The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review
Abstract
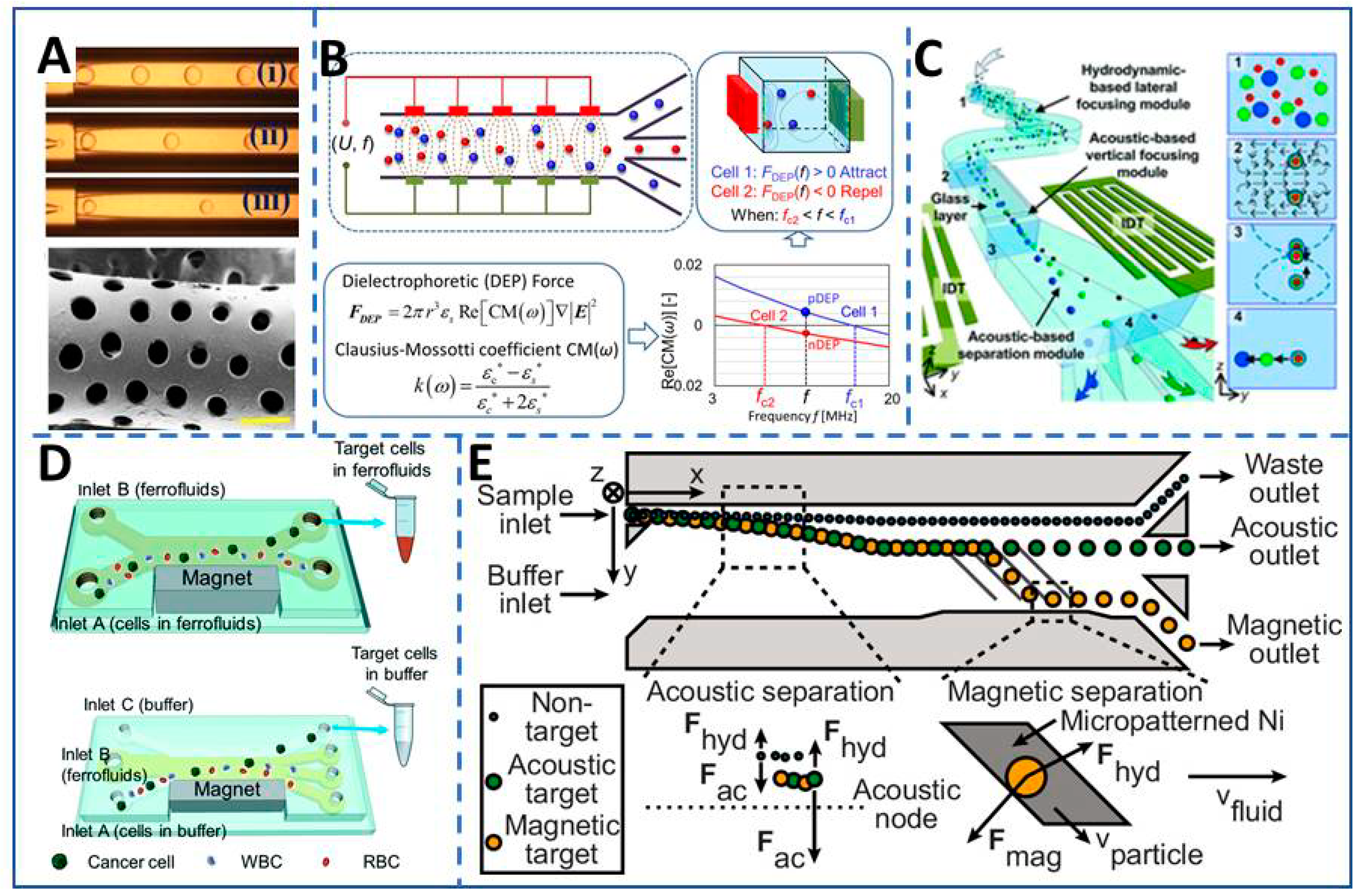
:1. Introduction
2. The Fabrication of High-Throughput Biomedical Microfluidic Systems (HTBMS)
2.1. The Scaffold Materials and Manufacturing Methods for HTBMS
2.2. The Construction of 3D Microenvironment in HTBMS
2.2.1. The Microenvironment Construction of 3D HTBMS for Cell Culture
2.2.2. The Microenvironment Construction of 3D HTBMS for Organ-on-a-Chip
3. The Application and Mechanism of HTBMS
3.1. Biomedical Synthesis and Separation in HTMS without Matrix Environment
3.1.1. Biomedical Synthesis and Detection
3.1.2. Biomedical Sorting and Diagnostics
3.2. The Application of Droplet and Cell/Organ-Based Drug Screening of HTBMS with Matrix Environment
3.2.1. Hydrogel Droplet for Biomedical Cell Encapsulation and Analysis
3.2.2. Cell/Organ Based Drug Screening
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Atiyat, S.A.; Karabsheh, S.M. Liver-on-a-Chip for Evaluating Hepatic Activation of Clopidogrel in Patients with Coronary Stents. In Proceedings of the 2017 13th Iasted International Conference on Biomedical Engineering (Biomed), Innsbruck, Austria, 20–21 February 2017. [Google Scholar]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8. [Google Scholar] [CrossRef]
- Decsi, B.; Krammer, R.; Hegedus, K.; Ender, F.; Gyarmati, B.; Szilagyi, A.; Totos, R.; Katona, G.; Paizs, C.; Balogh, G.T.; et al. Liver-on-a-ChipMagnetic Nanoparticle Bound Synthetic Metalloporphyrin-Catalyzed Biomimetic Oxidation of a Drug in a Magnechip Reactor. Micromachines 2019, 10, 668. [Google Scholar] [CrossRef] [Green Version]
- Kizilkurtlu, A.A.; Polat, T.; Aydin, G.B.; Akpek, A. Lung on a Chip for Drug Screening and Design. Curr. Pharm. Des. 2018, 24, 5386–5396. [Google Scholar] [CrossRef]
- Stucki, A.O.; Raggi, G.; Sigrist, S.; Zamprogno, P.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Stucki, J.D.; Hobi, N.; Guenat, O.T. Lung-on-a-Chip: The interplay of primary human epithelial and endothelial cells improves the alveolar barrier function. Toxicol. Lett. 2018, 295, S67–S68. [Google Scholar] [CrossRef]
- Zamprogno, P.; Achenbach, S.; Stucki, J.; Hobi, N.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Schmid, R.; Guenat, O. Lung alveoli array-on-chip with a bioartificial membrane. Toxicol. Lett. 2017, 280, S277. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Skelton, J.K.; Cherry, C.; Briones-Orta, M.A.; Hateley, C.A.; Dorner, M. “Liver-on-a-Chip” Cultures of Primary Hepatocytes and Kupffer Cells for Hepatitis B Virus Infection. JoVE-J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [Green Version]
- Frega, M.; Mossink, B.; Linda, K.; Keller, J.; Schubert, D.; Kasri, N.N. Brain-on-a chip technologies for investigating neuronal diseases: Toward precision medicine applications. In Proceedings of the 2018 Ieee International Symposium on Circuits and Systems (Iscas), Florence, Italy, 27–30 May 2018. [Google Scholar]
- Kilic, O.; Pamies, D.; Lavell, E.; Schiapparelli, P.; Feng, Y.; Hartung, T.; Bal-Price, A.; Hogberg, H.T.; Quinones-Hinojosa, A.; Guerrero-Cazares, H.; et al. Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip 2016, 16, 4152–4162. [Google Scholar] [CrossRef] [Green Version]
- Kozicz, T.; Klein-Gunnewiek, T.; Cassiman, D.; Nelson, T.; Rodenburg, R.; Perales-Clemente, E.; Morava, E.; Kasri, N.N. Brain-on-a-chip—A neurophysiological model of MELAS disease and comorbid psychopathology. Eur. J. Hum. Genet. 2019, 27, 1161–1162. [Google Scholar]
