grafting Density Gradients on Solid Substrates: 2. Theory. Macromolecules 2007, 40, 8765–8773. [Google Scholar] [CrossRef]
- Dong, R.; Lindau, M.; Ober, C.K. Dissociation behavior of weak polyelectrolyte brushes on a planar surface. Langmuir ACS J. Surf. Colloids 2009, 25, 4774–4779. [Google Scholar] [CrossRef]
- Uhlík, F.; Limpouchová, Z.; Jelínek, K.; Procházka, K. Polyelectrolyte shells of copolymer micelles in aqueous solutions: A Monte Carlo study. J. Chem. Phys. 2004, 121, 2367–2375. [Google Scholar] [CrossRef]
- Ren, Y.; Lam, D.C.C. Properties and Microstructures of Low-Temperature-Processable Ultralow-Dielectric Porous Polyimide Films. J. Electron. Mater. 2008, 37, 955–961. [Google Scholar] [CrossRef]
- Inoue, H.; Kuwahara, S.; Katayama, K. The whole process of phase transition and relaxation of poly(N-isopropylacrylamide) aqueous solution. Phys. Chem. Chem. Phys. PCCP 2013, 15. [Google Scholar] [CrossRef]
- Matsubara, T.; DiResta, G.R.; Kakunaga, S.; Li, D.; Healey, J.H. Additive Influence of Extracellular pH, Oxygen Tension, and Pressure on Invasiveness and Survival of Human Osteosarcoma Cells. Front. Oncol. 2013, 3, 199. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, R.A. Cell acidification in apoptosis. Apoptosis 1996, 1, 40–48. [Google Scholar] [CrossRef]
- Taylor, A.C. Responses of cells to pH changes in the mesium. J. Cell Biol. 1962, 15, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaatinen, L.; Young, E.; Hyttinen, J.; Vörös, J.; Zambelli, T.; Demkó, L. Quantifying the effect of electric current on cell adhesion studied by single-cell force spectroscopy. Biointerphases 2016, 11, 011004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, N.C.; Ricci, J.; Breger, L.; Zychlinsky, A.; Solomon, H.; Chen, G.-G.; Kuznetsov, D.; Dorfman, R. Effects of low-intensity AC and/or DC electromagnetic fields on cell attachment and induction of apoptosis. Bioelectromagnetics 1997, 18, 264–272. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinghammer, S.; Rauch, S.; Pregl, S.; Uhlmann, P.; Baraban, L.; Cuniberti, G. Surface Modification of Silicon Nanowire Based Field Effect Transistors with Stimuli Responsive Polymer Brushes for Biosensing Applications. Micromachines 2020, 11, 274. https://doi.org/10.3390/mi11030274
Klinghammer S, Rauch S, Pregl S, Uhlmann P, Baraban L, Cuniberti G. Surface Modification of Silicon Nanowire Based Field Effect Transistors with Stimuli Responsive Polymer Brushes for Biosensing Applications. Micromachines. 2020; 11(3):274. https://doi.org/10.3390/mi11030274
Chicago/Turabian StyleKlinghammer, Stephanie, Sebastian Rauch, Sebastian Pregl, Petra Uhlmann, Larysa Baraban, and Gianaurelio Cuniberti. 2020. "Surface Modification of Silicon Nanowire Based Field Effect Transistors with Stimuli Responsive Polymer Brushes for Biosensing Applications" Micromachines 11, no. 3: 274. https://doi.org/10.3390/mi11030274
APA StyleKlinghammer, S., Rauch, S., Pregl, S., Uhlmann, P., Baraban, L., & Cuniberti, G. (2020). Surface Modification of Silicon Nanowire Based Field Effect Transistors with Stimuli Responsive Polymer Brushes for Biosensing Applications. Micromachines, 11(3), 274. https://doi.org/10.3390/mi11030274





