Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Azide-Gelatin and Photocleabable Crosslinker
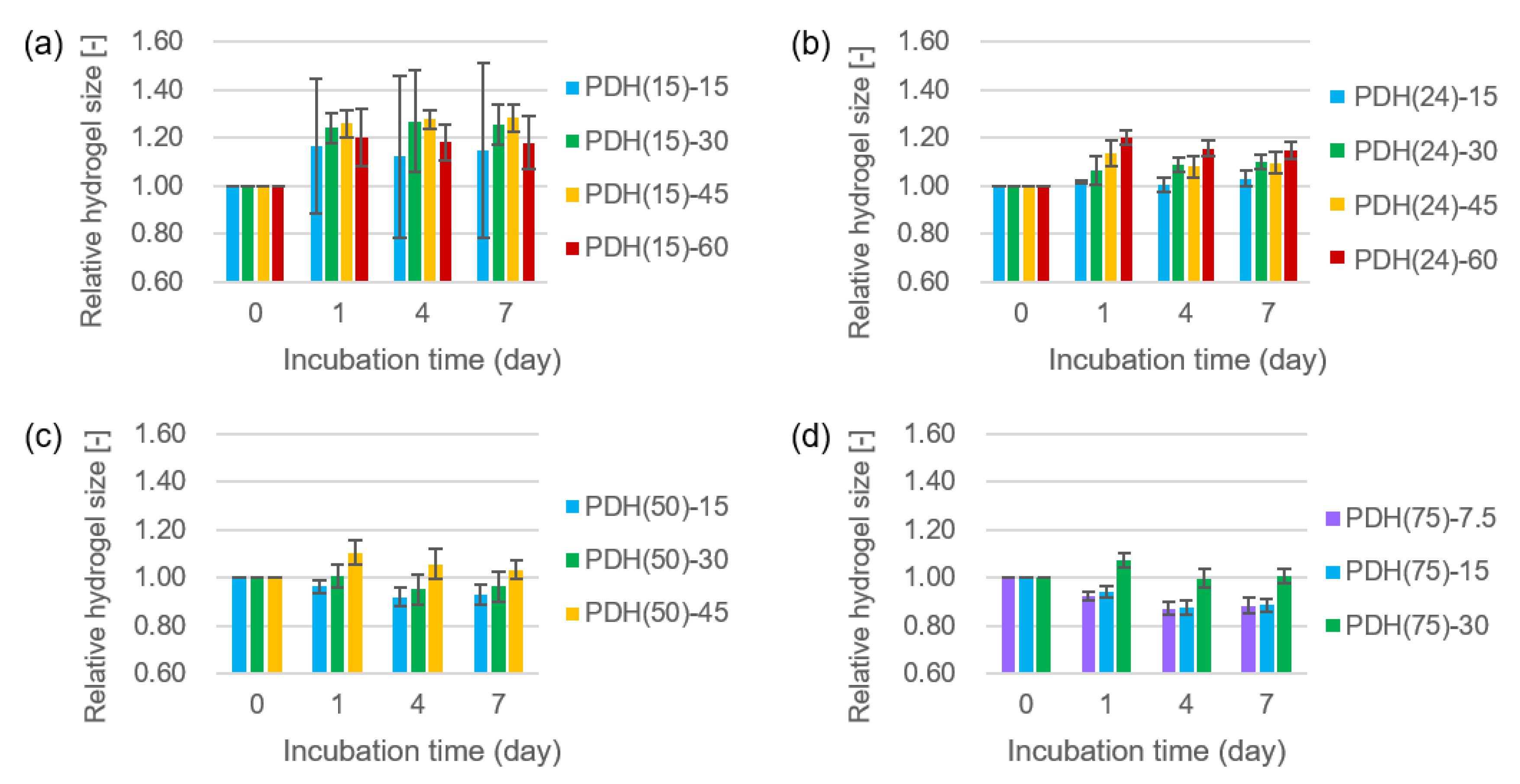
2.2. Size Change of Photodegradable Hydrogels
2.3. Cell Adhesion and Growth on Photodegradable Hydrogels
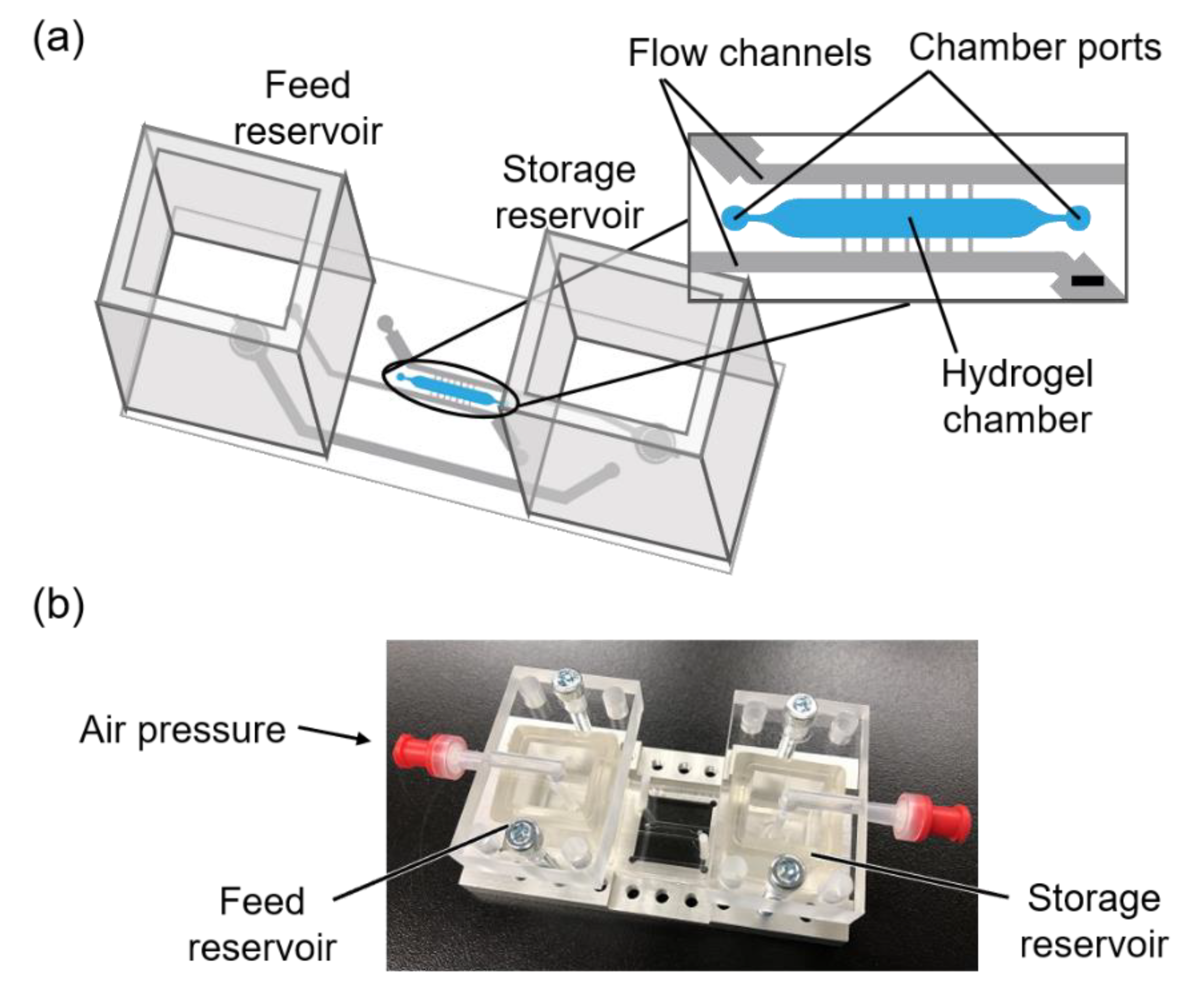
2.4. Fabrication of Microfluidic Device
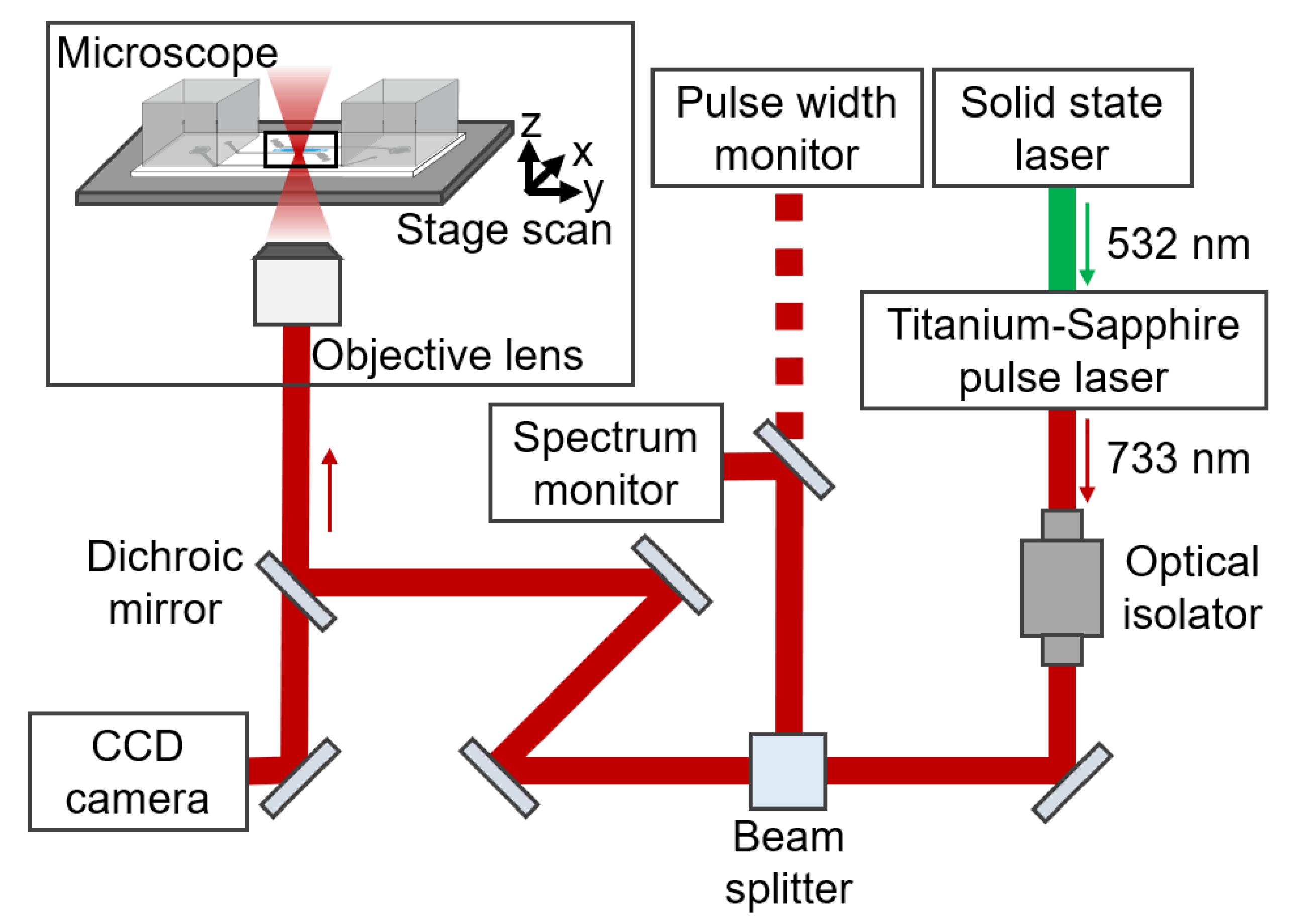
2.5. Setup of Laser Scanning
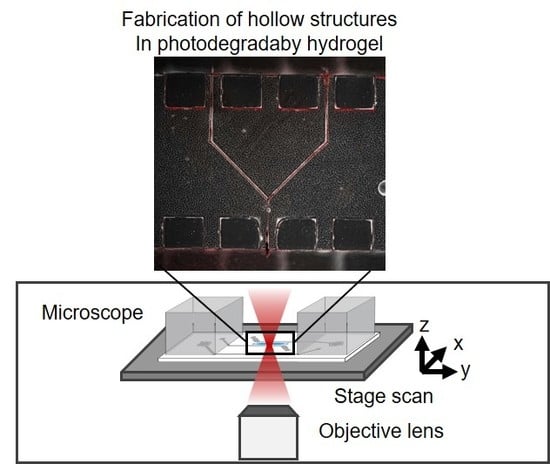
2.6. Fabrication of a Hollow Structure in a Photodegradable Hydrogel in the Microfluidic Device
2.7. Perfusion of Buffer in the Hollow Structure Fabricated in the Photodegradable Hydrogel
3. Results
3.1. Size Change of Photodegradable Hydrogels
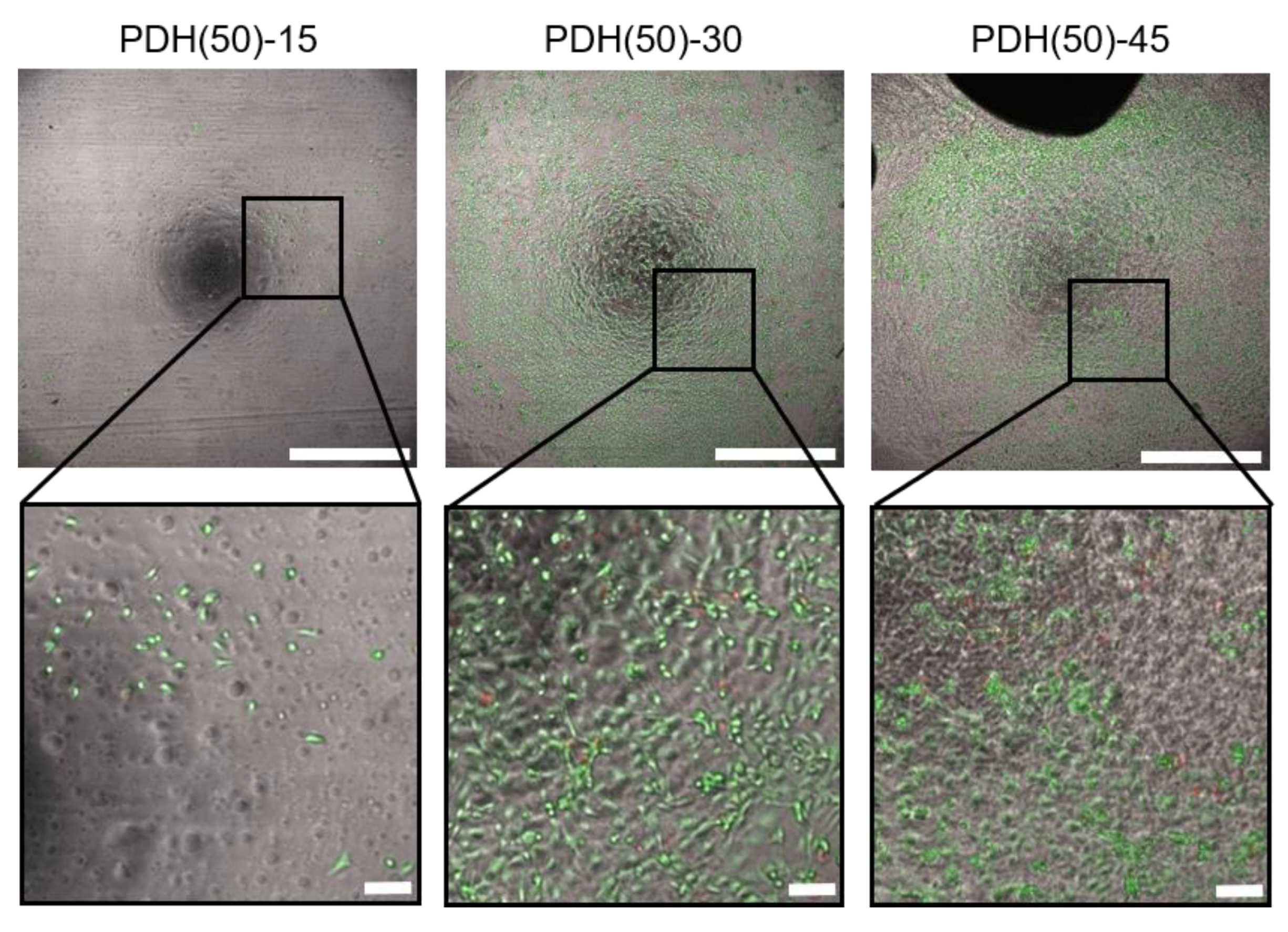
3.2. HUVEC Adhesion and Growth on Photodegradable Hydrogels
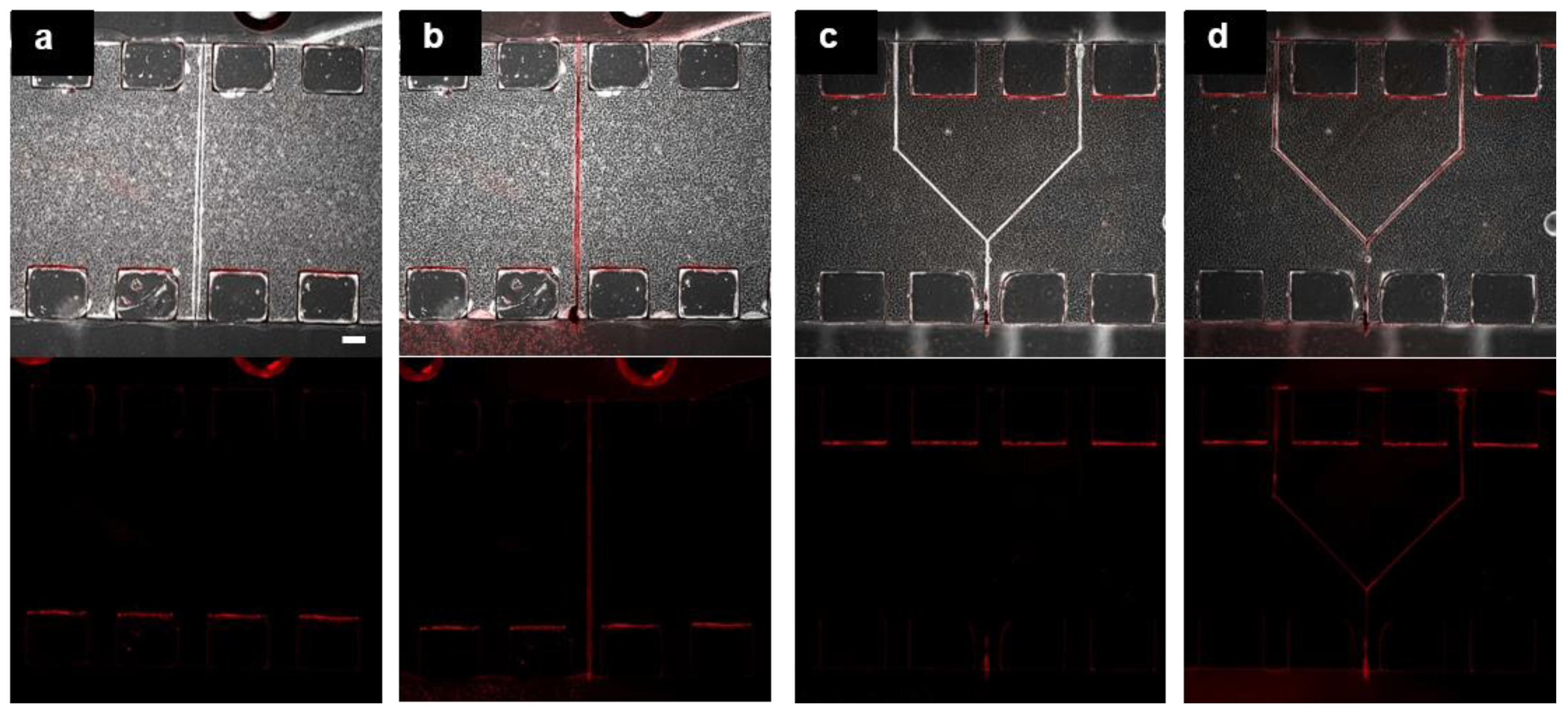
3.3. Fabrication of Hollow Structure Using Multi-Photon Excitation Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the Decline in Pharmaceutical R&D Efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [PubMed]
- Pammolli, F.; Magazzini, L.; Riccaboni, M. The Productivity Crisis in Pharmaceutical R&D. Nat. Rev. Drug Discov. 2011, 10, 428–438. [Google Scholar] [PubMed]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach, C.; López, J.A.; et al. In Vitro Microvessels for the Study of Angiogenesis and Thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Kataoka, N.; Ujita, S.; Sato, M. Effect of Flow Direction on the Morphological Responses of Cultured Bovine Aortic Endothelial Cells. Med. Biol. Eng. Comput. 1998, 36, 122–128. [Google Scholar] [CrossRef]