- Lassus, B.; Naude, N.; Faure, F.; la Cour, M.M.; Millan, M.; Peyrin, J. A “Brain-on-a-Chip” approach to characterizing glutamatergic and dopaminergic modulation of cortico-striatal networks. Eur. Neuropsychopharm 2017, 27, S537. [Google Scholar] [CrossRef]
- Miccoli, B.; Braeken, D.; Li, Y.C.E. Brain-on-a-chip Devices for Drug Screening and Disease Modeling Applications. Curr. Pharm. Des. 2018, 24, 5419–5436. [Google Scholar] [CrossRef]
- Shahdoost, S.; Frost, S.; Guggenmos, D.J.; Borrell, J.A.; Dunham, C.; Barbay, S.; Nudo, R.J.; Mohseni, P. A brain-spinal interface (BSI) system-on-chip (SoC) for closed-loop cortically-controlled intraspinal microstimulation. Analog Integr. Circuits Signal Process. 2018, 95, 1–16. [Google Scholar] [CrossRef]
- Aslan, M.K.; Yalcin, Y.D.; Ozgur, E.; Gunduz, U.; Eminoglu, S.; Kulah, H.; Akin, T. A High Throughput Lab-On-A-Chip System for Label Free Quantification of Breast Cancer Cells under Continuous Flow. Procedia Technol. 2017, 27, 59–61. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gao, D.; Wang, Y.W.; Lin, S.; Jiang, Y.Y. A novel 3D breast-cancer-on-chip platform for therapeutic evaluation of drug delivery systems. Anal. Chim. Acta 2018, 1036, 97–106. [Google Scholar] [CrossRef]
- Gioiella, F.; Urciuolo, F.; Imparato, G.; Brancato, V.; Netti, P.A. An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression. Adv. Healthc. Mater. 2016, 5, 3074–3084. [Google Scholar] [CrossRef]
- Grafton, M.M.G.; Wang, L.; Vidi, P.A.; Leary, J.; Lelievre, S.A. Breast on-a-chip: Mimicry of the channeling system of the breast for development of theranostics. Integr. Biol. 2011, 3, 451–459. [Google Scholar] [CrossRef]
- Wan, L.; LeDuc, P.R.; Neumann, C. 3D Vasculature Structure in Breast Cancer on a Chip Approaches through Micromilling. FASEB J. 2017, 31, 942. [Google Scholar]
- Anonymous. Human-on-a-chip with Barrier Organ Model. Atla-Altern. Lab. Anim. 2015, 43, 299. [Google Scholar]
- Sporn, M.B. The war on cancer. Lancet 1996, 347, 1377–1381. [Google Scholar] [CrossRef]
- Domachuk, P.; Tsioris, K.; Omenetto, F.G.; Kaplan, D.L. Bio-microfluidics: Biomaterials and Biomimetic Designs. Adv. Mater. 2010, 22, 249–260. [Google Scholar] [CrossRef]
- Han, J.P.; Sun, J.; Wang, L.; Liu, P.; Zhuang, B.; Zhao, L.; Liu, Y.; Li, C.X. The Optimization of Electrophoresis on a Glass Microfluidic Chip and its Application in Forensic Science. J. Forensic Sci. 2017, 62, 1603–1612. [Google Scholar] [CrossRef]
- Alrifaiy, A.; Lindahl, O.A.; Ramser, K. Polymer-Based Microfluidic Devices for Pharmacy, Biology and Tissue Engineering. Polymers 2012, 4, 1349–1398. [Google Scholar] [CrossRef]
- Bélanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef]
- Giridharan, G.A.; Nguyen, M.D.; Estrada, R.; Parichehreh, V.; Hamid, T.; Ismahil, M.A.; Prabhu, S.D.; Sethu, P. Microfluidic Cardiac Cell Culture Model (mu CCCM). Anal. Chem. 2010, 82, 7581–7587. [Google Scholar] [CrossRef]
- Thompson, A.J.; Ma, L.J.; Plegue, T.J.; Potkay, J.A. Design Analysis and Optimization of a Single-Layer PDMS Microfluidic Artificial Lung. IEEE Trans. Biomed. Eng. 2019, 66, 1082–1093. [Google Scholar] [CrossRef]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. An ultra-thin, all PDMS-based microfluidic lung assist device with high oxygenation capacity. Biomicrofluidics 2019, 13. [Google Scholar] [CrossRef]
