Recent Progress in Optical Sensors for Biomedical Diagnostics
Abstract
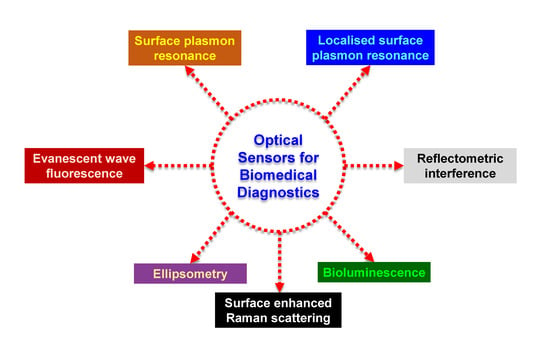
:1. Introduction
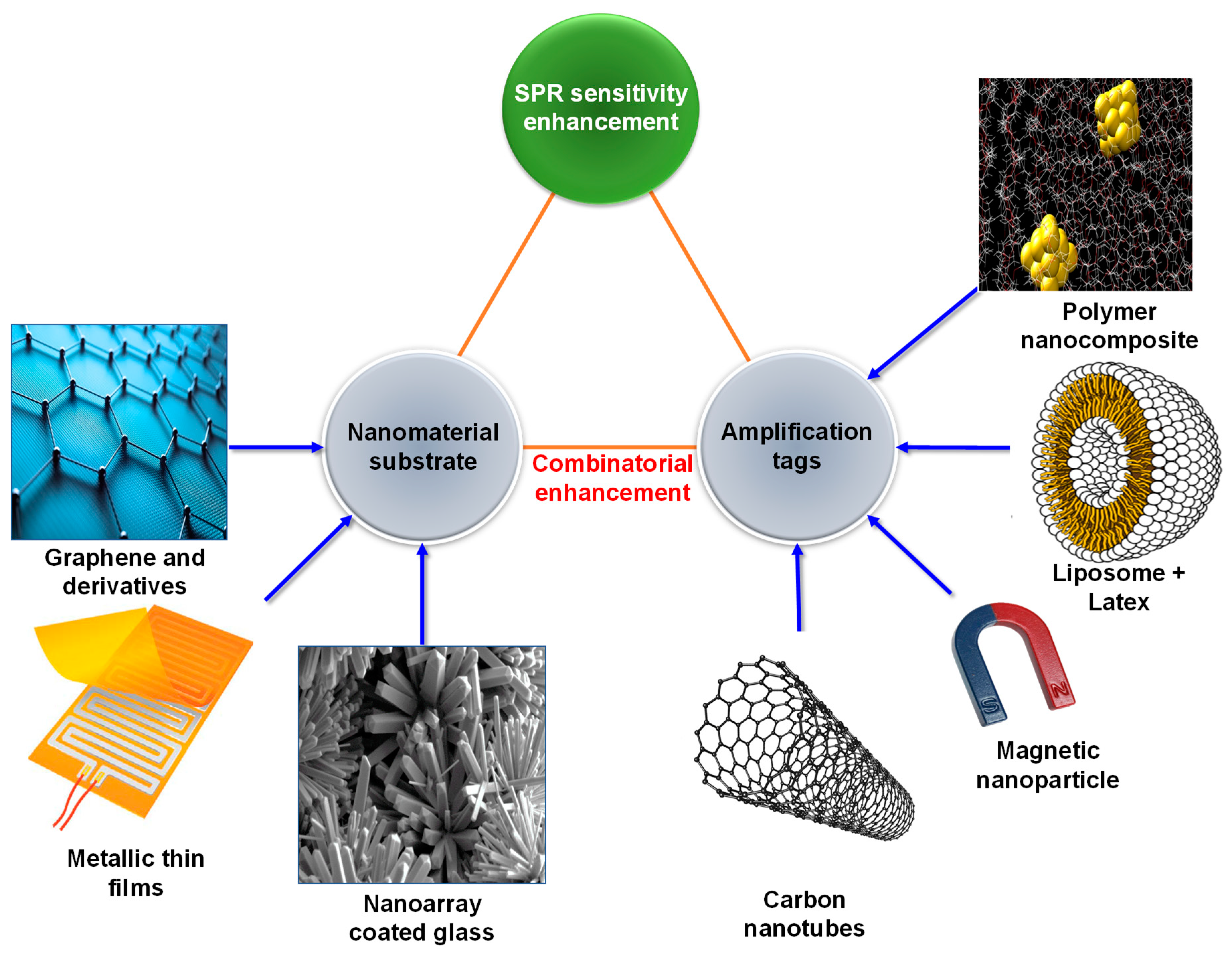
2. Surface Plasmon Resonance Sensors for Biomedical Diagnostics
3. Localised Surface Plasmon Resonance Sensors for Biomedical Diagnostics
4. Evanescent Wave-Based Sensors for Biomedical Diagnostics
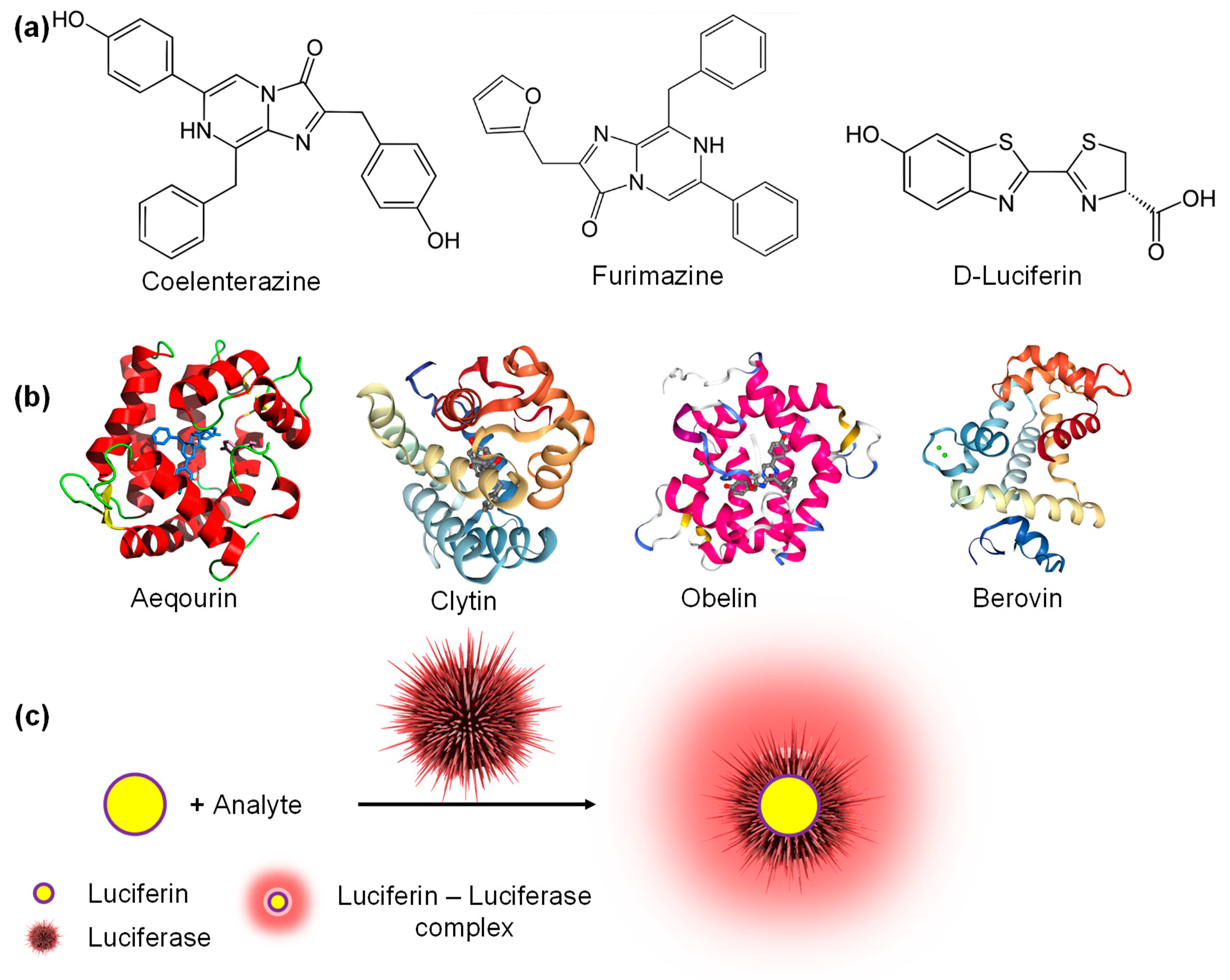
5. Bioluminescence-Based Sensors for Biomedical Diagnostics
6. Miscellaneous Optical Sensors for Biomedical Diagnostics
6.1. Ellipsometric Biosensors
6.2. SERS Biosensors
6.3. Reflectometric Interference Spectroscopy Biosensors
7. Summary and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [PubMed] [Green Version]
- Altintas, Z. Biosensors and Nanotechnology—Applications in Health Care Diagnostics; Altintas, Z., Ed.; John Wiley & Sons Press: Hoboken, NJ, USA, 2017; ISBN 978-1-119-06501-2. [Google Scholar]
- Altintas, Z. Optical Biosensors and Applications to Drug Discovery for Cancer Cases. In Biosensors and Nanotechnology: Applications in Health Care Diagnostics; Altintas, Z., Ed.; John Wiley & Sons Press: Hoboken, NJ, USA, 2017; pp. 327–348. [Google Scholar]
- Dey, D.; Goswami, T. Optical biosensors: A revolution towards quantum nanoscale electronics device fabrication. J. Biomed. Biotechnol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical biosensors for label-free detection of small molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Altintas, Z. Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 2019, 12, 2189. [Google Scholar] [CrossRef] [Green Version]
- Waffo, A.F.T.; Yesildag, C.; Caserta, G.; Katz, S.; Zebger, I.; Lensen, M.C.; Wollenberger, U.; Scheller, F.W.; Altintas, Z. Fully electrochemical MIP sensor for artemisinin. Sens. Actuators B Chem. 2018, 275, 163–173. [Google Scholar] [CrossRef]
- Gardikis, K.; Signorelli, M.; Ferrario, C.; Schiraldi, A.; Fortina, M.G.; Hatziantoniou, S.; Demetzos, C.; Fessas, D. Microbial biosensors to monitor the encapsulation effectiveness of doxorubicin in chimeric advanced drug delivery nano systems: A calorimetric approach. Int. J. Pharm. 2017, 516, 178–184. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Yao, Q.; He, F. A novel method for the rapid detection of microbes in blood using pleurocidin antimicrobial peptide functionalized piezoelectric sensor. J. Microbiol. Methods 2017, 133, 69–75. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Hussein, E.; Aljumaili, O.; Al Zoubi, M.; Altrad, B.; Albatayneh, K.; Abd Al-Razaq, M.A. Rapid magnetic nanobiosensor for the detection of Serratia marcescen. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012005. [Google Scholar] [CrossRef] [Green Version]
- Altintas, Z.; Guerreiro, A.; Piletsky, S.A.; Tothill, I.E. NanoMIP based optical sensor for pharmaceuticals monitoring. Sens. Actuators B Chem. 2015, 213, 305–313. [Google Scholar] [CrossRef]
- Pirzada, M.M. Recent Trends and Modifications in Glass Fibre Composites – A Review. Int. J. Mater. Chem. 2015, 5, 117–122. [Google Scholar]
- Zhang, H.; Miller, B.L. Immunosensor-based label-free and multiplex detection of influenza viruses: State of the art. Biosens. Bioelectron. 2019, 141, 111476. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. Trends Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Havlík, M.; Bříza, T.; Jakubek, M.; Králová, J.; Mikula, I.; Martásek, P.; Král, V. Optical probes and sensors as perspective tools in epigenetics. Bioorg. Med. Chem. 2017, 25, 2295–2306. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Peptide based biosensors. Trends Anal. Chem. 2018, 107, 1–20. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Zeng, S.; Yu, X.; Law, W.; Zhang, Y.; Hu, R.; Dinh, X.; Ho, H.; Yong, K. Chemical Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sens. Actuators B. Chem. 2013, 176, 1128–1133. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured Plasmonic Sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef]
- Law, W.; Markowicz, P.; Yong, K.; Roy, I.; Baev, A.; Patskovsky, S.; Kabashin, A.V.; Ho, H.; Prasad, P.N. Wide dynamic range phase-sensitive surface plasmon resonance biosensor based on measuring the modulation harmonics. Biosens. Bioelectron. 2007, 23, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.D.; Verma, R.K. Surface plasmon resonance-based fiber optic sensors: Principle, probe designs, and some applications. J. Sens. 2009, 2009, 1. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansuriya, M.; Altintas, A. Applications of graphene quantum dots in biomedical sensors. Sensors 2020, 20(4), 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Yong, K.; Roy, I. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Nie, W.; Wang, Q.; Yang, X.; Zhang, H.; Li, Z.; Gao, L.; Zheng, Y.; Liu, X.; Wang, K. High sensitivity surface plasmon resonance biosensor for detection of microRNA based on gold nanoparticles-decorated molybdenum sulfide. Anal. Chim. Acta 2017, 993, 55–62. [Google Scholar] [CrossRef]
- Law, W.; Yong, K.; Baev, A.; Prasad, P.N.; Al, L.A.W.E.T. Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. ACS Nano 2011, 5, 4858–4864. [Google Scholar] [CrossRef]
- Hao, K.; He, Y.; Lu, H.; Pu, S.; Zhang, Y.; Dong, H.; Zhang, X. High-sensitive surface plasmon resonance microRNA biosensor based on streptavidin functionalized gold nanorods-assisted signal amplification. Anal. Chim. Acta 2017, 954, 114–120. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Song, D. Magnetic field-assisted SPR biosensor based on carboxyl-functionalized graphene oxide sensing film and Fe3O4-hollow gold nanohybrids probe. Biosens. Bioelectron. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Zhang, Y.; Ma, P.; Yu, Y.; Wang, X.; Song, D. Ultrasensitive magnetic field-assisted surface plasmon resonance immunoassay for human cardiac troponin I. Biosens. Bioelectron. 2017, 96, 288–293. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhang, Y.; Fang, H.; Min, C.; Zhu, S.; Yuan, X. Investigation of phase SPR biosensor for efficient targeted drug screening with high sensitivity and stability. Sens. Actuators B. Chem. 2015, 209, 313–322. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Su, R.; Liu, B.; Huang, R.; Qi, W.; He, Z. A polydopamine-modified optical fiber SPR biosensor using electro-less-plated gold films for immunoassays. Biosens. Bioelectron. 2015, 74, 454–460. [Google Scholar] [CrossRef]
- Heidarzadeh, H. Analysis and simulation of a plasmonic biosensor for hemoglobin concentration detection using noble metal nano-particles resonances. Opt. Commun. 2019, 459, 124940. [Google Scholar] [CrossRef]
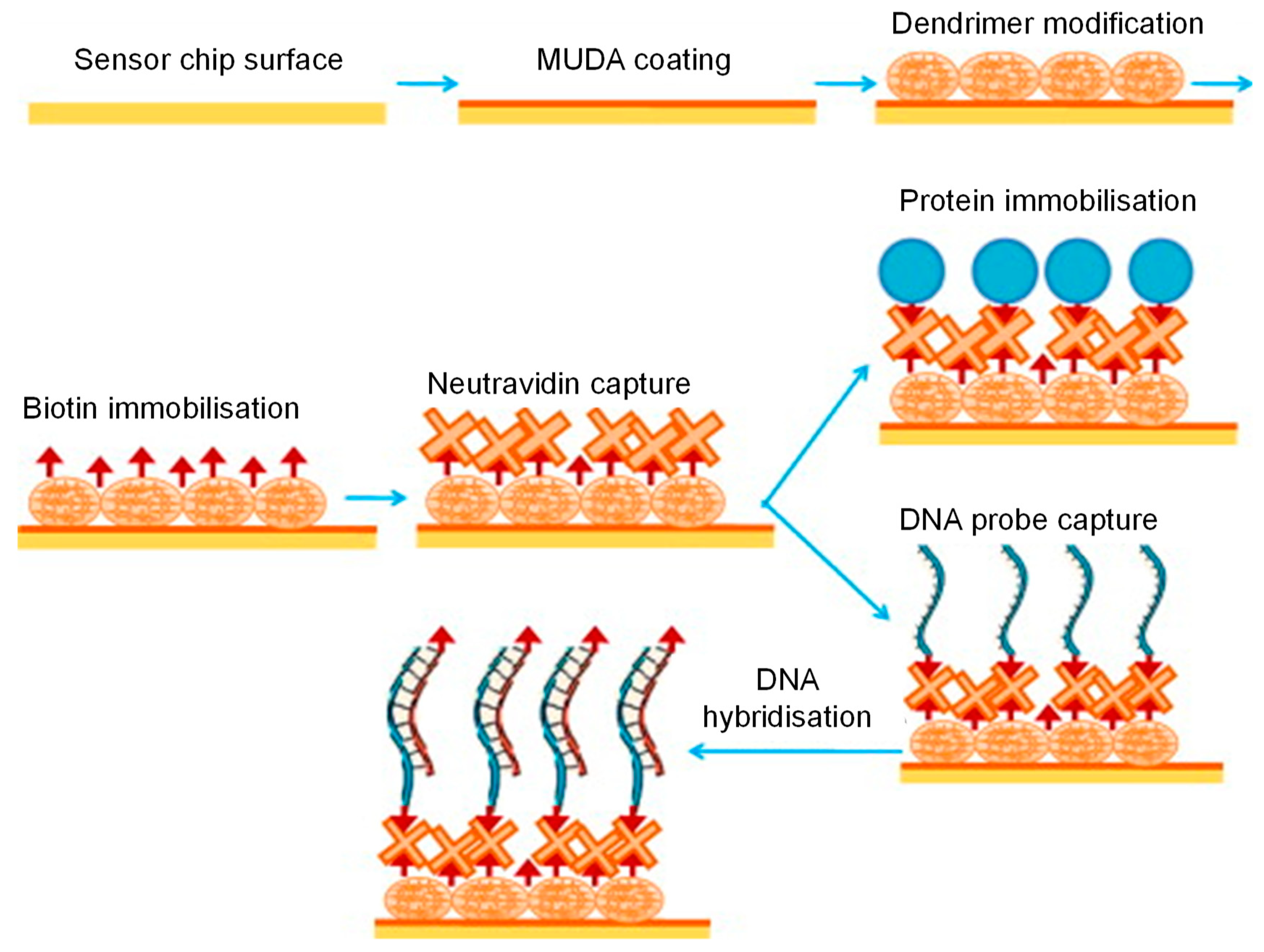
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I. Development of surface chemistry for surface plasmon resonance based sensors for the detection of proteins and DNA molecules. Anal. Chim. Acta 2012, 712, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Sun, Y.; Ma, P.; Zhang, D.; Li, S.; Wang, X.; Song, D. Gold nanostar-enhanced surface plasmon resonance biosensor based on carboxyl-functionalized graphene oxide. Anal. Chim. Acta 2016, 913, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Abdin, M.J.; Tothill, A.M.; Karim, K.; Tothill, I.E. Ultrasensitive detection of endotoxins using computationally designed nanoMIPs. Anal. Chim. Acta 2016, 935, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Abdin, M.J.; Altintas, Z.; Tothill, I.E. In silico designed nanoMIP based optical sensor for endotoxins monitoring. Biosens. Bioelectron. 2015, 67, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; France, B.; Ortiz, J.O.; Tothill, I.E. Computationally modelled receptors for drug monitoring using an optical based biomimetic SPR sensor. Sens. Actuators B Chem. 2016, 224, 726–737. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Guerreiro, A.; Thompson, K.A.; Walker, J.; Piletsky, S.; Tothill, I.E. Detection of waterborne viruses using high affinity molecularly imprinted polymers. Anal. Chem. 2015, 87, 6801–6807. [Google Scholar] [CrossRef]