- Nishida, K.; Harrison, D.G.; Navas, J.P.; Fisher, A.; Dockery, S.P.; Uematsu, M.; Nerem, R.M.; Alexander, R.W.; Murphy, T.J. Molecular Cloning and Characterization of the Constitutive Bovine Aortic Endothelial Cell Nitric Oxide Synthase. J. Clin. Investig. 1992, 90, 2092–2096. [Google Scholar] [CrossRef]
- Levesque, M.J.; Nerem, R.M. Nerem. The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef]
- Duval, K.E.A.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2d Vs. 3d Cell Culture. Physiology (Bethesda) 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of Functional, Perfusable 3d Microvascular Networks on a Chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Chen, M.B.; Whisler, J.A.; Fröse, J.; Yu, C.; Shin, Y.; Kamm, R.D. Kamm. On-Chip Human Microvasculature Assay for Visualization and Quantification of Tumor Cell Extravasation Dynamics. Nat. Protoc. 2017, 12, 865–880. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3d Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F.; Tripathi, S.C. Mechanical Stress Mechanisms and the Cell. An Endothelial Paradigm. Circ. Res. 1993, 72, 239–245. [Google Scholar] [CrossRef]
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial Cellular Response to Altered Shear Stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, N. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Hattori, K.; Munehira, Y.; Kobayashi, H.; Satoh, T.; Sugiura, S.; Kanamori, T. Microfluidic Perfusion Culture Chip Providing Different Strengths of Shear Stress for Analysis of Vascular Endothelial Function. J. Biosci. Bioeng. 2014, 118, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Narazaki, G.; Sugita, R.; Kobayashi, H.; Sugiura, S.; Kanamori, T. A Pneumatic Pressure-Driven Multi-Throughput Microfluidic Circulation Culture System. Lab Chip 2016, 16, 2339–2348. [Google Scholar] [CrossRef]