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Fu, H.Y.; Li, Y.P.; Chen, X.M.; Cheuk, W.; Li, C.W.; Zou, H.; Yue, W.Q.; Au, S.K.; Wang, Y.; et al. Single cell target gene mutation analysis by arc-edge-channel monolithic valve microfluidic cell isolation and locked nucleic acid-based PCR detection. Sens. Actuators B Chem. 2019, 293, 224–234. [Google Scholar] [CrossRef]
- Liu, Y.W.; Hu, K.; Wang, Y.H. Primary Hepatocytes Cultured on a Fiber-Embedded PDMS Chip to Study Drug Metabolism. Polymers 2017, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Oderinde, O.; Liu, S.; Li, K.; Kang, M.; Imtiaz, H.; Yao, F.; Fu, G. Multifaceted polymeric materials in three-dimensional processing (3DP) technologies: Current progress and prospects. Polym. Adv. Technol. 2018, 29, 1586–1602. [Google Scholar] [CrossRef]
- Ng, J.D.; Clark, P.J.; Stevens, R.C.; Kuhn, P. In situ X-ray analysis of protein crystals in low-birefringent and X-ray transmissive plastic microchannels. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 189–197. [Google Scholar] [CrossRef]
- Guha, S.; Perry, S.L.; Pawate, A.S.; Kenis, P.J.A. Fabrication of X-ray compatible microfluidic platforms for protein crystallization. Sens. Actuators B Chem. 2012, 174, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Maeki, M.; Pawate, A.S.; Yamashita, K.; Kawamoto, M.; Tokeshi, M.; Kenis, P.J.A.; Miyazaki, M. A Method of Cryoprotection for Protein Crystallography by Using a Microfluidic Chip and Its Application for in Situ X-ray Diffraction Measurements. Anal. Chem. 2015, 87, 4194–4200. [Google Scholar] [CrossRef]
- Pawate, A.S.; Srajer, V.; Schieferstein, J.; Guha, S.; Henning, R.; Kosheleva, I.; Schmidt, M.; Ren, Z.; Kenis, P.J.A.; Perry, S.L. Towards time-resolved serial crystallography in a microfluidic device. Acta Crystallogr. F 2015, 71, 823–830. [Google Scholar] [CrossRef]
- Denz, M.; Brehm, G.; Hemonnot, C.Y.J.; Spears, H.; Wittmeier, A.; Cassini, C.; Saldanha, O.; Perego, E.; Diaz, A.; Burghammer, M.; et al. Cyclic olefin copolymer as an X-ray compatible material for microfluidic devices. Lab Chip 2018, 18, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Jellali, R.; Paullier, P.; Fleury, M.J.; Leclerc, E. Liver and kidney cells cultures in a new perfluoropolyether biochip. Sens. Actuators B Chem. 2016, 229, 396–407. [Google Scholar] [CrossRef]
- Soper, S.A.; Hashimoto, M.; Situma, C.; Murphy, M.C.; McCarley, R.L.; Cheng, Y.W.; Barany, F. Fabrication of DNA microarrays onto polymer substrates using UV modification protocols with integration into microfluidic platforms for the sensing of low-abundant DNA point mutations. Methods 2005, 37, 103–113. [Google Scholar] [CrossRef]
- Su, S.S.; Jing, G.S.; Zhang, M.Q.; Liu, B.X.; Zhu, X.R.; Wang, B.; Fu, M.Z.; Zhu, L.X.; Cheng, J.; Guo, Y. One-step bonding and hydrophobic surface modification method for rapid fabrication of polycarbonate-based droplet microfluidic chips. Sens. Actuators B Chem. 2019, 282, 60–68. [Google Scholar] [CrossRef]
- Yeung, C.; Chen, S.; King, B.; Lin, H.S.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.X.; Sun, W.J.; et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 2019, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Fiorini, G.S.; Yim, M.; Jeffries, G.D.; Schiro, P.G.; Mutch, S.A.; Lorenz, R.M.; Chiu, D.T. Fabrication improvements for thermoset polyester (TPE) microfluidic devices. Lab Chip 2007, 7, 923–926. [Google Scholar] [CrossRef]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef]
