Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes
Abstract
:1. Introduction
2. Materials and Methods
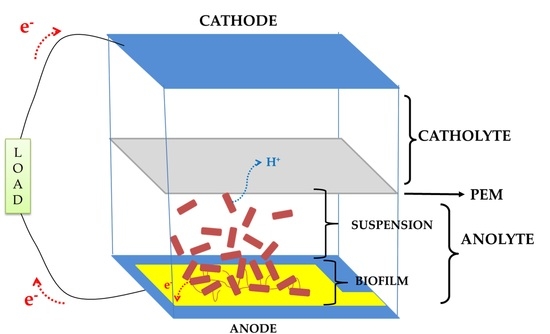
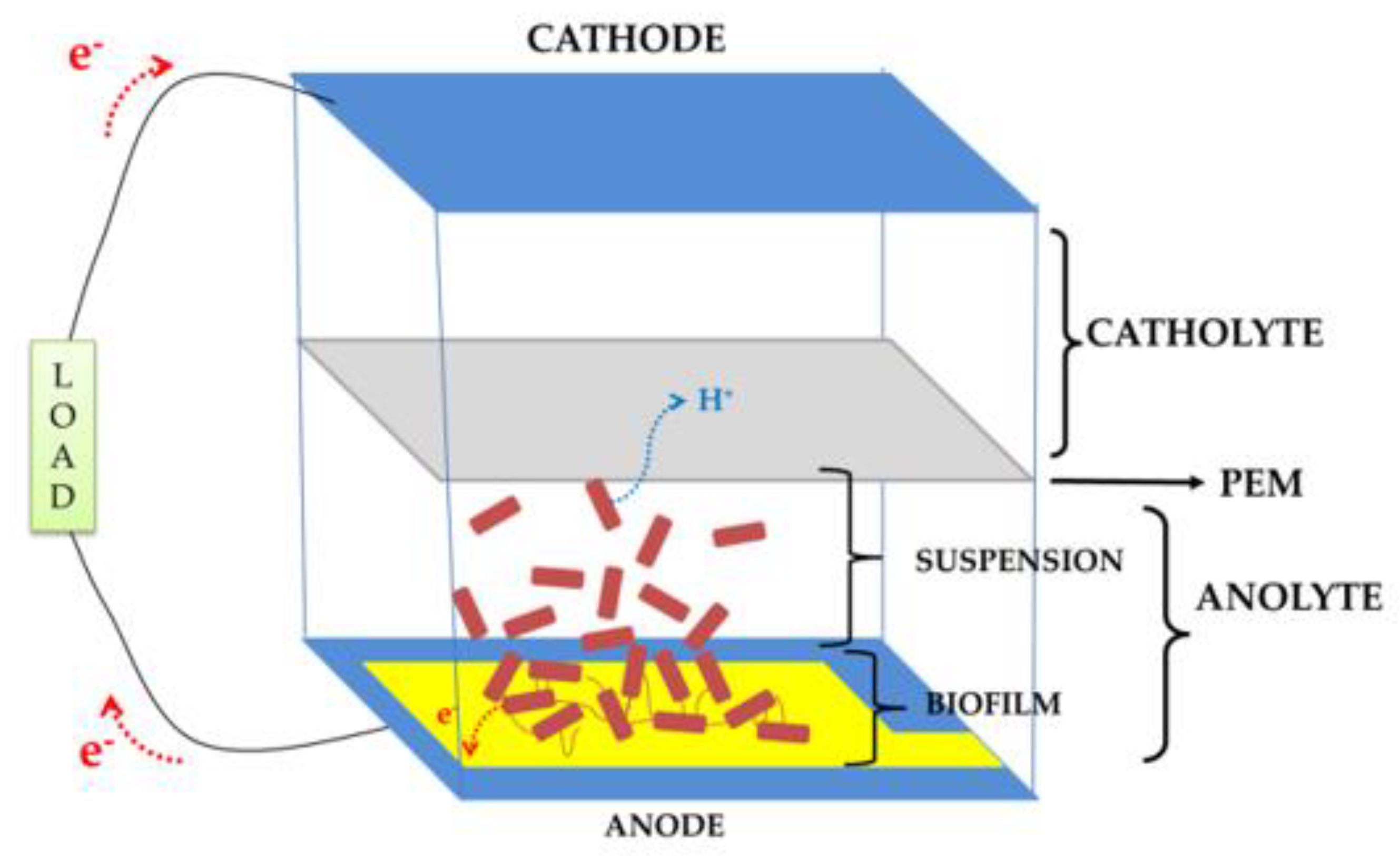
2.1. Device Design, Fabrication, and Assembly
2.2. Surface Modification with Thiols
2.3. Inoculum
2.4. MFC Operation
2.5. Performance Evaluation
2.6. Biofilm Analysis
3. Results and Discussion
3.1. Characterization of Thiolated Gold Surfaces
3.2. µMFC Performance
3.3. EIS Characterization
3.4. Characterization of Biofilms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, Z.; Li, H.; Gu, T. A State of the Art Review on Microbial Fuel Cells: A Promising Technology for Wastewater Treatment and Bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug Juice: Harvesting Electricity with Microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial Fuel Cells: Novel Biotechnology for Energy Generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter Sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dávila, D.; Esquivel, J.P.; Sabaté, N.; Masa, J. Silicon-based microfabricated microbial fuel cell toxicity sensor. Biosens. Bioelectron. 2011, 26, 2426–2430. [Google Scholar] [CrossRef]
- Choi, S. Microscale microbial fuel cells: Advances and challenges. Biosens. Bioelectron. 2015, 69, 8–25. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, B.; Tang, X. Microbial Fuel Cell-Based Biosensors. Biosensors 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Qian, F.; Baum, M.; Gu, Q.; Morse, D.E. A 1.5 µL microbial fuel cell for on-chip bioelectricity generation. Lab Chip 2009, 9, 3076–3081. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.-S.; Yang, Y.; Parameswaran, P.; Torres, C.I.; Rittmann, B.E.; Chae, J. A μL-scale Micromachined Microbial Fuel Cell Having High Power Density. Lab Chip 2011, 11, 1110–1117. [Google Scholar] [CrossRef]
- Ren, H.; Lee, H.-S.; Chae, J. Miniaturizing microbial fuel cells for potential portable power sources: Promises and challenges. Microfluid. Nanofluidics 2012, 13, 353–381. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bernarda, A.; Huang, C.Y.; Lee, D.J.; Chang, J.S. Micro-sized microbial fuel cell: A mini-review. Bioresour. Technol. 2011, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chae, J. Optimal biofilm formation and power generation in a micro-sized microbial fuel cell (MFC). Sens. Actuators A Phys. 2013, 195, 206–212. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in microbial fuel cells toward bioelectricity generation: A review. Int. J. Energy Res. 2015, 39, 1048–1067. [Google Scholar] [CrossRef]