- Kim, L.; Vahey, M.D.; Lee, H.-Y.; Voldman, J. Microfluidic Arrays for Logarithmically Perfused Embryonic Stem Cell Culture. Lab Chip 2006, 6, 394–406. [Google Scholar] [CrossRef]
- Hattori, K.; Sugiura, S.; Kanamori, T. Generation of Arbitrary Monotonic Concentration Profiles by a Serial Dilution Microfluidic Network Composed of Microchannels with a High Fluidic-Resistance Ratio. Lab Chip 2009, 9, 1763–1772. [Google Scholar] [CrossRef]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Lin, R.; Lee, L.P. Continuous Perfusion Microfluidic Cell Culture Array for High-Throughput Cell-Based Assays. Biotechnol. Bioeng. 2005, 89, 1–8. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing Complex 3d Tissue Physiology in Vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Bersini, S.; Moretti, M. 3d Functional and Perfusable Microvascular Networks for Organotypic Microfluidic Models. J. Mater. Sci. -Mater. Med. 2015, 26, 180. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-Dimensional Tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-in Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In Vitro Modeling of the Microvascular Occlusion and Thrombosis That Occur in Hematologic Diseases Using Microfluidic Technology. J. Clin. Investig. 2012, 122, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel Microfabrication Technology toward Three Dimensional Tissue Engineering. Regen. Ther. 2016, 3, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Stereolithography-Based Hydrogel Microenvironments to Examine Cellular Interactions. Adv. Funct. Mater. 2011, 21, 3642–3651. [Google Scholar] [CrossRef]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in Tissue Engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef]
- Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Camci-Unal, G.; Patel, A.; Khademhosseini, A.; Kanamori, T. Activated-Ester-Type Photocleavable Crosslinker for Preparation of Photodegradable Hydrogels Using a Two-Component Mixing Reaction. Adv. Healthc. Mater. 2015, 4, 246–254. [Google Scholar] [CrossRef]
- Deforest, C.A.; Anseth, K.S. Cytocompatible Click-Based Hydrogels with Dynamically Tunable Properties through Orthogonal Photoconjugation and Photocleavage Reactions. Nat. Chem. 2011, 3, 925–931. [Google Scholar] [CrossRef]
- Pradhan, S.; Keller, K.; Sperduto, J.L.; Slater, J.H. Fundamentals of Laser-Based Hydrogel Degradation and Applications in Cell and Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700681. [Google Scholar] [CrossRef]
- Arakawa, C.; Gunnarsson, C.; Howard, C.; Bernabeu, M.; Phong, K.; Yang, E.; Deforest, C.A.; Smith, J.D.; Zheng, Y. Biophysical and Biomolecular Interactions of Malaria-Infected Erythrocytes in Engineered Human Capillaries. Sci. Adv. 2020, 6, eaay7243. [Google Scholar] [CrossRef]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-Based Three-Dimensional Multiscale Micropatterning of Biocompatible Hydrogels for Customized Tissue Engineering Scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052. [Google Scholar] [CrossRef]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3d Biomimetic Microfluidic Networks in Hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M. In Situ Patterning of Microfluidic Networks in 3d Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. Laser Photoablation of Guidance Microchannels into Hydrogels Directs Cell Growth in Three Dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Lunzer, M.; Shi, L.; Andriotis, O.G.; Gruber, P.; Markovic, M.; Thurner, P.J.; Ossipov, D.A.; Liska, R.; Ovsianikov, A. A Modular Approach to Sensitized Two-Photon Patterning of Photodegradable Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 15122–15127. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Arakawa, C.; Badeau, B.A.; Zheng, Y.; Deforest, C.A. DeForest. Multicellular Vascularized Engineered Tissues through User-Programmable Biomaterial Photodegradation. Adv. Mater. 2017, 29, 1703156. [Google Scholar] [CrossRef]
- Tamura, M.; Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Kanamori, T. Click-Crosslinkable and Photodegradable Gelatin Hydrogels for Cytocompatible Optical Cell Manipulation in Natural Environment. Sci. Rep. 2015, 5, 15060. [Google Scholar] [CrossRef] [PubMed]
- Miedel, M.C.; Hulmes, J.D.; Pan, Y.-C.E. The Use of Fluorescamine as a Detection Reagent in Protein Microcharacterization. J. Biochem. Biophys. Methods 1989, 18, 37–52. [Google Scholar] [CrossRef]
- Shibuta, M.; Tamura, M.; Kanie, K.; Yanagisawa, M.; Matsui, H.; Satoh, T.; Takagi, T.; Kanamori, T.; Sugiura, S.; Kato, R. Imaging Cell Picker: A Morphology-Based Automated Cell Separation System on a Photodegradable Hydrogel Culture Platform. J. Biosci. Bioeng. 2018, 126, 653–660. [Google Scholar] [CrossRef]
- Deng, T.; Wu, H.; Brittain, S.T.; Whitesides, G.M. Whitesides. Prototyping of Masks, Masters, and Stamps/Molds for Soft Lithography Using an Office Printer and Photographic Reduction. Anal. Chem. 2000, 72, 3176–3180. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Whitesides. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 551–575. [Google Scholar] [CrossRef]
- Jie, M.; Li, H.-F.; Lin, L.; Zhang, J.; Lin, J.-M. Integrated Microfluidic System for Cell Co-Culture and Simulation of Drug Metabolism. RSC Adv. 2016, 6, 54564–54572. [Google Scholar] [CrossRef]