- Song, K.N.; Wang, Z.R.; Liu, R.C.; Chen, G.; Liu, L.Y. Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases. Int. J. Mol. Sci. 2018, 19, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Choi, J.W.; Yu, J.; Park, D.; Ha, J.; Son, K.; Lee, S.; Chung, M.; Kim, H.Y.; Jeon, N.L. Microfluidics within a well: An injection-molded plastic array 3D culture platform. Lab Chip 2018, 18, 2433–2440. [Google Scholar] [CrossRef]
- Kim, J.A.; Hong, S.; Rhee, W.J. Microfluidic three-dimensional cell culture of stem cells for high-throughput analysis. World J. Stem Cells 2019, 11, 803–816. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Park, D.; Son, K.; Hwang, Y.; Ko, J.; Lee, Y.; Doh, J.; Jeon, N.L. High-Throughput Microfluidic 3D Cytotoxicity Assay for Cancer Immunotherapy (CACI-IMPACT Platform). Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Sung, K.E.; Su, G.; Pehlke, C.; Trier, S.M.; Eliceiri, K.W.; Keely, P.J.; Friedl, A.; Beebe, D.J. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 2009, 30, 4833–4841. [Google Scholar] [CrossRef] [Green Version]
- Lii, J.; Hsu, W.-J.; Parsa, H.; Das, A.; Rouse, R.; Sia, S.K. Real-time microfluidic system for studying mammalian cells in 3D microenvironments. Anal. Chem. 2008, 80, 3640–3647. [Google Scholar] [CrossRef]
- Lanz, H.L.; Saleh, A.; Kramer, B.; Cairns, J.; Ng, C.P.; Yu, J.; Trietsch, S.J.; Hankemeier, T.; Joore, J.; Vulto, P.; et al. Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform. BMC Cancer 2017, 17. [Google Scholar] [CrossRef] [Green Version]
- Beachley, V.Z.; Wolf, M.T.; Sadtler, K.; Manda, S.S.; Jacobs, H.; Blatchley, M.R.; Bader, J.S.; Pandey, A.; Pardoll, D.; Elisseeff, J.H. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods 2015, 12, 1197. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Soker, S.; Hall, A.R. In situ patterned micro 3D liver constructs for parallel toxicology testing in a fluidic device. Biofabrication 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Karamikamkar, S.; Behzadfar, E.; Cheung, K.C. A novel approach to producing uniform 3-D tumor spheroid constructs using ultrasound treatment. Biomed. Microdevices 2018, 20. [Google Scholar] [CrossRef]
- Alessandri, K.; Feyeux, M.; Gurchenkov, B.; Delgado, C.; Trushko, A.; Krause, K.-H.; Vignjevic, D.; Nassoy, P.; Roux, A. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 2016, 16, 1593–1604. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.W. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019, 11, 12. [Google Scholar] [CrossRef]
- Hegde, M.; Jindal, R.; Bhushan, A.; Bale, S.S.; McCarty, W.J.; Golberg, I.; Usta, O.B.; Yarmush, M.L. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip 2014, 14, 2033–2039. [Google Scholar] [CrossRef]
- Humayun, M.; Chow, C.-W.; Young, E.W.K. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef]
- Fan, Q.H.; Liu, R.C.; Jiao, Y.; Tian, C.X.; Farrell, J.D.; Diao, W.W.; Wang, X.C.; Zhang, F.R.; Yuan, W.; Han, H.B.; et al. A novel 3-D bio-microfluidic system mimicking in vivo heterogeneous tumour microstructures reveals complex tumour-stroma interactions. Lab Chip 2017, 17, 2852–2860. [Google Scholar] [CrossRef]
- Du, X.; Li, W.; Du, G.; Cho, H.; Yu, M.; Fang, Q.; Lee, L.P.; Fang, J. Droplet Array-Based 3D Coculture System for High-Throughput Tumor Angiogenesis Assay. Anal. Chem. 2018, 90, 3253–3261. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef]