- Altintas, Z. Surface plasmon resonance based sensor for the detection of glycopeptide antibiotics in milk using rationally designed nanoMIPs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Mir, T.A.; Wani, S.I.; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; Chinnappan, R.; et al. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Pundir, C.S. Detection of tumor suppressor protein p53 with special emphasis on biosensors: A review. Anal. Biochem. 2020, 588, 113473. [Google Scholar]
- Qian, L.; Li, Q.; Baryeh, K.; Qiu, W.; Li, K.; Zhang, J.; Yu, Q.; Xu, D.; Liu, W.; Brand, R.E.; et al. Biosensors for early diagnosis of pancreatic cancer: A review. Transl. Res. 2019, 213, 67–89. [Google Scholar] [CrossRef]
- Mohammadzadeh-Asl, S.; Keshtkar, A.; Ezzati Nazhad Dolatabadi, J.; de la Guardia, M. Nanomaterials and phase sensitive based signal enhancment in surface plasmon resonance. Biosens. Bioelectron. 2018, 110, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Rashidi, M.-R.; Omidi, Y. Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 2019, 192, 118–127. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef]
- La Franier, B.D.; Thompson, M. Early stage detection and screening of ovarian cancer: A research opportunity and significant challenge for biosensor technology. Biosens. Bioelectron. 2019, 135, 71–81. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Lin, T.-L.; Kuo, C.-T. Highly sensitive carboxyl-graphene oxide-based surface plasmon resonance immunosensor for the detection of lung cancer for cytokeratin 19 biomarker in human plasma. Sens. Actuators B Chem. 2018, 265, 264–272. [Google Scholar] [CrossRef]
- Loyez, M.; Larrieu, J.-C.; Chevineau, S.; Remmelink, M.; Leduc, D.; Bondue, B.; Lambert, P.; Devière, J.; Wattiez, R.; Caucheteur, C. In situ cancer diagnosis through online plasmonics. Biosens. Bioelectron. 2019, 131, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Ribaut, C.; Loyez, M.; Larrieu, J.-C.; Chevineau, S.; Lambert, P.; Remmelink, M.; Wattiez, R.; Caucheteur, C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: Towards in vivo diagnosis. Biosens. Bioelectron. 2017, 92, 449–456. [Google Scholar] [CrossRef]
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens. Actuators B Chem. 2016, 224, 823–832. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B Chem. 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Khan, Y.; Li, A.; Chang, L.; Li, L.; Guo, L. Gold nano disks arrays for localized surface plasmon resonance based detection of PSA cancer marker. Sens. Actuators B Chem. 2018, 255, 1298–1307. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Q.; Li, Q.; Yang, X.; Wang, K.; Nie, W. Surface plasmon resonance biosensor for sensitive detection of microRNA and cancer cell using multiple signal amplification strategy. Biosens. Bioelectron. 2017, 87, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Giamblanco, N.; Petralia, S.; Conoci, S.; Messineo, C.; Marletta, G. Ionic strength-controlled hybridization and stability of hybrids of KRAS DNA single-nucleotides: A surface plasmon resonance study. Colloids Surfaces B Biointerfaces 2017, 158, 41–46. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pagneux, Q.; Larroulet, I.; Serrano, A.Y.; Pesquera, A.; Zurutuza, A.; Mandler, D.; Boukherroub, R.; Szunerits, S. Label-free femtomolar cancer biomarker detection in human serum using graphene-coated surface plasmon resonance chips. Biosens. Bioelectron. 2017, 89, 606–611. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Y.; Zhang, L.; Jiang, L.; Zhou, Z.; Chen, H.; Zhou, J. Aluminum nanopyramid array with tunable ultraviolet–visible–infrared wavelength plasmon resonances for rapid detection of carbohydrate antigen 199. Biosens. Bioelectron. 2016, 79, 500–507. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, R.; Wang, Y.; Lu, M.; Yang, D.; Fa, M.; Yao, X. Surface plasmon resonance biosensor for the accurate and sensitive quantification of O-GlcNAc based on cleavage by β-D-N-acetylglucosaminidase. Anal. Chim. Acta 2018, 1040, 90–98. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Laudanski, P.; Romanowicz, L.; Hermanowicz, A.; Roszkowska-Jakimiec, W.; Debek, W.; Gorodkiewicz, E. Development of surface plasmon resonance imaging biosensors for detection of ubiquitin carboxyl-terminal hydrolase L1. Anal. Biochem. 2015, 469, 4–11. [Google Scholar] [CrossRef]
- Fathi, F.; Rahbarghazi, R.; Movassaghpour, A.A.; Rashidi, M.-R. Detection of CD133-marked cancer stem cells by surface plasmon resonance: Its application in leukemia patients. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1575–1582. [Google Scholar] [CrossRef]
- Chen, H.; Hou, Y.; Ye, Z.; Wang, H.; Koh, K.; Shen, Z.; Shu, Y. Label-free surface plasmon resonance cytosensor for breast cancer cell detection based on nano-conjugation of monodisperse magnetic nanoparticle and folic acid. Sens. Actuators B Chem. 2014, 201, 433–438. [Google Scholar] [CrossRef]
- Chen, H.; Jia, S.; Qi, F.; Zou, F.; Hou, Y.; Koh, K.; Yin, Y. Fabrication of a simple and convenient surface plasmon resonance cytosensor based on oriented peptide on calix[4]arene crownether monolayer. Sens. Actuators B. Chem. 2016, 225, 504–509. [Google Scholar] [CrossRef]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Barderas, R.; Pingarrón, J.M.; Campuzano, S.; Merino, S. Surface plasmon resonance immunosensor for ErbB2 breast cancer biomarker determination in human serum and raw cancer cell lysates. Anal. Chim. Acta 2016, 905, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Narayan, T.; Kumar, S.; Kumar, S.; Augustine, S.; Yadav, B.K.; Malhotra, B.D. Protein functionalised self assembled monolayer based biosensor for colon cancer detection. Talanta 2019, 201, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef]
- Szymańska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A biosensor for determination of the circulating biomarker CA125/MUC16 by surface plasmon resonance imaging. Talanta 2020, 206, 120187. [Google Scholar] [CrossRef]
- Pal, M.K.; Rashid, M.; Bisht, M. Multiplexed magnetic nanoparticle-antibody conjugates (MNPs-ABS) based prognostic detection of ovarian cancer biomarkers, CA-125, β-2M and ApoA1 using fluorescence spectroscopy with comparison of surface plasmon resonance (SPR) analysis. Biosens. Bioelectron. 2015, 73, 146–152. [Google Scholar] [CrossRef]
- Teotia, P.K.; Kaler, R.S. 1-D grating based SPR biosensor for the detection of lung cancer biomarkers using Vroman e ff ect. Opt. Commun. 2018, 406, 188–191. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; Cui, X. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef]
- Li, R.; Feng, F.; Chen, Z.; Bai, Y.; Guo, F.; Wu, F.-Y.; Zhou, G. Sensitive detection of carcinoembryonic antigen using surface plasmon resonance biosensor with gold nanoparticles signal amplification. Talanta 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Guo, C.; Su, F.; Song, Y.; Hu, B.; Wang, M.; He, L.; Peng, D.; Zhang, Z. Aptamer-templated silver nanoclusters embedded in zirconium metal-organic framework for bifunctional electrochemical and SPR aptasensors toward carcinoembryogenic antigen. ACS Appl. Mater. Interfaces 2017, 9, 41188–41199. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Wang, J.; Fu, W.; Yao, C. A SPR biosensor based on signal amplification using antibody-QD conjugates for quantitative determination of multiple tumor markers. Nat. Publ. Gr. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weremijewicz, A.; Matuszczak, E.; Sankiewicz, A.; Tylicka, M.; Komarowska, M.; Tokarzewicz, A.; Debek, W.; Gorodkiewicz, E.; Hermanowicz, A. Matrix metalloproteinase-2 and its correlation with basal membrane components laminin-5 and collagen type IV in paediatric burn patients measured with Surface Plasmon Resonance Imaging (SPRI) biosensors. Burns 2018, 44, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Breveglieri, G.; D’Aversa, E.; Gallo, T.E.; Pellegatti, P.; Guerra, G.; Cosenza, L.C.; Finotti, A.; Gambari, R.; Borgatti, M. A novel and efficient protocol for Surface Plasmon Resonance based detection of four β-thalassemia point mutations in blood samples and salivary swabs. Sens. Actuators B Chem. 2018, 260, 710–718. [Google Scholar] [CrossRef]