- Read, S.T.; Dutta, P.; Bond, P.L.; Keller, J.; Rabaey, K. Initial Development and Structure of Biofilms on Microbial Fuel Cell Anodes. BMC Microbiol. 2010, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef] [Green Version]
- Angelaalincy, M.J.; Krishnaraj, R.N.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm Engineering Approaches for Improving the Performance of Microbial Fuel Cells and Bioelectrochemical Systems. Front. Energy Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Friman, H.; Schechter, A.; Ioffe, Y.; Nitzan, Y.; Cahan, R. Current production in a microbial fuel cell using a pure culture of C upriavidus basilensis growing in acetate or phenol as a carbon source. Microb. Biotechnol. 2013, 6, 425–434. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 60–81. [Google Scholar] [CrossRef]
- Mink, J.E.; Qaisi, R.M.; Logan, B.E.; Hussain, M.M. Energy harvesting from organic liquids in micro-sized microbial fuel cells. NPG Asia Mater. 2014, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems†. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crittenden, S.R.; Sund, C.J.; Sumner, J.J. Mediating Electron Transfer from Bacteria to a Gold Electrode via a Self-Assembled Monolayer. Langmuir 2006, 22, 9473–9476. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of Surface Charge and Hydrophobicity on Anodic Biofilm Formation, Community Composition, and Current Generation in Bioelectrochemical Systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Füeg, M.; Borjas, Z.; Estevez-Canales, M.; Esteve-Núñez, A.; Pobelov, I.V.; Broekmann, P.; Kuzume, A. Interfacial electron transfer between Geobacter sulfurreducens and gold electrodes via carboxylate-alkanethiol linkers: Effects of the linker length. Bioelectrochemistry 2019, 126, 130–136. [Google Scholar] [CrossRef] [PubMed]
- La, J.A.; Jeon, J.-M.; Sang, B.-I.; Yang, Y.-H.; Cho, E.C. A Hierarchically Modified Graphite Cathode with Au Nanoislands, Cysteamine, and Au Nanocolloids for Increased Electricity-Assisted Production of Isobutanol by Engineered Shewanella oneidensis MR-1. Appl. Mater. Interfaces 2017, 9, 43563–43574. [Google Scholar] [CrossRef]
- Lowy, D.A.; Tender, L.M.; Zeikus, J.G.; Park, D.H.; Lovley, D.R. Harvesting Energy From the Marine Sediment-Water Interface II. Kinetic Activity of Anode Materials. Biosens. Bioelectron. 2006, 15, 2058–2063. [Google Scholar] [CrossRef]
- Şen Doğan, B.; Afşar Erkal, N.; Özgür, E.; Zorlu, Ö.; Külah, H. Performance Enhancement of Mems-Based Microbial Fuel Cells (μMFC) For Microscale Power Generation. J. Phys. Conf. Ser. 2016, 773, 012018. [Google Scholar] [CrossRef]