- Hassan, N.; Oyarzun-Ampuero, F.; Lara, P.; Guerrero, S.; Cabuil, V.; Abou-Hassan, A.; Kogan, M.J. Flow Chemistry to Control the Synthesis of Nano and Microparticles for Biomedical Applications. Curr. Top. Med. Chem. 2014, 14, 676–689. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Chen, K.-J.; Lu, Y.; Wang, S.; Lin, W.-Y.; Guo, F.; Kamei, K.-I.; Chen, Y.-C.; Ohashi, M.; et al. A Rapid Pathway Toward a Superb Gene Delivery System: Programming Structural and Functional Diversity into a Supramolecular Nanoparticle Library. ACS Nano 2010, 4, 6235–6243. [Google Scholar] [CrossRef]
- Ran, R.; Middelberg, A.P.J.; Zhao, C.X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic Platform for Combinatorial Synthesis and Optimization of Targeted Nanoparticles for Cancer Therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.S.; Chuang, F.C.; Chen, C.K.; Lee, M.H.; Chen, S.W.; Su, T.L.; Yang, Y.C. A high-performance micromixer using three-dimensional Tesla structures for bio-applications. Chem. Eng. J. 2015, 263, 444–451. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 17. [Google Scholar] [CrossRef]
- Fernandez-Carballo, B.L.; McBeth, C.; McGuiness, I.; Kalashnikov, M.; Baum, C.; Borros, S.; Sharon, A.; Sauer-Budge, A.F. Continuous-flow, microfluidic, qRT-PCR system for RNA virus detection. Anal. Bioanal. Chem. 2018, 410, 33–43. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.-C.; Choi, J.-W.; Ahn, C.H. A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab Chip 2004, 4, 109–113. [Google Scholar] [CrossRef]
- Duarte-Guevara, P.; Duarte-Guevara, C.; Ornob, A.; Bashir, R. On-chip PMA labeling of foodborne pathogenic bacteria for viable qPCR and qLAMP detection. Microfluid. Nanofluid. 2016, 20, 9. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Streck, S.; Clulow, A.J.; Nielsen, H.M.; Rades, T.; Boyd, B.; McDowell, A. The distribution of cell-penetrating peptides on polymeric nanoparticles prepared using microfluidics and elucidated with small angle X-ray scattering. J. Colloid Interface Sci. 2019, 555, 438–448. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Maerkl, S.J. A 1024-sample serum analyzer chip for cancer diagnostics. Lab Chip 2014, 14, 2642–2650. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, J.W. Lab on a Chip for in situ Diagnosis: From Blood to Point of Care. Biomed. Eng. Lett. (BMEL) 2013, 3, 59–66. [Google Scholar] [CrossRef]
- Cai, Y.L.; Wu, F.Y.; Yu, Y.R.; Liu, Y.X.; Shao, C.M.; Gu, H.C.; Li, M.L.; Zhao, Y.J. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019, 84, 222–230. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Lou, C.P.; Gong, H.Q. Fabrication of multi-layer polymeric micro-sieve having narrow slot pores with conventional ultraviolet-lithography and micro-fabrication techniques. Biomicrofluidics 2011, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Warkiani, M.E.; Wu, L.D.; Tay, A.K.P.; Han, J. Large-Volume Microfluidic Cell Sorting for Biomedical Applications. In Annual Review of Biomedical Engineering; Yarmush, M.L., Ed.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 17, pp. 1–34. [Google Scholar]
- Xia, Y.Q.; Wan, Y.; Hao, S.J.; Nisic, M.; Harouaka, R.A.; Chen, Y.Z.; Zou, X.; Zheng, S.Y. Nucleus of Circulating Tumor Cell Determines Its Translocation Through Biomimetic Microconstrictions and Its Physical Enrichment by Microfiltration. Small 2018, 14, 11. [Google Scholar] [CrossRef]
- VanDelinder, V.; Groisman, A. Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Anal. Chem. 2006, 78, 3765–3771. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.C.; Sul, O.; Kim, C.G.; Kim, W.Y.; et al. Continuous Separation of Circulating Tumor Cells from Whole Blood Using a Slanted Weir Microfluidic Device. Cancers 2019, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef]