- Fathi, F.; Rezabakhsh, A.; Rahbarghazi, R.; Rashidi, M.-R. Early-stage detection of VE-cadherin during endothelial differentiation of human mesenchymal stem cells using SPR biosensor. Biosens. Bioelectron. 2017, 96, 358–366. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Siddiqui, A.A.; Banerjee, C.; Nag, S.; Mazumder, S.; De, R.; Saha, S.J.; Karri, S.K.; Bandyopadhyay, U. Detection of retromer assembly in Plasmodium falciparum by immunosensing coupled to Surface Plasmon Resonance. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 722–730. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef]
- Chen, H.; Qi, F.; Zhou, H.; Jia, S.; Gao, Y.; Koh, K.; Yin, Y. Fe3O4@Au nanoparticles as a means of signal enhancement in surface plasmon resonance spectroscopy for thrombin detection. Sens. Actuators B Chem. 2015, 212, 505–511. [Google Scholar] [CrossRef]
- Ataman Sadık, D.; Boyacı, İ.H.; Mutlu, M. Mixed monolayer decorated SPR sensing surface for thrombin detection. J. Pharm. Biomed. Anal. 2019, 176, 112822. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Yang, X.; Wang, K.; Zhang, H.; Nie, W. High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide-gold nanoparticles composites. Talanta 2017, 174, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Jatschka, J.; Dathe, A.; Csáki, A.; Fritzsche, W.; Stranik, O. Propagating and localized surface plasmon resonance sensing—A critical comparison based on measurements and theory. Sens. Bio-Sens. Res. 2016, 7, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Singh, P. LSPR Biosensing: Recent Advances and Approaches. In Reviews in Plasmonics 2016; Geddes, C.D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 211–238. [Google Scholar]
- Csáki, A.; Stranik, O.; Fritzsche, W. Localized surface plasmon resonance based biosensing. Expert Rev. Mol. Diagn. 2018, 18, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Renganathan, V.; Chen, S.-M.; Singh, V. Ingenious design and development of recyclable 2D BiOCl nanotiles attached tri-functional robust strips for high performance selective electrochemical sensing, SERS and heterogenous dip catalysis. Chem. Eng. J. 2020, 385, 123974. [Google Scholar] [CrossRef]
- Asha, S.; Ananth, A.N.; Jose, S.P.; Jothi Rajan, M.A. Temperature assisted reorganization of silver nanoparticles in free-standing, flexible chitosan functionalized reduced graphene oxide thick films: A potential SERS probe for folic acid sensing. Mater. Sci. Eng. B 2020, 252, 114454. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Hou, C.; Huo, D.; Wang, W.; Zhao, J.; Lei, Y. Rapid and fingerprinted monitoring of pesticide methyl parathion on the surface of fruits/leaves as well as in surface water enabled by gold nanorods based casting-and-sensing SERS platform. Talanta 2019, 200, 84–90. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, S.; Chen, J.; Feng, J.; Hou, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Gold nanoparticles/electrochemically expanded graphite composite: A bifunctional platform toward glucose sensing and SERS applications. J. Electroanal. Chem. 2019, 851, 113471. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, D.; Zhang, Q.; Lu, Y.; Li, N.; Chen, Q.; Liu, Q. Electrophoresis-enhanced localized surface plasmon resonance sensing based on nanocup array for thrombin detection. Sens. Actuators B Chem. 2016, 232, 219–225. [Google Scholar] [CrossRef]
- Sun, W.; Yuan, S.; Huang, H.; Liu, N.; Tan, Y. A label-free biosensor based on localized surface plasmon resonance for diagnosis of tuberculosis. J. Microbiol. Methods 2017, 142, 41–45. [Google Scholar] [CrossRef]
- Li, W.; Ren, K.; Zhou, J. Aluminum-based localized surface plasmon resonance for biosensing. TrAC Trends Anal. Chem. 2016, 80, 486–494. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, W.; Wang, S.; Qi, Z.; Luo, Z.; Chen, C.; Dai, J. Effect of sapphire substrate on the localized surface plasmon resonance of aluminum triangular nanoparticles. Opt. Commun. 2017, 395, 175–182. [Google Scholar] [CrossRef]
- Xu, C.; Lan, L.; Yao, Y.; Ping, J.; Li, Y.; Ying, Y. An unmodified gold nanorods-based DNA colorimetric biosensor with enzyme-free hybridization chain reaction amplification. Sens. Actuators B Chem. 2018, 273, 642–648. [Google Scholar] [CrossRef]
- Wang, D.; Guo, R.; Wei, Y.; Zhang, Y.; Zhao, X.; Xu, Z. Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of Au nanorods. Biosens. Bioelectron. 2018, 122, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rao, H.; Xue, X.; An, P.; Gao, M.; Luo, M.; Liu, X.; Xue, Z. Target-mediated surface chemistry of gold nanorods for breaking the low color resolution limitation of monocolorimetric sensor. Anal. Chim. Acta 2020, 1097, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Z.; Chuang, P.-C.; Liao, P.-C.; Chen, J.-P.; Chen, Y.-F. Increasing the spectral shifts in LSPR biosensing using DNA-functionalized gold nanorods in a competitive assay format for the detection of interferon-γ. Biosens. Bioelectron. 2016, 81, 221–228. [Google Scholar] [CrossRef]
- Tadimety, A.; Zhang, Y.; Kready, K.M.; Palinski, T.J.; Tsongalis, G.J.; Zhang, J.X.J. Design of peptide nucleic acid probes on plasmonic gold nanorods for detection of circulating tumor DNA point mutations. Biosens. Bioelectron. 2019, 130, 236–244. [Google Scholar] [CrossRef]
- Pai, J.-H.; Yang, C.-T.; Hsu, H.-Y.; Wedding, A.B.; Thierry, B. Development of a simplified approach for the fabrication of localised surface plasmon resonance sensors based on gold nanorods functionalized using mixed polyethylene glycol layers. Anal. Chim. Acta 2017, 974, 87–92. [Google Scholar] [CrossRef]
- Peixoto, L.P.F.; Santos, J.F.L.; Andrade, G.F.S. Plasmonic nanobiosensor based on Au nanorods with improved sensitivity: A comparative study for two different configurations. Anal. Chim. Acta 2019, 1084, 71–77. [Google Scholar] [CrossRef]
- Kong, W.; Guo, X.; Jing, M.; Qu, F.; Lu, L. Highly sensitive photoelectrochemical detection of bleomycin based on Au/WS2 nanorod array as signal matrix and Ag/ZnMOF nanozyme as multifunctional amplifier. Biosens. Bioelectron. 2020, 150, 111875. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wang, Y.; Sun, K.; Chen, X.; Chen, H.; Zhou, J. Reproducible plasmonic nanopyramid array of various metals for highly sensitive refractometric and surface-enhanced Raman biosensing. ACS Omega 2018, 3, 14181–14187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Wang, Y.; Fu, Q.; Tan, Y.; Wang, H.; Chen, H.; Zhou, J. Plasmonic Al nanopyramid array sensor for monitoring the attaching and spreading of cells. Sens. Actuators B Chem. 2019, 279, 503–508. [Google Scholar] [CrossRef]
- Kim, G.W.; Ha, J.W. Effect of adsorbate electrophilicity and spiky uneven surfaces on single gold nanourchin-based localized surface plasmon resonance sensors. Chem. Phys. Lett. 2018, 697, 38–42. [Google Scholar] [CrossRef]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic gold nanoparticles in biomedical applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.C.; Chang, Y.F.; Wang, H.Y.; Lin, Y.X.; Kuo, C.C.; Annie Ho, J.A.; Lee, C.C.; Su, L.C. An innovative application of time-domain spectroscopy on localized surface plasmon resonance sensing. Sci. Rep. 2017, 7, 44555. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Xu, M.; Lu, M.; Chen, G.; Tang, D. Urchin-like (gold core)@(platinum shell) nanohybrids: A highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens. Bioelectron. 2015, 70, 194–201. [Google Scholar] [CrossRef]