- Khan, A.; Jawaid, M.; Neppolian, B.; Asiri, A.M. Graphene Functionalization and Nanopolymers. In Graphene Functionalization Strategies: From Synthesis to Applications; Springer: Berlin, Germany, 2019; p. 170. [Google Scholar]
- Abaci, S.; Shannon, C. The influence of decanethiol/4-aminothiophenol mixed monolayers on the electrodeposition of polyaniline thin films. Electrochim. Acta 2005, 50, 2967–2973. [Google Scholar] [CrossRef]
- Hayes, W.A.; Shannon, C. Electrochemistry of Surface-Confined Mixed Monolayers of 4-Aminothiophenol and Thiophenol on Au. Langmuir 1996, 12, 3688–3694. [Google Scholar] [CrossRef]
- Sigma-Aldrich Technical Bulletin AL-266. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/Instructions/1/al-266.pdf?utm_source=redirect&utm_medium=promotional&utm_campaign=insite_al_techbull_al266 (accessed on 29 June 2020).
- Shen, H.; Mark, J.E.; Seliskar, C.J.; Mark, H.B., Jr.; Heineman, W.R. Blocking behavior of self-assembled monolayers on gold electrodes. J. Solid State Electrochem. 1997, 1, 148–154. [Google Scholar] [CrossRef]
- Ashaduzzaman, M.; Antony, A.A.; Murugan, N.A.; Deshpande, S.R.; Turner, A.; Tiwari, A. Studies on an on/off-switchable immunosensor for troponin T. Biosens. Bioelectron. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Afara, N.; Omanovic, S.; Asghari-Khiavi, M. Functionalization of a gold surface with fibronectin (FN) covalently bound to mixed alkanethiol self-assembled monolayers (SAMs): The influence of SAM composition on its physicochemical properties and FN surface secondary structure. Thin Solid Films 2012, 522, 381–389. [Google Scholar] [CrossRef]
- Veiseh, M.; Zareie, M.H.; Zhang, M. Highly selective protein patterning on gold-silicon substrates for biosensor applications. Langmuir 2002, 14, 6671–6678. [Google Scholar] [CrossRef]
- Dharanivasan, G.; Rajamuthuramalingam, T.; Jesse, D.M.I.; Rajendiran, N.; Kathiravan, K. Gold nanoparticles assisted characterization of amine functionalized polystyrene multiwell plate and glass slide surfaces. Appl. Nanosci. 2015, 5, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Borole, A.P.; Reguera, G.; Ringeisen, B.; Wang, Z.-W.; Feng, Y.; Kim, B.H. Electroactive biofilms: Current status and future research needs. Energy Environ. Sci. 2011, 4, 4813–4834. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Hendeson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Rozenfeld, S.; Hirsch, L.O.; Gandu, B.; Farber, R.; Schechter, A.; Cahan, R. Improvement of Microbial Electrolysis Cell Activity by Using Anode Based on Combined Plasma-Pretreated Carbon Cloth and Stainless Steel. Energies 2019, 12, 1968. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology†. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Jia, Y.; Feng, H.; Shen, D.; Zhou, Y.; Chen, T.; Wang, M.; Chen, W.; Ge, Z.; Huang, L.; Zheng, S. High-performance microbial fuel cell anodes obtained from sewage sludge mixed with fly ash. J. Hazard. Mater. 2018, 354, 27–32. [Google Scholar] [CrossRef]
- Du, Q.; An, J.; Li, J.; Zhou, L.; Li, N.; Wang, X. Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells. J. Power Sources 2017, 343, 477–482. [Google Scholar] [CrossRef]
- Vigolo, D.; Al-Housseiny, T.T.; Shen, Y.; Akinlawon, F.O.; Al-Housseiny, S.T.; Hobson, R.K.; Sahu, A.; Bedkowski, K.I.; DiChristina, T.J.; Stone, H.A. Flow dependent performance of microfluidic microbial fuel cells†. Phys. Chem. Chem. Phys. 2014, 16, 12535–12543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ali, M.A.; Xu, Z.; Halverson, L.J.; Dong, L. Integrated Microfluidic Flow-Through Microbial Fuel Cells. Sci. Rep. 2017, 7, 41208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 15, 3388–3394. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Modification of anode electrode in microbial fuel cell for electrochemical recovery of energy and copper metal. Electrochim. Acta 2018, 275, 8–17. [Google Scholar] [CrossRef]
- Torres, C.I.; Kato Marcus, A.; Rittmann, B. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 2008, 100, 872–881. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Li, J.; Liao, Q.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zheng, J.; Zhang, L.; Fu, Q.; Zhu, X.; Liao, Q. Response of anodic biofilm and the performance of microbial fuel cells to different discharging current densities. Bioresour. Technol. 2017, 233, 1–6. [Google Scholar] [CrossRef]







| System | Surface Treatment | Acclimatization Condition |
|---|---|---|
| BG-OCV | Bare gold | Under OCV conditions |
| BG-25 | Bare gold | Under 25 kΩ load |
| CYS-OCV | Gold surface modified with Cysteamine | Under OCV conditions |
| CYS-25 | Gold surface modified with Cysteamine | Under 25 kΩ load |
| MUA-OCV | Gold surface modified with 11-MUA | Under OCV conditions |
| MUA-25 | Gold surface modified with 11-MUA | Under 25 kΩ load |
| ATP-OCV | Gold surface modified with 4-ATP | Under OCV conditions |
| Surface | Contact Angle |
|---|---|
| Bare gold | 65° |
| Gold surface modified with cysteamine | 52° |
| Gold surface modified with 11-MUA | 53.7° |
| Gold surface modified with 4-ATP | 52° |