- Imura, Y.; Sato, K.; Yoshimura, E. Micro Total Bioassay System for Ingested Substances: Assessment of Intestinal Absorption, Hepatic Metabolism, and Bioactivity. Anal. Chem. 2010, 82, 9983–9988. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. A Micro Cell Culture Analog (Mu Cca) with 3-D Hydrogel Culture of Multiple Cell Lines to Assess Metabolism-Dependent Cytotoxicity of Anti-Cancer Drugs. Lab Chip 2009, 9, 1385–1394. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Rahim, N.A.A.; Van Noort, D.; Yu, H. Towards a Human-on-Chip: Culturing Multiple Cell Types on a Chip with Compartmentalized Microenvironments. Lab Chip 2009, 9, 3185–3192. [Google Scholar] [CrossRef]
- Prot, J.M.; Maciel, L.; Bricks, T.; Merlier, F.; Cotton, J.; Paullier, P.; Bois, F.Y.; Leclerc, E. First Pass Intestinal and Liver Metabolism of Paracetamol in a Microfluidic Platform Coupled with a Mathematical Modeling as a Means of Evaluating Adme Processes in Humans. Biotechnol. Bioeng. 2014, 111, 2027–2040. [Google Scholar] [CrossRef]
- Satoh, T.; Narazaki, G.; Sugita, R.; Kobyashi, H.; Sugiura, S.; Kanamori, T. Pneumatic Pressure-Driven Circulation Cell Culture Microfluidic Device for Investigation of Vascular Endothelial Cells under Shear Stress. In Proceedings of the 19th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Gyeongju, Korea, 25–29 October 2015; pp. 618–620. [Google Scholar]