- Li, N.; Zhang, D.; Zhang, Q.; Lu, Y.; Jiang, J.; Liu, G.L.; Liu, Q. Combining localized surface plasmon resonance with anodic stripping voltammetry for heavy metal ion detection. Sens. Actuators B Chem. 2016, 231, 349–356. [Google Scholar] [CrossRef]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are plasmonic optical biosensors ready for use in point-of-need applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef] [Green Version]
- Focsan, M.; Craciun, A.M.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and Tunable 3D Gold Nanocups Platform as Plasmonic Biosensor for Specific Dual LSPR-SERS Immuno-Detection. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Liu, Q. Nanoplasmonic biosensor using localized surface plasmon resonance spectroscopy for biochemical detection. In Biosensors and Biodetection: Methods and Protocols Volume 1: Optical-Based Detectors; Rasooly, A., Prickril, B., Eds.; Springer Science+Business Media LLC: New York, NY, USA, 2017; Vol. 1571, pp. 89–107. ISBN 978-1-4939-6846-6. [Google Scholar]
- Agharazy Dormeny, A.; Abedini Sohi, P.; Kahrizi, M. Design and simulation of a refractive index sensor based on SPR and LSPR using gold nanostructures. Results Phys. 2020, 16, 102869. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Luo, J.; Ai, S. Sensitive detection of adenosine triphosphate by exonuclease III-assisted cyclic amplification coupled with surface plasmon resonance enhanced fluorescence based on nanopore. Sens. Actuators B Chem. 2016, 228, 509–514. [Google Scholar] [CrossRef]
- Thakur, A.; Qiu, G.; NG, S.-P.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.-M.L. Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, Y.; Li, S.; Zhang, Q.; Wu, J.; Jiang, J.; Liu, G.L.; Liu, Q. Monitoring the electrochemical responses of neurotransmitters through localized surface plasmon resonance using nanohole array. Biosens. Bioelectron. 2017, 93, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.Y.; Law, A.H.L.; Ng, S.P.; Wu, C.M.L. Label-free detection of lead(II) ion using differential phase modulated localized surface plasmon resonance sensors. Procedia Eng. 2016, 168, 533–536. [Google Scholar] [CrossRef]
- Qiu, G.; Ng, S.P.; Wu, C.-M.L. Bimetallic Au-Ag alloy nanoislands for highly sensitive localized surface plasmon resonance biosensing. Sens. Actuators B Chem. 2018, 265, 459–467. [Google Scholar] [CrossRef]
- Qiu, G.; Ng, S.P.; Wu, L.C.-M. Dielectric functionalization for differential phase detecting localized surface plasmon resonance biosensor. Sens. Actuators B Chem. 2016, 234, 247–254. [Google Scholar] [CrossRef]
- Yang, Z.; Sassa, F.; Hayashi, K. A robot equipped with a high-speed LSPR gas sensor module for collecting spatial odor information from on-ground invisible odor sources. ACS Sens. 2018, 3, 1174–1181. [Google Scholar] [CrossRef]
- Chung, T.; Lee, Y.; Ahn, M.S.; Lee, W.; Bae, S.I.; Hwang, C.S.H.; Jeong, K.H. Nanoislands as plasmonic materials. Nanoscale 2019, 11, 8651–8664. [Google Scholar] [CrossRef]
- Langelüddecke, L.; Singh, P.; Deckert, V. Exploring the Nanoscale: Fifteen Years of Tip-Enhanced Raman Spectroscopy. Appl. Spectrosc. 2015, 69, 1357–1371. [Google Scholar] [CrossRef] [Green Version]
- Gühlke, M.; Heiner, Z.; Kneipp, J. Surface-enhanced hyper-Raman and Raman hyperspectral mapping. Phys. Chem. Chem. Phys. 2016, 18, 14228–14233. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Chattopadhyay, N. Gold and silver nanoparticles based superquenching of fluorescence: A review. J. Lumin. 2015, 160, 223–232. [Google Scholar] [CrossRef]
- Jin, H.Y.; Li, D.W.; Zhang, N.; Gu, Z.; Long, Y.T. Analyzing carbohydrate-protein interaction based on single plasmonic nanoparticle by conventional dark field microscopy. ACS Appl. Mater. Interfaces 2015, 7, 12249–12253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, P.; Liu, X.; Di, Y.; Han, S.; Cui, X.; He, L. Sensing performance analysis on Fano resonance of metallic double-baffle contained MDM waveguide coupled ring resonator. Opt. Laser Technol. 2018, 101, 273–278. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, S.; Ji, B.; Zou, P.; Liu, L.; Li, Y. Fano resonance in Al nano-dolmen plasmonic structure for enhanced biosensing. Sens. Bio-Sens. Res. 2017, 15, 5–11. [Google Scholar] [CrossRef]
- Zarrabi, F.B.; Naser-Moghadasi, M. Plasmonic split ring resonator with energy enhancement for the application of bio-sensing and energy harvesting based on the second harmonic generation and multi Fano resonance. J. Alloys Compd. 2017, 706, 568–575. [Google Scholar] [CrossRef]
- Shaimanov, A.N.; Orlikovsky, N.A.; Khabushev, E.M.; Zverev, A.V.; Pishimova, A.A.; Sharonov, G.V.; Yankovskii, G.M.; Rodionov, I.A.; Baryshev, A.V. Interfering surface and localized plasmon: Tuning the Wood anomaly for biosensing. Photonics Nanostructures Fundam. Appl. 2018, 32, 1–5. [Google Scholar] [CrossRef]
- Zheng, G.; Zou, X.; Chen, Y.; Xu, L.; Rao, W. Fano resonance in graphene-MoS2 heterostructure-based surface plasmon resonance biosensor and its potential applications. Opt. Mater. (Amst). 2017, 66, 171–178. [Google Scholar] [CrossRef]
- Jo, S.; Lee, W.; Park, J.; Kim, W.; Kim, W.; Lee, G.; Lee, H.-J.; Hong, J.; Park, J. Localized surface plasmon resonance aptasensor for the highly sensitive direct detection of cortisol in human saliva. Sens. Actuators B Chem. 2020, 304, 127424. [Google Scholar] [CrossRef]
- Seok Kim, Y.; Ahmad Raston, N.H.; Bock Gu, M. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef]
- Faridli, Z.; Mahani, M.; Torkzadeh-Mahani, M.; Fasihi, J. Development of a localized surface plasmon resonance-based gold nanobiosensor for the determination of prolactin hormone in human serum. Anal. Biochem. 2016, 495, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Uthaman, S.; Lee, J.; Hwang, H.; Kim, G.; Yoo, P.J.; Hammock, B.D.; Kim, C.S.; Park, Y.-S.; Park, I.-K. In-direct localized surface plasmon resonance (LSPR)-based nanosensors for highly sensitive and rapid detection of cortisol. Sens. Actuators B Chem. 2018, 266, 710–716. [Google Scholar] [CrossRef]
- Nasrin, F.; Chowdhury, A.D.; Takemura, K.; Lee, J.; Adegoke, O.; Deo, V.K.; Abe, F.; Suzuki, T.; Park, E.Y. Single-step detection of norovirus tuning localized surface plasmon resonance-induced optical signal between gold nanoparticles and quantum dots. Biosens. Bioelectron. 2018, 122, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, A.; Sakač, N.; Lenyk, B.; Campanile, R.; Mayer, D.; Offenhäusser, A.; Velotta, R.; Della Ventura, B. LSPR-based colorimetric immunosensor for rapid and sensitive 17β-estradiol detection in tap water. Sens. Actuators B Chem. 2020, 308, 127699. [Google Scholar] [CrossRef]
- Kim, H.-M.; Uh, M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens. Actuators B Chem. 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Salahvarzi, A.; Mahani, M.; Torkzadeh-Mahani, M.; Alizadeh, R. Localized surface plasmon resonance based gold nanobiosensor: Determination of thyroid stimulating hormone. Anal. Biochem. 2017, 516, 1–5. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; De Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular imprinting of complex matrices at localized surface plasmon resonance biosensors for screening of global interactions of polyphenols and proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Teixeira, N.; De Freitas, V.; Sales, M.G.F.; Sutherland, D.S. A saliva molecular imprinted localized surface plasmon resonance biosensor for wine astringency estimation. Food Chem. 2017, 233, 457–466. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Ge, L.; Hayashi, K. Localized surface plasmon resonance gas sensor of Au nano-islands coated with molecularly imprinted polymer: Influence of polymer thickness on sensitivity and selectivity. Sens. Actuators B Chem. 2016, 231, 787–792. [Google Scholar] [CrossRef]