| Parameter | BG-OCV | BG-25 | CYS-OCV | CYS-25 | MUA-OCV |
|---|---|---|---|---|---|
| Load during operation | OCV | 25 kΩ | OCV | 25 kΩ | OCV |
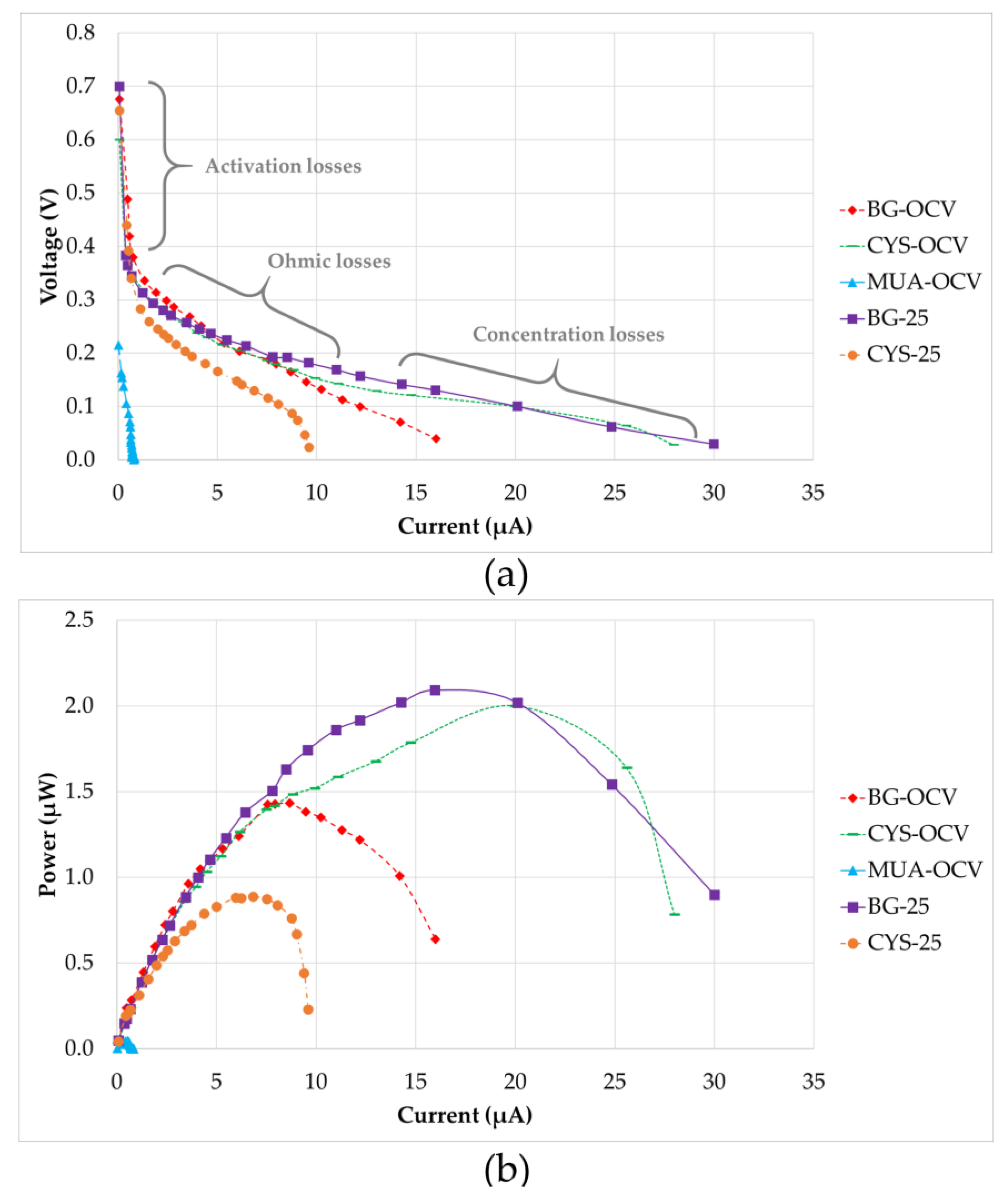
| Start-up time | 70 h | 48 h | 30 h | 42 h | 27 h |
| Internal resistance | 19 kΩ | 8 kΩ | 6 kΩ | 23 kΩ | 214 kΩ |
| Maximum power | 1.433 µW | 2.093 µW | 2.000 µW | 0.889 µW | 0.046 µW |
| Current at max. power | 8.684 µA | 15.976 µA | 20.000 µA | 6.842 µA | 0.527 µA |
| Parameter | BG-25 | CYS-OCV | Qian et al., 2009 [7] | Vigolo et al., 2014 [44] |
|---|---|---|---|---|
| Bacteria | S. oneidensis MR-1 | S. oneidensis MR-1 | S. oneidensis MR-1 | S. oneidensis MR-1 |
| Fuel cell geometry | Double chamber with Nafion-117 | Double chamber with Nafion-117 | Double chamber with Nafion-117 | Double chamber with Nafion-117 |
| Process type | Continuous | Continuous | Batch | Continuous |
| Anode area | 0.61 cm2 | 0.61 cm2 | 0.15 cm2 | 0.5 cm2 |
| Anode volume | 10.4 µL | 10.4 µL | 5 µL | 5 µL |
| Chamber depth | 170 µm | 170 µm | 100 µm | 100 µm |
| Anode/Cathode materials | Au/Au | Au/Au | Au/Carbon cloth | Carbon paper/Carbon paper |
| Anolyte/Catholyte | TSB/K3Fe(CN)6 | TSB/K3Fe(CN) | TSB/K3Fe(CN) | TSB/K3Fe(CN) |
| Load | 25 kΩ | OCV | 100 Ω | 100 kΩ |
| Start-up time | 48 h | 30 h | 47 h | 160 h(15 h lag time) |
| Internal resistance | 8 kΩ | 6 kΩ | 30 kΩ | 49 kΩ |
| Volumetric power density | 330 µW/cm3 | 315 µW/cm3 | 15 µW/cm3 | 900 µW/cm3 |
| Areal power density | 3.4 µW/cm2 | 3.3 µW/cm2 | 0.15 µW/cm2 | 9 µW/cm2 |
| Volumetric current density | 2515 µA/cm3 | 3148 µA/cm3 | 1300 µA/cm3 | 6200 µA/cm3 |
| Areal current density | 26.1 µA/cm2 | 32.7 µA/cm2 | 13 µA/cm2 | 62 µA/cm2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen-Doğan, B.; Okan, M.; Afşar-Erkal, N.; Özgür, E.; Zorlu, Ö.; Külah, H. Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes. Micromachines 2020, 11, 703. https://doi.org/10.3390/mi11070703
Şen-Doğan B, Okan M, Afşar-Erkal N, Özgür E, Zorlu Ö, Külah H. Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes. Micromachines. 2020; 11(7):703. https://doi.org/10.3390/mi11070703
Chicago/Turabian StyleŞen-Doğan, Begüm, Meltem Okan, Nilüfer Afşar-Erkal, Ebru Özgür, Özge Zorlu, and Haluk Külah. 2020. "Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes" Micromachines 11, no. 7: 703. https://doi.org/10.3390/mi11070703
APA StyleŞen-Doğan, B., Okan, M., Afşar-Erkal, N., Özgür, E., Zorlu, Ö., & Külah, H. (2020). Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes. Micromachines, 11(7), 703. https://doi.org/10.3390/mi11070703