- Sugiura, S.; Edahiro, J.-I.; Kikuchi, K.; Sumaru, K.; Kanamori, T. Pressure-Driven Perfusion Culture Microchamber Array for Parallel Drug Cytotoxicity Assay. Biotechnol. Bioeng. 2008, 100, 1156–1165. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.; Yue, K.; Levett, P.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacryloyl-Based Hydrogels as Modular Tissue Culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Yang, X.J.; Zheng, P.J.; Cui, Z.D.; Zhao, N.Q.; Wang, Y.F.; De Yao, K. Swelling Behaviour and Elastic Properties of Gelatin Gels. Polym. Int. 1997, 44, 448–452. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Huynh, V.; Jesmer, A.H.; Shoaib, M.M.; Wylie, R.G. Influence of Hydrophobic Cross-Linkers on Carboxybetaine Copolymer Stimuli Response and Hydrogel Biological Properties. Langmuir 2019, 35, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of Cell Binding to Collagen and Gelatin: A Study of the Effect of 2d and 3d Architecture and Surface Chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Massimi, M.; Di Rosario, B.; Nardecchia, S.; De Colli, M.; Devirgiliis, L.C.; Dentini, M. Emulsion Templated Scaffolds That Include Gelatin and Glycosaminoglycans. Biomacromolecules 2008, 9, 2844–2856. [Google Scholar] [CrossRef]
| Type of the Photodegradable Hydrogel | Type of Azide-Gelatin | Concentration (mg/mL) | |
|---|---|---|---|
| Azide-Gelatin | DBCO-PC-4arm PEG Crosslinker | ||
| PDH(15)-7.5 | Azide-gelatin (15) | 7.5 | 2.9 |
| PDH(15)-15 | 15 | 5.9 | |
| PDH(15)-30 | 30 | 11.7 | |
| PDH(15)-45 | 45 | 17.6 | |
| PDH(15)-60 | 60 | 23.5 | |
| PDH(24)-7.5 | Azide-gelatin (24) | 7.5 | 4.9 |
| PDH(24)-15 | 15 | 9.8 | |
| PDH(24)-30 | 30 | 19.5 | |
| PDH(24)-45 | 45 | 29.3 | |
| PDH(24)-60 | 60 | 39.1 | |
| PDH(50)-7.5 | Azide-gelatin (50) | 7.5 | 9.8 |
| PDH(50)-15 | 15 | 19.5 | |
| PDH(50)-30 | 30 | 39.1 | |
| PDH(50)-45 | 45 | 58.6 | |
| PDH(50)-60 | 60 | 78.2 | |
| PDH(75)-7.5 | Azide-gelatin (75) | 7.5 | 14.7 |
