Biomimetic Functional Surfaces towards Bactericidal Soft Contact Lenses
Abstract
:1. Introduction
2. Bacterial Adhesion and Rupture Mechanism
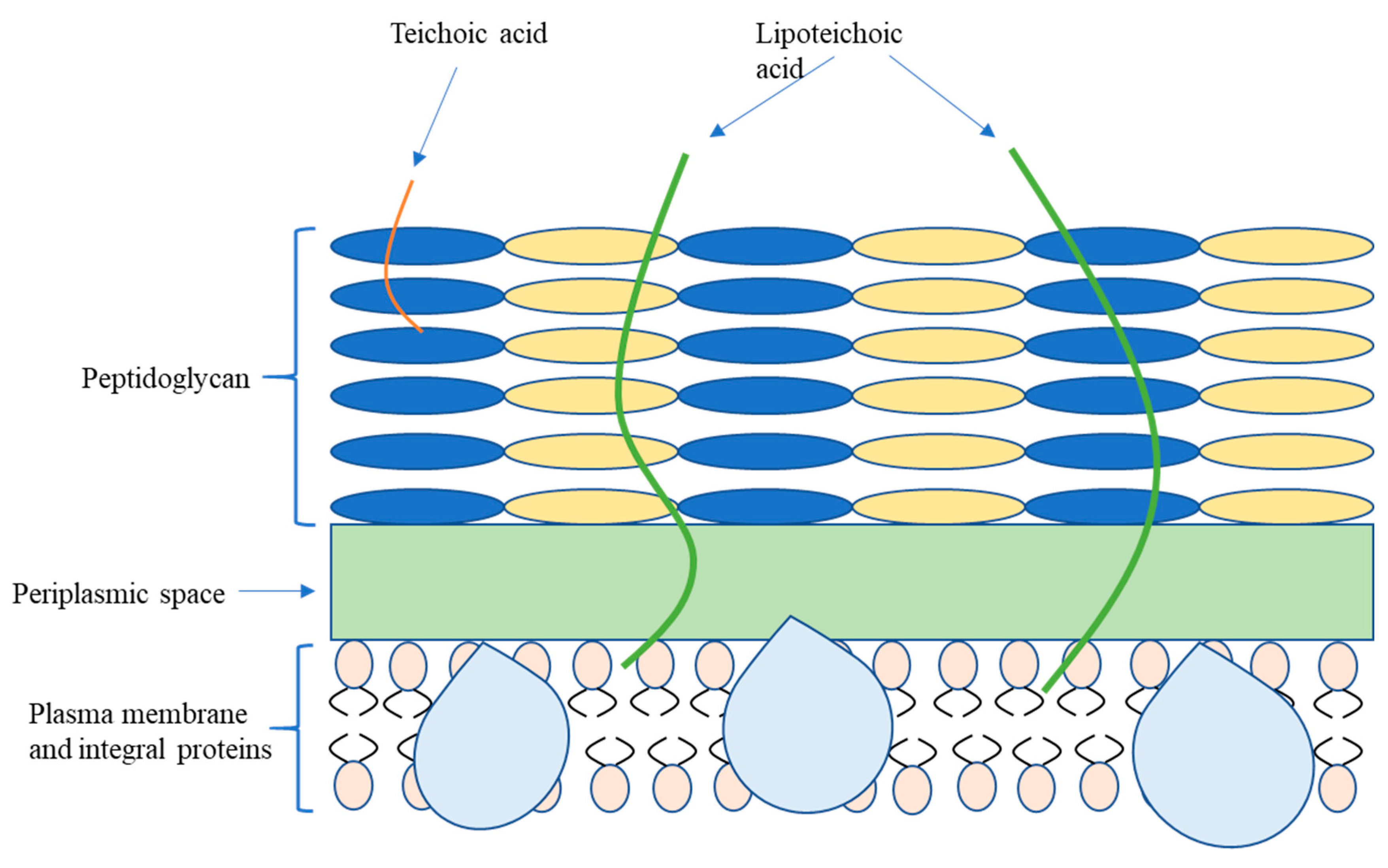
2.1. Bacterial Classification and Membrane Structure
2.2. Pathogenic Microorganism on Various Exterior
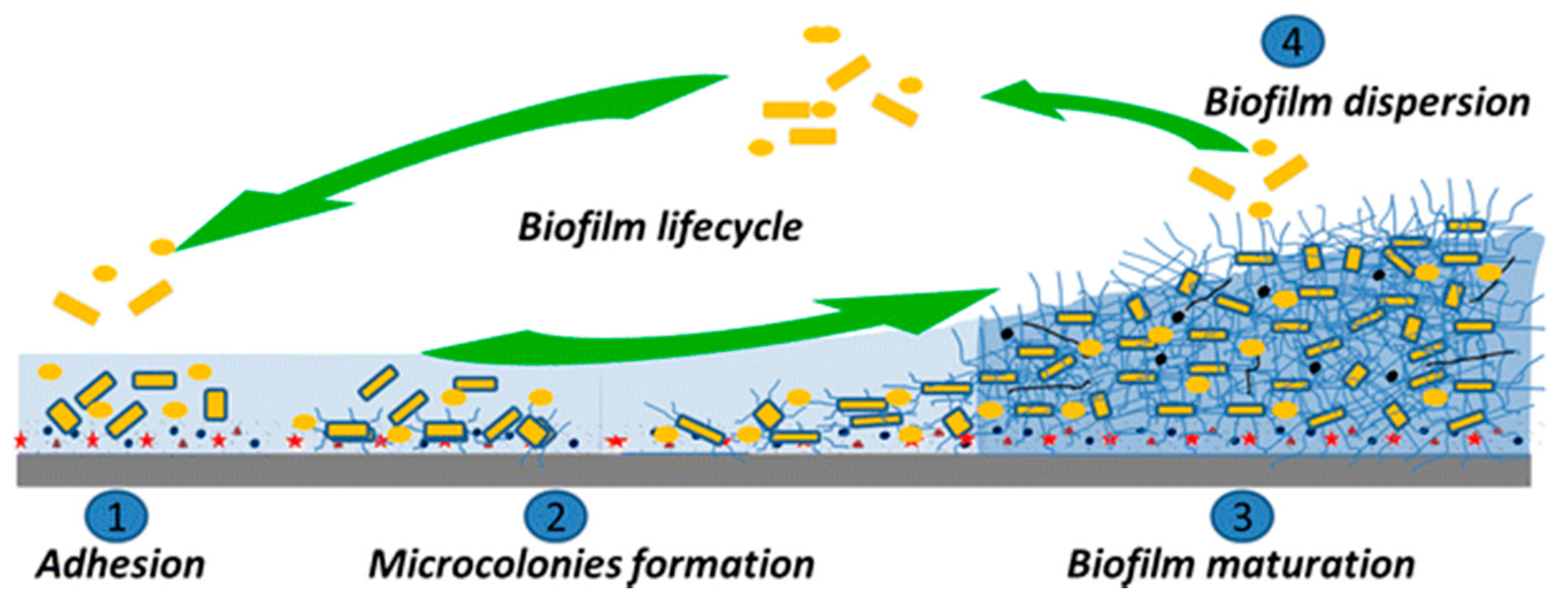
2.3. Bacterial Colonization and Biofilm Formation
2.4. Interaction between Bacteria and Topographical Surface
2.4.1. Conventional Bacterial Adhesion Mechanism
2.4.2. Perspectives of Contemporary Nanostructure–Membrane Interaction
3. Bactericidal Properties of Natural Antimicrobial Surfaces
3.1. Naturally Occurring Antibiofouling Surfaces
3.2. Bactericidal Efficacy of Natural Nanostructure Surface
4. Artificial Biomimetic Surface Development
4.1. Characterization of Bio-Inspired Surfaces
4.2. Systematic Analysis on Biomimetic Basis and Derivates
5. Prospects for the Development of Biomimetic Bactericidal Surfaces in Soft Contact Lenses
5.1. Bacterial Infection on SCL
5.2. Predisposing Factors of SCL-Induced Biofouling
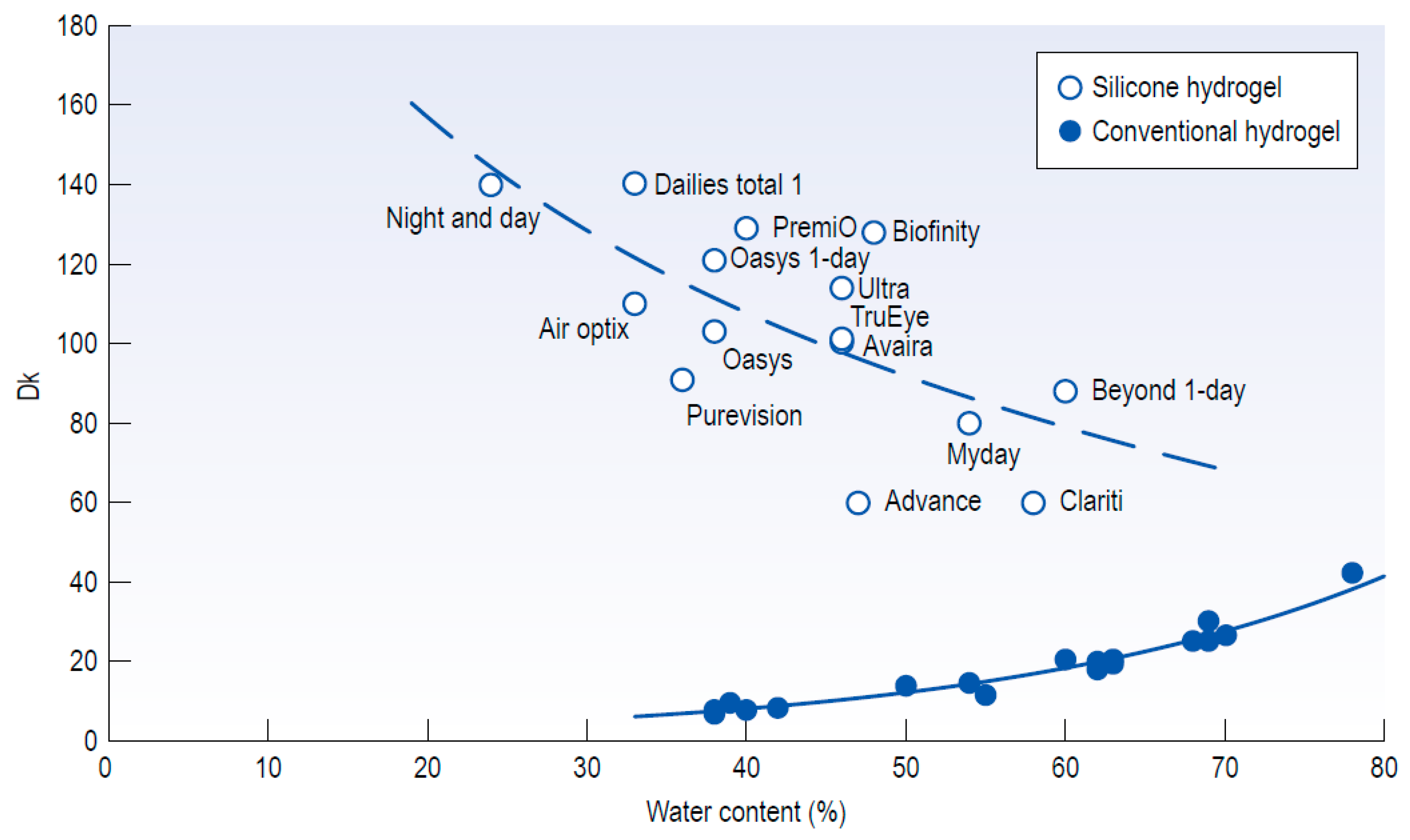
5.3. Nanostructure Fabrication on the Hydrogel Materials
6. Conclusions
Funding
Conflicts of Interest
References
- Huang, C.; Yu, F.P.; Feters, G.A.M.C.; Stewart, P.S. Nonuniform Spatial Patterns of Respiratory Activity within Biofilms during Disinfection. Appl. Environ. Microbiol. 1995, 61, 2252–2256. [Google Scholar] [CrossRef] [Green Version]