- Iliescu, F.S.; Sim, W.J.; Heidari, H.; Poenar, D.P.; Miao, J.; Taylor, H.K.; Iliescu, C. Highlighting the uniqueness in dielectrophoretic enrichment of circulating tumor cells. Electrophoresis 2019, 40, 1457–1477. [Google Scholar] [CrossRef]
- Jubery, T.Z.; Srivastava, S.K.; Dutta, P. Dielectrophoretic separation of bioparticles in microdevices: A review. Electrophoresis 2014, 35, 691–713. [Google Scholar] [CrossRef]
- Xavier, M.; Oreffo, R.O.C.; Morgan, H. Skeletal stem cell isolation: A review on the state-of-the-art microfluidic label-free sorting techniques. Biotechnol. Adv. 2016, 34, 908–923. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, G.; Zhao, T.; Takei, M. Microfluidic device embedding electrodes for dielectrophoretic manipulation of cells-A review. Electrophoresis 2019, 40, 1166–1177. [Google Scholar] [CrossRef]
- Park, J.; Komori, T.; Uda, T.; Miyajima, K.; Fujii, T.; Kim, S.H. Sequential Cell-Processing System by Integrating Hydrodynamic Purification and Dielectrophoretic Trapping for Analyses of Suspended Cancer Cells. Micromachines 2019, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Xu, J.; Shamul, J.G.; Lu, X.; Husain, S.; He, X. Creating a capture zone in microfluidic flow greatly enhances the throughput and efficiency of cancer detection. Biomaterials 2019, 197, 161–170. [Google Scholar] [CrossRef]
- Skjeltorp, T.A. One- and Two-Dimensional Crystallization of Magnetic Holes. Phys. Rev. Lett. 1985, 51, 2306–2309. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, R.; Lim, S.H.; Miller, J.R.; Zhang, W.; Tang, W.; Xie, J.; Mao, L. Biocompatible and Label-Free Separation of Cancer Cells of Cell Culture Lines from White Blood Cells in Ferrofluids. Lab Chip 2017, 17, 2243–2255. [Google Scholar] [CrossRef]
- Alnaimat, F.; Dagher, S.; Mathew, B.; Hilal-Alnqbi, A.; Khashan, S. Microfluidics Based Magnetophoresis: A Review. Chem. Rec. 2018, 18, 1596–1612. [Google Scholar] [CrossRef]
- Gourikutty, S.B.N.; Chang, C.P.; Puiu, P.D. Microfluidic immunomagnetic cell separation from whole blood. J. Chromatogr. B 2016, 1011, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Besanjideh, M. Investigation of a Novel Microfluidic Device for Label-Free Ferrohydrodynamic Cell Separation on a Rotating Disk. IEEE Trans. Biomed. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Moore, L.; Xue, W.; Kim, J.; Zborowski, M.; Chalmers, J.J. Correlation of simulation/finite element analysis to the separation of intrinsically magnetic spores and red blood cells using a microfluidic magnetic deposition system. Biotechnol. Bioeng. 2018, 115, 1288–1300. [Google Scholar] [CrossRef]
- Leigh, D.R.; Steinert, S.; Moore, L.R.; Chalmers, J.J.; Zborowski, M. Cell tracking velocimetry as a tool for defining saturation binding of magnetically conjugated antibodies. Cytom. Part A J. Int. Soc. Anal. Cytol. 2005, 66, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Moore, L.R.; Jing, Y.; Haam, S.; Williams, P.S.; Fleischman, A.J.; Roy, S.; Chalmers, J.J.; Zborowski, M. Continuous flow magnetic cell fractionation based on antigen expression level. J. Biochem. Biophys. Methods 2006, 68, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Moore, L.R.; Schneider, T.; Williams, P.S.; Chalmers, J.J.; Farag, S.S.; Bolwell, B.; Zborowski, M. Negative selection of hematopoietic progenitor cells by continuous magnetophoresis. Exp. Hematol. 2007, 35, 662–672. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, K.E.; Comella, K.; Chalmers, J.J.; Margel, S.; Zborowski, M. Mobility measurements of immunomagnetically labeled cells allow quantitation of secondary antibody binding amplification. Biotechnol. Bioeng. 2001, 75, 642–655. [Google Scholar] [CrossRef]