| PDH(75)-15 | 15 | 29.3 | |
| PDH(75)-30 | 30 | 58.6 | |
| PDH(75)-45 | 45 | 88.0 | |
| PDH(75)-60 | 60 | 117.3 | |
| Concentration of Azide-Gelatin Solutions (mg/mL) | Type of Azide-Gelatin | |||
|---|---|---|---|---|
| Azide-Gelatin (15) | Azide-Gelatin (24) | Azide-Gelatin (50) | Azide-Gelatin (75) | |
| 7.5 | d | d | d | 0.88/Partial |
| 15 | 1.15/Uniform | 1.03/Uniform | 0.93/Partial | 0.89/Partial |
| 30 | 1.26/Uniform | 1.10/Uniform | 0.96/Uniform | 1.01/Uniform |
| 45 | 1.28/Nonuniform | 1.09/Nonuniform | 1.03/Nonuniform | i |
| 60 | 1.18/Nonuniform | 1.15/Nonuniform | i | i |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, U.; Sugiura, S.; Kakehata, M.; Yanagawa, F.; Takagi, T.; Sumaru, K.; Satoh, T.; Tamura, M.; Hosokawa, Y.; Torizuka, K.; et al. Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering. Micromachines 2020, 11, 679. https://doi.org/10.3390/mi11070679
Watanabe U, Sugiura S, Kakehata M, Yanagawa F, Takagi T, Sumaru K, Satoh T, Tamura M, Hosokawa Y, Torizuka K, et al. Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering. Micromachines. 2020; 11(7):679. https://doi.org/10.3390/mi11070679
Chicago/Turabian StyleWatanabe, Uran, Shinji Sugiura, Masayuki Kakehata, Fumiki Yanagawa, Toshiyuki Takagi, Kimio Sumaru, Taku Satoh, Masato Tamura, Yoichiroh Hosokawa, Kenji Torizuka, and et al. 2020. "Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering" Micromachines 11, no. 7: 679. https://doi.org/10.3390/mi11070679
APA StyleWatanabe, U., Sugiura, S., Kakehata, M., Yanagawa, F., Takagi, T., Sumaru, K., Satoh, T., Tamura, M., Hosokawa, Y., Torizuka, K., & Kanamori, T. (2020). Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering. Micromachines, 11(7), 679. https://doi.org/10.3390/mi11070679