- He, H.; Muhammad, P.; Guo, Z.; Peng, Q.; Lu, H.; Liu, Z. Controllably prepared molecularly imprinted core-shell plasmonic nanostructure for plasmon-enhanced fluorescence assay. Biosens. Bioelectron. 2019, 146, 111733. [Google Scholar] [CrossRef]
- Rapisarda, A.; Giamblanco, N.; Marletta, G. Kinetic discrimination of DNA single-base mutations by localized surface plasmon resonance. J. Colloid Interface Sci. 2017, 487, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Morita, M.; Kato, T.; Ito, M.; Suzuki, T.; Park, E.Y. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens. Bioelectron. 2017, 94, 513–522. [Google Scholar] [CrossRef]
- Nasrin, F.; Chowdhury, A.D.; Takemura, K.; Kozaki, I.; Honda, H.; Adegoke, O.; Park, E.Y. Fluorometric virus detection platform using quantum dots-gold nanocomposites optimizing the linker length variation. Anal. Chim. Acta 2020. [Google Scholar] [CrossRef]
- Austin Suthanthiraraj, P.P.; Sen, A.K. Localized surface plasmon resonance (LSPR) biosensor based on thermally annealed silver nanostructures with on-chip blood-plasma separation for the detection of dengue non-structural protein NS1 antigen. Biosens. Bioelectron. 2019, 132, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Adegoke, O.; Takahashi, N.; Kato, T.; Li, T.-C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Versatility of a localized surface plasmon resonance-based gold nanoparticle-alloyed quantum dot nanobiosensor for immunofluorescence detection of viruses. Biosens. Bioelectron. 2017, 89, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Dadfarnia, S.; Haji Shabani, A.M.; Sadjadi, S. Non-enzymatic sensing of dopamine by localized surface plasmon resonance using carbon dots-functionalized gold nanoparticles. J. Pharm. Biomed. Anal. 2019, 172, 223–229. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Jabbari, A.; Kowsari, E.; Niroumand, R.; Booth, T.J. Colorimetric sensing of dopamine using hexagonal silver nanoparticles decorated by task-specific pyridinum based ionic liquid. Sens. Actuators B Chem. 2018, 271, 64–72. [Google Scholar] [CrossRef]
- Shen, J.; Sun, C.; Wu, X. Silver nanoprisms-based Tb(III) fluorescence sensor for highly selective detection of dopamine. Talanta 2017, 165, 369–376. [Google Scholar] [CrossRef]
- Ranganathan, V.; Srinivasan, S.; Singh, A.; DeRosa, M.C. An aptamer-based colorimetric lateral flow assay for the detection of human epidermal growth factor receptor 2 (HER2). Anal. Biochem. 2020, 588, 113471. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; de Melo, L.S.A.; Farooq, S.; Baptista, A.; Kato, I.T.; Núñez, S.C.; de Araujo, R.E. Photodynamic inactivation assisted by localized surface plasmon resonance of silver nanoparticles: In vitro evaluation on Escherichia coli and Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2018, 22, 191–196. [Google Scholar] [CrossRef]
- Savariraj, A.D.; Vinoth, V.; Mangalaraja, R.V.; Arun, T.; Contreras, D.; Akbari-Fakhrabadi, A.; Valdés, H.; Banat, F. Microwave-assisted synthesis of localized surface plasmon resonance enhanced bismuth selenide (Bi2Se3) layers for non-enzymatic glucose sensing. J. Electroanal. Chem. 2020, 856, 113629. [Google Scholar] [CrossRef]
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors: Advances of the last decade. Biosens. Bioelectron. 2016, 76, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adányi, N.; Majer-Baranyi, K.; Székács, A. Evanescent field effect–based nanobiosensors for agro-environmental and food safety. In Nanobiosensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 429–474. ISBN 9780128043011. [Google Scholar]
- Sinibaldi, A.; Sampaoli, C.; Danz, N.; Munzert, P.; Sibilio, L.; Sonntag, F.; Occhicone, A.; Falvo, E.; Tremante, E.; Giacomini, P.; et al. Detection of soluble ERBB2 in breast cancer cell lysates using a combined label-free/fluorescence platform based on Bloch surface waves. Biosens. Bioelectron. 2017, 92, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, R.; Xia, K.; Zhou, X.; Shi, H. Nucleic acid functionalized fiber optic probes for sensing in evanescent wave: Optimization and application. RSC Adv. 2019, 9, 2316–2324. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Tang, Y.; Li, B.; He, M. Rapid detection of cocaine using aptamer-based biosensor on an evanescent wave fibre platform. R. Soc. Open Sci. 2018, 5, 180821. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Long, F.; Gu, C.; Wang, C.; Han, S.; He, M. Reusable split-aptamer-based biosensor for rapid detection of cocaine in serum by using an all-fiber evanescent wave optical biosensing platform. Anal. Chim. Acta 2016, 933, 182–188. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Evanescent wave aptasensor for continuous and online aminoglycoside antibiotics detection based on target binding facilitated fluorescence quenching. Biosens. Bioelectron. 2018, 102, 646–651. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Huang, T.-T.; Wang, C.-H.; Huang, C.-J.; Tsai, T.-H.; Yu, S.-N.; Chen, Y.-T.; Hong, S.-W.; Hsu, C.-W.; Chang, T.-C.; et al. Fiber optic nanogold-linked immunosorbent assay for rapid detection of procalcitonin at femtomolar concentration level. Biosens. Bioelectron. 2020, 151, 111871. [Google Scholar] [CrossRef]
- Liu, L.; Marques, L.; Correia, R.; Morgan, S.P.; Lee, S.-W.; Tighe, P.; Fairclough, L.; Korposh, S. Highly sensitive label-free antibody detection using a long period fibre grating sensor. Sens. Actuators B Chem. 2018, 271, 24–32. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Lu, Y.; Shan, D.; Xu, B.; He, M.; Shi, H.; Qian, Y. Facile screening of potential xenoestrogens by an estrogen receptor-based reusable optical biosensor. Biosens. Bioelectron. 2017, 97, 16–20. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, A.; Lou, X.; Song, D.; Yang, R.; Shi, H.; Long, F. Universal quantum dot-based sandwich-like immunoassay strategy for rapid and ultrasensitive detection of small molecules using portable and reusable optofluidic nano-biosensing platform. Anal. Chim. Acta 2016, 905, 140–148. [Google Scholar] [CrossRef]
- Lou, X.; Zhu, A.; Wang, H.; Wu, J.; Zhou, L.; Long, F. Direct and ultrasensitive optofluidic-based immunosensing assay of aflatoxin M1 in dairy products using organic solvent extraction. Anal. Chim. Acta 2016, 940, 120–127. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Song, B.; Zhang, J.; Shi, H. Highly sensitive and simultaneous detection of melamine and aflatoxin M1 in milk products by multiplexed planar waveguide fluorescence immunosensor (MPWFI). Food Chem. 2016, 197, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, W.; Xiao, P.; Yang, M.; Sun, L.-P.; Zhang, Y.; Xue, W.; Guan, B.-O. Polydopamine-based molecular imprinted optic microfiber sensor enhanced by template-mediated molecular rearrangement for ultra-sensitive C-reactive protein detection. Chem. Eng. J. 2020, 387, 124074. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Sai, V.V.R. Evanescent wave absorbance based U-bent fiber probe for immunobiosensor with gold nanoparticle labels. Sens. Actuators B Chem. 2016, 226, 184–190. [Google Scholar] [CrossRef]
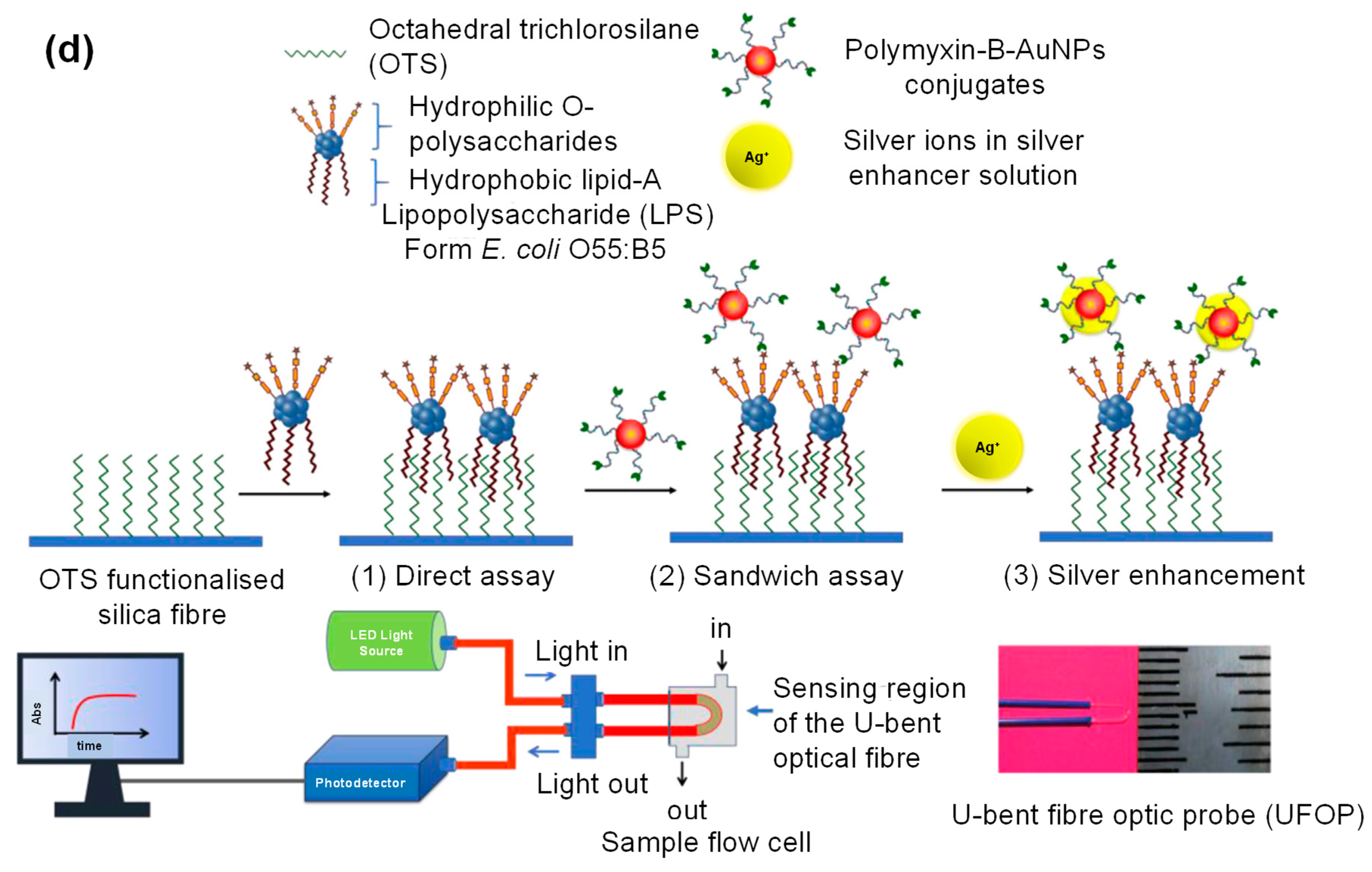
- Manoharan, H.; Kalita, P.; Gupta, S.; Sai, V.V.R. Plasmonic biosensors for bacterial endotoxin detection on biomimetic C-18 supported fiber optic probes. Biosens. Bioelectron. 2019, 129, 79–86. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Fu, C.; Chen, Y.-T.; Fang-Ju Jou, A.; Chen, C.-C.; Chou, C.; Annie Ho, J. Use of liposomal amplifiers in total internal reflection fluorescence fiber-optic biosensors for protein detection. Biosens. Bioelectron. 2016, 77, 1201–1207. [Google Scholar] [CrossRef]