- Gourikutty, S.B.N.; Chia-Pin, C.; Poenar, D.P. An integrated on-chip platform for negative enrichment of tumour cells. J. Chromatogr. B 2016, 1028, 153–164. [Google Scholar] [CrossRef]
- Barani, A.; Paktinat, H.; Janmaleki, M.; Mohammadi, A.; Mosaddegh, P.; Fadaei-Tehrani, A.; Sanati-Nezhad, A. Microfluidic integrated acoustic waving for manipulation of cells and molecules. Biosens. Bioelectron. 2016, 85, 714–725. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.S.; Oh, J.H. Microfluidic Technology for Cell Manipulation. Appl. Sci. 2018, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.F.; Wang, T.B.; Wang, H.S.; Zheng, Y.Z. Microfluidic techniques for tumor cell detection. Electrophoresis 2019, 40, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-Y.; Notton, T.; Fong, E.; Shusteff, M.; Weinberger, L.S. Spatial tuning of acoustofluidic pressure nodes by altering net sonic velocity enables high-throughput, efficient cell sorting. Lab Chip 2015, 15, 1000–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbansky, A.; Ohlsson, P.; Lenshof, A.; Garofalo, F.; Scheding, S.; Laurell, T. Rapid and effective enrichment of mononuclear cells from blood using acoustophoresis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Yang, S.; Wang, Z.; Huang, P.-H.; Mai, J.; Li, Z.-Y.; Huang, T.J. High-throughput cell focusing and separation via acoustofluidic tweezers. Lab Chip 2018, 18, 3003–3010. [Google Scholar] [CrossRef]
- Adams, J.D.; Thevoz, P.; Bruus, H.; Soh, H.T. Integrated acoustic and magnetic separation in microfluidic channels. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, W.; Huang, F.; Feng, H.; Shu, W.; Xu, X.; Chen, Y. High throughput capture of circulating tumor cells using an integrated microfluidic system. Biosens. Bioelectron. 2013, 47, 113–119. [Google Scholar] [CrossRef]
- Tang, M.; Wen, C.-Y.; Wu, L.-L.; Hong, S.-L.; Hu, J.; Xu, C.-M.; Pang, D.-W.; Zhang, Z.-L. A chip assisted immunomagnetic separation system for the efficient capture and in situ identification of circulating tumor cells. Lab Chip 2016, 16, 1214–1223. [Google Scholar] [CrossRef]
- Augustsson, P.; Magnusson, C.; Nordin, M.; Lilja, H.; Laurell, T. Microfluidic, Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis. Anal. Chem. 2012, 84, 7954–7962. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Yang, C.J. Hydrogel Droplet Microfluidics for High-Throughput Single Molecule/Cell Analysis. Acc. Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Shembekar, N.; Chaipan, C.; Utharala, R.; Merten, C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 2016, 16, 1314–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allazetta, S.; Hausherr, T.C.; Lutolf, M.P. Microfluidic Synthesis of Cell-Type-Specific Artificial Extracellular Matrix Hydrogels. Biomacromolecules 2013, 14, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Yang, S.; Kim, P. Microdroplet-based cell culture models and their application. BiocChip J. 2016, 10, 310–317. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.F.; Li, N.; Lin, L.; Huang, Q.S.; Uchiyama, K.; Lin, J.M. Concentrating Single Cells in Picoliter Droplets for Phospholipid Profiling on a Microfluidic System. Small 2019. [Google Scholar] [CrossRef]