- Redfield, R.R. Antibiotic resistance threats in the United States. Centers Dis. Control Prev. 2019, 148, 938–941. [Google Scholar]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Sun, Y.; Gleichauf, K.; Lou, J.; Li, Q. Nanostructure on taro leaves resists fouling by colloids and bacteria under submerged conditions. Langmuir 2011, 27, 10035–10040. [Google Scholar] [CrossRef]
- Chien, H.W.; Chen, X.Y.; Tsai, W.P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces 2020, 186, 110738. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [Green Version]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowlin, K.; Boseman, A.; Covell, A.; LaJeunesse, D. Adhesion-dependent rupturing of Saccharomyces cerevisiae on biological antimicrobial nanostructured surfaces. J. R. Soc. Interface 2014, 12, 20140999. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, S.; Truong, V.K.; Walter, V.; Thalmann, F.; Marques, C.M.; Hanssen, E.; Vongsvivut, J.; Tobin, M.J.; Baulin, V.A.; Juodkazis, S.; et al. Interaction of Giant Unilamellar Vesicles with the Surface Nanostructures on Dragonfly Wings. Langmuir 2019, 35, 2422–2430. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Webb, H.K.; Hasan, J.; Truong, V.K.; Lamb, R.N.; Duan, X.; Tobin, M.J.; Mahon, P.J.; Crawford, R.J. Molecular Organization of the Nanoscale Surface Structures of the Dragonfly Hemianax papuensis Wing Epicuticle. PLoS ONE 2013, 8, e67893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Tsibouklis, J.; Stone, M.; Thorpe, A.A.; Graham, P.; Peters, V.; Heerlien, R.; Smith, J.R.; Green, K.L.; Nevell, T.G. Preventing bacterial adhesion onto surfaces: The low-surface-energy approach. Biomaterials 1999, 20, 1229–1235. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Szczotka-Flynn, L.B.; Pearlman, E.; Ghannoum, M. Microbial contamination of contact lenses, lens care solutions, and their accessories: A literature review. Eye Contact Lens 2010, 36, 116–129. [Google Scholar] [CrossRef]
- Ofek, I.; Doyle, R.J. Bacterial Adhesion to Cells and Tissues; Springer Science & Business Media: Berlin, Germany, 1994. [Google Scholar]
- Allison, D.G.; Lambert, P.A. Modes of Action of Antibacterial Agents; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 1–3. [Google Scholar]
- Martinez de Tejada, G.; Sanchez-Gomez, S.; Razquin-Olazaran, I.; Kowalski, I.; Kaconis, Y.; Heinbockel, L.; Andra, J.; Schurholz, T.; Hornef, M.; Dupont, A.; et al. Bacterial Cell Wall Compounds as Promising Targets of Antimicrobial Agents I. Antimicrobial Peptides and Lipopolyamines. Curr. Drug Targets 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.; Bassler, B.L. Bacterial Quorum-Sensing Network Architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, D.E. Symbiosis Therapy: The Potential of Using Human Protozoa for Molecular Therapy. Mol. Ther. 2000, 2, 535–538. [Google Scholar] [CrossRef]
- Jiang, R.H.Y. Dynamics of effectors in host–pathogen interactions. Mycology 2011, 2, 210–217. [Google Scholar]