- Lopes, R.N.; Rodrigues, D.M.C.; Allil, R.C.S.B.; Werneck, M.M. Plastic optical fiber immunosensor for fast detection of sulfate-reducing bacteria. Measurement 2018, 125, 377–385. [Google Scholar] [CrossRef]
- Sun, X.; Li, N.; Zhou, B.; Zhao, W.; Liu, L.; Huang, C.; Ma, L.; Kost, A.R. Non-enzymatic glucose detection based on phenylboronic acid modified optical fibers. Opt. Commun. 2018, 416, 32–35. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Gan, L.; Liu, Q.; Yan, Z.; Liu, D.; Sun, Q. Immobilized optical fiber microprobe for selective and high sensitive glucose detection. Sens. Actuators B Chem. 2018, 255, 3004–3010. [Google Scholar] [CrossRef]
- Liu, T.; Liang, L.-L.; Xiao, P.; Sun, L.-P.; Huang, Y.-Y.; Ran, Y.; Jin, L.; Guan, B.-O. A label-free cardiac biomarker immunosensor based on phase-shifted microfiber Bragg grating. Biosens. Bioelectron. 2018, 100, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Alvaro, M.; Danz, N.; Napione, L.; Descrovi, E.; Schmieder, S.; Sinibaldi, A.; Chandrawati, R.; Rana, S.; Munzert, P.; et al. Bloch surface wave label-free and fluorescence platform for the detection of VEGF biomarker in biological matrices. Sens. Actuators B Chem. 2018, 255, 2143–2150. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Metkar, S.K.; Girigoswami, A.; Girigoswami, K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C 2017, 78, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Liu, J.; Xu, W.; Long, F.; Zhu, A. A novel dual-color total internal reflection fluorescence detecting platform using compact optical structure and silicon-based photodetector. Talanta 2019, 196, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, B.; Liu, S.; Tian, T.; Xu, G.; Cai, B. An optical fiber-waveguide-fiber platform for ppt level evanescent field-based sensing. Sens. Actuators B Chem. 2020, 306, 127548. [Google Scholar] [CrossRef]
- Sharifian, S.; Homaei, A.; Hemmati, R.B.; Luwor, R.; Khajeh, K. The emerging use of bioluminescence in medical research. Biomed. Pharmacother. 2018, 101, 74–86. [Google Scholar] [CrossRef]
- Yeh, H.-W.; Ai, H.-W. Development and applications of bioluminescent and chemiluminescent reporters and biosensors. Annu. Rev. Anal. Chem. 2019, 12, 129–150. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Griss, R.; Schena, A.; Johnsson, K. Highly modular bioluminescent sensors for small molecules and proteins. In Methods in Enzymology; Thompson, R.B., Fierke, C.A., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2017; Vol. 589, pp. 365–382. ISBN 9780128054062. [Google Scholar]
- Santangelo, M.F.; Libertino, S.; Turner, A.P.F.; Filippini, D.; Mak, W.C. Integrating printed microfluidics with silicon photomultipliers for miniaturised and highly sensitive ATP bioluminescence detection. Biosens. Bioelectron. 2018, 99, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Hsiao, K.; Goueli, S.A. A bioluminescent assay for monitoring conjugation of ubiquitin and ubiquitin-like proteins. Anal. Biochem. 2016, 510, 41–51. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, H.R.; Hwang, J. Continuous and real-time bioaerosol monitoring by combined aerosol-to-hydrosol sampling and ATP bioluminescence assay. Anal. Chim. Acta 2016, 941, 101–107. [Google Scholar] [CrossRef]
- Su, X.; Wang, M.; Ouyang, H.; Yang, S.; Wang, W.; He, Y.; Fu, Z. Bioluminescent detection of the total amount of viable Gram-positive bacteria isolated by vancomycin-functionalized magnetic particles. Sens. Actuators B Chem. 2017, 241, 255–261. [Google Scholar] [CrossRef]
- Rasooly, R.; Do, P.; Hernlem, B.J. Low cost bioluminescence imaging as an alternative to in vivo bioassays for quantifying biologically active staphylococcal enterotoxin type E. Sens. Actuators B Chem. 2018, 259, 387–393. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, H.R.; Jung, J.H.; Lee, K.-B.; Kim, B.C. The development of paper discs immobilized with luciferase/D-luciferin for the detection of ATP from airborne bacteria. Sens. Actuators B Chem. 2018, 260, 274–281. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-lantern on paper for smartphone-based ATP detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef] [PubMed]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Costantini, F.; Roda, A.; Gallinella, G.; Caputo, D.; et al. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sens. Actuators B Chem. 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Xiang, D.; Li, C.; Zhang, C. A universal DNAzyme-based bioluminescent sensor for label-free detection of biomolecules. Anal. Chim. Acta 2018, 1043, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Nishikawa, M.; Yasuda, K.; Ikushiro, S.; Saito, N.; Sawada, D.; Honzawa, S.; Takano, M.; Kittaka, A.; Sakaki, T. Novel screening system for high-affinity ligand of heredity vitamin D-resistant rickets-associated vitamin D receptor mutant R274L using bioluminescent sensor. J. Steroid Biochem. Mol. Biol. 2017, 167, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cevenini, L.; Calabretta, M.M.; Tarantino, G.; Michelini, E.; Roda, A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sens. Actuators B Chem. 2016, 225, 249–257. [Google Scholar] [CrossRef]
- Michelini, E.; Calabretta, M.M.; Cevenini, L.; Lopreside, A.; Southworth, T.; Fontaine, D.M.; Simoni, P.; Branchini, B.R.; Roda, A. Smartphone-based multicolor bioluminescent 3D spheroid biosensors for monitoring inflammatory activity. Biosens. Bioelectron. 2019, 123, 269–277. [Google Scholar] [CrossRef]
- Liu, P.; Fang, X.; Cao, H.; Gu, M.; Kong, J.; Deng, A. Magnetic-bioluminescent-nanoliposomes for ultrasensitive and portable detection of protein biomarkers in blood. Anal. Chim. Acta 2018, 1039, 98–107. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Dong, M.; Zheng, W.; Sun, J.; Jiang, X. Double-enzymes-mediated bioluminescent sensor for quantitative and ultrasensitive point-of-care testing. Anal. Chem. 2017, 89, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bao, Y.; Denstedt, J.; Zhang, J. Nanostructured bioluminescent sensor for rapidly detecting thrombin. Biosens. Bioelectron. 2016, 77, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, L.; Tang, W.; Chen, P.; Zhu, H.; Yuan, Y.; Li, G.; Zhang, H.; Liang, G. Hydrazide D-luciferin for in vitro selective detection and intratumoral imaging of Cu2+. Biosens. Bioelectron. 2016, 83, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Jin, Q.; Zou, L.W.; Hou, J.; Lv, X.; Lei, W.; Cheng, H.L.; Ge, G.B.; Yang, L. A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples. Chem. Commun. 2016, 52, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Moehring, D.; Muñoz-Planillo, R.; Núñez, G.; Callaway, J.; Ting, J.; Scurria, M.; Ugo, T.; Bernad, L.; Cali, J.; et al. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. J. Immunol. Methods 2017, 447, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tenda, K.; van Gerven, B.; Arts, R.; Hiruta, Y.; Merkx, M.; Citterio, D. Paper-based antibody detection devices Using bioluminescent BRET-switching sensor proteins. Angew. Chemie-Int. Ed. 2018, 57, 15369–15373. [Google Scholar] [CrossRef] [Green Version]
- Arts, R.; Den Hartog, I.; Zijlema, S.E.; Thijssen, V.; Van Der Beelen, S.H.E.; Merkx, M. Detection of antibodies in blood plasma using bioluminescent sensor proteins and a smartphone. Anal. Chem. 2016, 88, 4525–4532. [Google Scholar] [CrossRef]
- Van Rosmalen, M.; Ni, Y.; Vervoort, D.F.M.; Arts, R.; Ludwig, S.K.J.; Merkx, M. Dual-color bioluminescent sensor proteins for therapeutic drug monitoring of antitumor antibodies. Anal. Chem. 2018, 90, 3592–3599. [Google Scholar] [CrossRef]