- Linsenmeier, M.; Kopp, M.R.G.; Grigolato, F.; Liu, D.; Zrcher, D.; Hondele, M.; Weis, K.; Palmiero, U.C.; Arosio, P. Dynamics of Synthetic Membraneless Organelles in Microfluidic Droplets. Angew. Chem. Int. Ed. 2019, 58, 14489–14494. [Google Scholar] [CrossRef]
- Lee, D.; Cha, C. The Combined Effects of Co-Culture and Substrate Mechanics on 3D Tumor Spheroid Formation within Microgels Prepared via Flow-Focusing Microfluidic Fabrication. Pharmaceutics 2018, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ho, K.L.; Fan, S.K. Liver microsystems in vitro for drug response. J. Biomed. Sci. 2019, 26, 10. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Chen, Q.; Liu, W.; He, Z.; Lin, J.-M. Recent advances in microfluidic 3D cellular scaffolds for drug assays. TrAC Trends Anal. Chem. 2017, 87, 19–31. [Google Scholar] [CrossRef]
- Lu, H.F.; Leong, M.; Lim, T.; Chua, Y.P.; Lim, J.K.; Du, C.; Wan, A.C.A. Engineering a functional three-dimensional human cardiac tissue model for drug toxicity screening. Biofabrication 2017, 9, 025011. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Ozturk, E.; Gulce-Iz, S.; Anil, M.; Yesil-Celiktas, O. Cytotoxic responses of carnosic acid and doxorubicin on breast cancer cells in butterfly-shaped microchips in comparison to 2D and 3D culture. Cytotechnology 2017, 69, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, S.H.; Chamberlain, M.D.; Mahesh, S.; Sefton, M.V.; Wheeler, A.R. Hepatic organoids for microfluidic drug screening. Lab Chip 2014, 14, 3290–3299. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-C.; Lin, C.-H.; Juang, D.; Wu, H.-W.; Lee, C.-Y.; Chen, C.; Hsu, C.-H. Multilayer architecture microfluidic network array for combinatorial drug testing on 3D-cultured cells. Biofabrication 2019, 11. [Google Scholar] [CrossRef]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci. Rep. 2018, 8, 2841. [Google Scholar] [CrossRef]




| Mechanism | Application | Throughput | Efficiency |
|---|---|---|---|
| Filtration | Capture LM2 MDAMB- 231 breast cancer cells from phosphate-buffered saline [83] | 2.5 mL/h [83] | 97.1% [83] |
| Dielectrophoretic | Capture CTCs from DEP buffer and patient blood [90] | 1 mL/h and 8 mL/h respectively [90] | ~100% and 28.3% ± 7.6% respectively [90] |
| Magnetophoresis | Capture cancer cell MCF-7 from PBS, and whole blood [111] | 1 μL/min [111] | 95.8% and >94% respectively [111] |
| Acoustophoresis | Separate tumor cells from white blood cells in Blood [112] | 70 μL/min [112] | 97.4%–98.4% [112] |
| Integrative techniques | Capture MCF-7cells from 10× diluted blood samples [110] | 9.6 mL/min [110]; | 90% [110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Li, G.; Zu, X.; Du, Z.; Liu, L.; Hu, Z. The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review. Micromachines 2020, 11, 297. https://doi.org/10.3390/mi11030297
Song K, Li G, Zu X, Du Z, Liu L, Hu Z. The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review. Micromachines. 2020; 11(3):297. https://doi.org/10.3390/mi11030297
Chicago/Turabian StyleSong, Kena, Guoqiang Li, Xiangyang Zu, Zhe Du, Liyu Liu, and Zhigang Hu. 2020. "The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review" Micromachines 11, no. 3: 297. https://doi.org/10.3390/mi11030297
APA StyleSong, K., Li, G., Zu, X., Du, Z., Liu, L., & Hu, Z. (2020). The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review. Micromachines, 11(3), 297. https://doi.org/10.3390/mi11030297