- Arts, R.; Ludwig, S.K.J.; Van Gerven, B.C.B.; Estirado, E.M.; Milroy, L.G.; Merkx, M. Semisynthetic bioluminescent sensor proteins for direct detection of antibodies and small molecules in solution. ACS Sens. 2017, 2, 1730–1736. [Google Scholar] [CrossRef]
- Yoshida, T.; Kakizuka, A.; Imamura, H. BTeam, a novel BRET-based biosensor for the accurate quantification of ATP concentration within living cells. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Inagaki, S.; Tsutsui, H.; Suzuki, K.; Agetsuma, M.; Arai, Y.; Jinno, Y.; Bai, G.; Daniels, M.J.; Okamura, Y.; Matsuda, T.; et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cumberbatch, D.; Centanni, S.; Shi, S.Q.; Winder, D.; Webb, D.; Johnson, C.H. Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca++ sensing. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aper, S.J.A.; Dierickx, P.; Merkx, M. Dual readout BRET/FRET sensors for measuring intracellular zinc. ACS Chem. Biol. 2016, 11, 2854–2864. [Google Scholar] [CrossRef] [Green Version]
- Krasitskaya, V.V.; Chaukina, V.V.; Abroskina, M.V.; Vorobyeva, M.A.; Ilminskaya, A.A.; Kabilov, M.R.; Prokopenko, S.V.; Nevinsky, G.A.; Venyaminova, A.G.; Frank, L.A. Bioluminescent aptamer-based sandwich-type assay of anti-myelin basic protein autoantibodies associated with multiple sclerosis. Anal. Chim. Acta 2019, 1064, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Den Hamer, A.; Dierickx, P.; Arts, R.; De Vries, J.S.P.M.; Brunsveld, L.; Merkx, M. Bright bioluminescent BRET sensor proteins for measuring intracellular caspase activity. ACS Sens. 2017, 2, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacres, H.; Wang, J.; Anderson, A.; Trowell, S.C. A rapid and sensitive biosensor for measuring plasmin activity in milk. Sens. Actuators B Chem. 2019, 301, 127141. [Google Scholar] [CrossRef]
- Weihs, F.; Peh, A.; Dacres, H. A red-shifted Bioluminescence Resonance Energy Transfer (BRET) biosensing system for rapid measurement of plasmin activity in human plasma. Anal. Chim. Acta 2020, 1102, 99–108. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Kumagai, A.; Miyawaki, A.; Khashirova, S.Y.; Zhansitov, A.; Rumyantsev, E.V. Magnetic polymer-silica composites as bioluminescent sensors for bilirubin detection. Mater. Chem. Phys. 2016, 183, 422–429. [Google Scholar] [CrossRef]
- Zamani, P.; Sajedi, R.H.; Hosseinkhani, S.; Zeinoddini, M.; Bakhshi, B. A luminescent hybridoma-based biosensor for rapid detection of V. cholerae upon induction of calcium signaling pathway. Biosens. Bioelectron. 2016, 79, 213–219. [Google Scholar] [CrossRef]
- Podraza, N.J.; Jellison, G.E. Ellipsometry. Encycl. Spectrosc. Spectrom. 2017, 482–489. [Google Scholar]
- Muth, M.; Schmid, R.P.; Schnitzlein, K. Ellipsometric study of molecular orientations of Thermomyces lanuginosus lipase at the air–water interface by simultaneous determination of refractive index and thickness. Colloids Surfaces B Biointerfaces 2016, 140, 60–66. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Rubaye, A.G.; Al-Jawdah, A.M.; Tsargorodska, A.; Marty, J.-L.; Catanante, G.; Szekacs, A.; Takacs, E. Novel optical biosensing technologies for detection of mycotoxins. Opt. Laser Technol. 2019, 109, 212–221. [Google Scholar] [CrossRef]
- Al-Rubaye, A.G.; Nabok, A.; Tsargorodska, A. Spectroscopic ellipsometry study of gold nanostructures for LSPR bio-sensing applications. Sens. Bio-Sens. Res. 2017, 12, 30–35. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Üstündağ, Z. Spectrophotometric ellipsometry based Tat-protein RNA-aptasensor for HIV-1 diagnosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117748. [Google Scholar] [CrossRef] [PubMed]
- Al Rubaye, A.; Nabok, A.; Catanante, G.; Marty, J.-L.; Takacs, E.; Szekacs, A. Detection of ochratoxin A in aptamer assay using total internal reflection ellipsometry. Sens. Actuators B Chem. 2018, 263, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Pant, U.; Mohapatra, S.; Moirangthem, R.S. Total internal reflection ellipsometry based SPR sensor for studying biomolecular interaction. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Paiva, T.O.; Almeida, I.; Marquês, J.T.; Liu, W.; Niu, Y.; Jin, G.; Viana, A.S. Nanostructured interfaces with site-specific bioreceptors for immunosensing. Appl. Surf. Sci. 2017, 412, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Caglayan, M.O.; Üstündağ, Z. Detection of zearalenone in an aptamer assay using attenuated internal reflection ellipsometry and it’s cereal sample applications. Food Chem. Toxicol. 2020, 136, 111081. [Google Scholar] [CrossRef]
- Kalas, B.; Nador, J.; Agocs, E.; Saftics, A.; Kurunczi, S.; Fried, M.; Petrik, P. Protein adsorption monitored by plasmon-enhanced semi-cylindrical Kretschmann ellipsometry. Appl. Surf. Sci. 2017, 421, 585–592. [Google Scholar] [CrossRef]
- Sohrabi, F.; Hamidi, S.M. Optical detection of brain activity using plasmonic ellipsometry technique. Sens. Actuators B Chem. 2017, 251, 153–163. [Google Scholar] [CrossRef]
- Dow, X.Y.; DeWalt, E.L.; Sullivan, S.Z.; Schmitt, P.D.; Ulcickas, J.R.W.; Simpson, G.J. Imaging the nonlinear susceptibility tensor of collagen by nonlinear optical Stokes ellipsometry. Biophys. J. 2016, 111, 1361–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diware, M.S.; Cho, H.M.; Chegal, W.; Cho, Y.J.; Kim, D.S.; Kim, K.S.; Paek, S.H. Ultrasensitive, label-free detection of cardiac biomarkers with optical SIS sensor. Biosens. Bioelectron. 2017, 87, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Das, N.K.; Dai, Y.; Liu, P.; Hu, C.; Tong, L.; Chen, X.; Smith, Z.J. Raman plus X: Biomedical applications of multimodal Raman spectroscopy. Sensors 2017, 17, 1592. [Google Scholar]
- Henry, A.I.; Sharma, B.; Cardinal, M.F.; Kurouski, D.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy biosensing: In vivo diagnostics and multimodal imaging. Anal. Chem. 2016, 88, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Yang, K.; Hu, Y.; Dong, N.; Zhu, G.; Zhu, T.; Jiang, N. A novel SERS-based magnetic aptasensor for prostate specific antigen assay with high sensitivity. Biosens. Bioelectron. 2017, 94, 286–291. [Google Scholar] [CrossRef]
- Chaloupková, Z.; Balzerová, A.; Bařinková, J.; Medříková, Z.; Šácha, P.; Beneš, P.; Ranc, V.; Konvalinka, J.; Zbořil, R. Label-free determination of prostate specific membrane antigen in human whole blood at nanomolar levels by magnetically assisted surface enhanced Raman spectroscopy. Anal. Chim. Acta 2018, 997, 44–51. [Google Scholar] [CrossRef]
- Yu, J.; Jeon, J.; Choi, N.; Lee, J.O.; Kim, Y.-P.; Choo, J. SERS-based genetic assay for amplification-free detection of prostate cancer specific PCA3 mimic DNA. Sens. Actuators B Chem. 2017, 251, 302–309. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, A.; Huang, Z.; Chen, Y.; Zhang, Q.; Cui, D. Monodisperse Au@Ag core-shell nanoprobes with ultrasensitive SERS-activity for rapid identification and Raman imaging of living cancer cells. Talanta 2019, 198, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.A.; Berus, S.; Szleszkowski, Ł.; Kamińska, A.; Kmiecik, A.; Ratajczak-Wielgomas, K.; Jurek, T.; Zadka, Ł. Brain tumour homogenates analysed by surface-enhanced Raman spectroscopy: Discrimination among healthy and cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 231, 117769. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.C.G.; Sousa-Castillo, A.; Correa-Duarte, M.A.; Sales, M.G.F. Dual biorecognition by combining molecularly-imprinted polymer and antibody in SERS detection. Application to carcinoembryonic antigen. Biosens. Bioelectron. 2019, 146, 111761. [Google Scholar] [CrossRef] [PubMed]
- Balzerová, A.; Opletalová, A.; Ranc, V.; Zbořil, R. Multiplex competitive analysis of HER2 and EpCAM cancer markers in whole human blood using Fe2O3@Ag nanocomposite. Appl. Mater. Today 2018, 13, 166–173. [Google Scholar] [CrossRef]
- Deng, R.; Yue, J.; Qu, H.; Liang, L.; Sun, D.; Zhang, J.; Liang, C.; Xu, W.; Xu, S. Glucose-bridged silver nanoparticle assemblies for highly sensitive molecular recognition of sialic acid on cancer cells via surface-enhanced raman scattering spectroscopy. Talanta 2018, 179, 200–206. [Google Scholar] [CrossRef]
- Gholami, M.D.; Sonar, P.; Ayoko, G.A.; Izake, E.L. A highly sensitive SERS quenching nanosensor for the determination of tumor necrosis factor alpha in blood. Sens. Actuators B Chem. 2020, 310, 127867. [Google Scholar] [CrossRef]
- Gjergjizi, B.; Çoğun, F.; Yıldırım, E.; Eryılmaz, M.; Selbes, Y.; Sağlam, N.; Tamer, U. SERS-based ultrafast and sensitive detection of luteinizing hormone in human serum using a passive microchip. Sens. Actuators B Chem. 2018, 269, 314–321. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, J.; Lee, S.; Lim, D.W.; Joo, H.; Choo, J. Quantitative analysis of thyroid-stimulating hormone (TSH) using SERS-based lateral flow immunoassay. Sens. Actuators B Chem. 2017, 240, 358–364. [Google Scholar] [CrossRef]
- Wen, G.; Liang, X.; Liu, Q.; Liang, A.; Jiang, Z. A novel nanocatalytic SERS detection of trace human chorionic gonadotropin using labeled-free Vitoria blue 4R as molecular probe. Biosens. Bioelectron. 2016, 85, 450–456. [Google Scholar] [CrossRef]
- Pu, H.; Xie, X.; Sun, D.-W.; Wei, Q.; Jiang, Y. Double strand DNA functionalized Au@Ag Nps for ultrasensitive detection of 17β-estradiol using surface-enhanced raman spectroscopy. Talanta 2019, 195, 419–425. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, R.; Chen, Y.; Shi, H.; Zhao, G. A simple one-step pretreatment, highly sensitive and selective sensing of 17β-estradiol in environmental water samples using surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2018, 254, 1157–1164. [Google Scholar] [CrossRef]
- Gao, S.; Pearson, B.; He, L. Mapping bacteria on filter membranes, an innovative SERS approach. J. Microbiol. Methods 2018, 147, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of Ag@TiO2 electrospinning nanofibrous felts as SERS substrate for direct and sensitive bacterial detection. Sens. Actuators B Chem. 2018, 273, 600–609. [Google Scholar] [CrossRef]
- Duan, N.; Yan, Y.; Wu, S.; Wang, Z. Vibrio parahaemolyticus detection aptasensor using surface-enhanced Raman scattering. Food Control 2016, 63, 122–127. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering(SERS)biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Sabharwal, P.K.; Jain, S.; Kaur, A.; Singh, H. Functionalized polymeric magnetic nanoparticle assisted SERS immunosensor for the sensitive detection of S. typhimurium. Anal. Chim. Acta 2019, 1067, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.; Sohi, A.N.; Khatoon, Z.; Berthiaume, V.R.; Alarcon, E.I.; Godin, M.; Anis, H. Optofluidic label-free SERS platform for rapid bacteria detection in serum. Sens. Actuators B Chem. 2019, 300, 126907. [Google Scholar] [CrossRef]
- Ali, A.; Hwang, E.Y.; Choo, J.; Lim, D.W. Nanoscale graphene oxide-induced metallic nanoparticle clustering for surface-enhanced Raman scattering-based IgG detection. Sens. Actuators B Chem. 2018, 255, 183–192. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.; Huang, Q. Label-free SERS diagnostics of radiation-induced injury via detecting the biomarker Raman signal in the serum and urine bio-samples based on Au-NPs array substrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117282. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Li, S.; Xu, L.; Tian, Y.; Jiao, A.; Liu, X.; Chen, F.; Chen, M. Boron nitride/gold nanocomposites for crystal violet and creatinine detection by surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2018, 457, 684–694. [Google Scholar] [CrossRef]
- El-Said, W.A.; Alshitari, W.; Choi, J. Controlled fabrication of gold nanobipyramids/polypyrrole for shell-isolated nanoparticle-enhanced Raman spectroscopy to detect γ-aminobutyric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117890. [Google Scholar] [CrossRef]
- Franco, D.; De Plano, L.M.; Rizzo, M.G.; Scibilia, S.; Lentini, G.; Fazio, E.; Neri, F.; Guglielmino, S.P.P.; Mezzasalma, A.M. Bio-hybrid gold nanoparticles as SERS probe for rapid bacteria cell identification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117394. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Krajczewski, J.; Kowalik, A.; Weyher, J.L.; Dzięcielewski, I.; Chłopek, M.; Góźdź, S.; Nowicka, A.M.; Kudelski, A. New strategy for the gene mutation identification using surface enhanced Raman spectroscopy (SERS). Biosens. Bioelectron. 2019, 132, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Proll, G.; Markovic, G.; Fechner, P.; Proell, F.; Gauglitz, G. Reflectometric interference spectroscopy. In Biosensors and Biodetection: Methods and Protocols Volume 1: Optical-Based Detectors; Rasooly, A., Prickril, B., Eds.; Springer Science+Business Media LLC: New York, NY, USA, 2017; Vol. 1571, pp. 207–220. ISBN 978-1-4939-6846-6. [Google Scholar]
- Makiyan, F.; Rahimi, F.; Hajati, M.; Shafiekhani, A.; Rezayan, A.H.; Ansari-Pour, N. Label-free discrimination of single nucleotide changes in DNA by reflectometric interference Fourier transform spectroscopy. Colloids Surfaces B Biointerfaces 2019, 181, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, D.; Wang, K.; Bai, X.; Zhang, C.; Zhao, W.; Feng, X.; Bai, J. Label-free detecting oligonucleotide hybridization melting temperature in real-time with a reflectometric interference spectroscopy–based nanosensor system. Optik 2019, 192, 162903. [Google Scholar] [CrossRef]
- Nagatsuka, T.; Uzawa, H.; Tanaka, D.; Oba, Y.; Nishida, Y.; Iwasa, T.; Tayama, K.; Yoshida, T.; Ezaki, T.; Seto, Y. Preparation of silicon nitride biochips for reflectometric interference spectroscopic (RIfS) analysis of biological toxins and E. coli O157:H7 strain. Sens. Actuators B Chem. 2017, 246, 937–942. [Google Scholar] [CrossRef]
- Weber, P.; Riegger, B.R.; Niedergall, K.; Tovar, G.E.M.; Bach, M.; Gauglitz, G. Nano-MIP based sensor for penicillin G: Sensitive layer and analytical validation. Sens. Actuators B Chem. 2018, 267, 26–33. [Google Scholar] [CrossRef]
- Yu, N.; Wu, J. Rapid and reagentless detection of thrombin in clinic samples via microfluidic aptasensors with multiple target-binding sites. Biosens. Bioelectron. 2019, 146, 111726. [Google Scholar] [CrossRef]
- Bender, J.; Bognar, S.; Camagna, M.; Donauer, J.A.M.; Eble, J.W.; Emig, R.; Fischer, S.; Jesser, R.; Keilholz, L.; Kokotek, D.M.U.; et al. Multiplexed antibody detection from blood sera by immobilization of in vitro expressed antigens and label-free readout via imaging reflectometric interferometry (iRIf). Biosens. Bioelectron. 2018, 115, 97–103. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Petrou, P.; Misiakos, K.; Drygiannakis, D.; Raptis, I.; Stefanitsis, G.; Martini, S.; Nikita, D.; Goustouridis, D.; Moser, I.; et al. Simultaneous determination of CRP and D-dimer in human blood plasma samples with White Light Reflectance Spectroscopy. Biosens. Bioelectron. 2016, 84, 89–96. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirzada, M.; Altintas, Z. Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines 2020, 11, 356. https://doi.org/10.3390/mi11040356
Pirzada M, Altintas Z. Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines. 2020; 11(4):356. https://doi.org/10.3390/mi11040356
Chicago/Turabian StylePirzada, Muqsit, and Zeynep Altintas. 2020. "Recent Progress in Optical Sensors for Biomedical Diagnostics" Micromachines 11, no. 4: 356. https://doi.org/10.3390/mi11040356
APA StylePirzada, M., & Altintas, Z. (2020). Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines, 11(4), 356. https://doi.org/10.3390/mi11040356