- Balloux, F.; van Dorp, L. Q&A: What are pathogens, and what have they done to and for us? BMC Biol. 2017, 15, 91. [Google Scholar]
- Heß, S.; Lüddeke, F.; Gallert, C. Concentration of facultative pathogenic bacteria and antibiotic resistance genes during sewage treatment and in receiving rivers. Water Sci. Technol. 2016, 74, 1753–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emori, T.G.; Gaynes, R.P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 1993, 6, 428–442. [Google Scholar] [CrossRef]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1039–1044. [Google Scholar] [PubMed]
- Lee, D.S.; Lee, S.-J.; Choe, H.-S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. Biomed Res. Int. 2018, 2018, 7656752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Michalska, M.; Gambacorta, F.; Divan, R.; Aranson, I.S.; Sokolov, A.; Noirot, P.; Laible, P.D. Tuning antimicrobial properties of biomimetic nanopatterned surfaces. Nanoscale 2018, 10, 6639–6650. [Google Scholar] [CrossRef]
- Lappin-Scott, H.M.; Costerton, J.W. Microbial Biofilms; Cambridge University Press: Cambridge, UK, 2003; Volume 5. [Google Scholar]
- Sharma, A.; Jamali, H.; Vaishnav, A.; Giri, B.S.; Srivastava, A.K. Chapter 15—Microbial biofilm: An Advanced Eco-Friendly Approach for Bioremediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 205–219. [Google Scholar]
- Yadav, S.; Chandra, R. Chapter 14—Biofilm-Mediated Bioremediation of Pollutants from the Environment for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–203. ISBN 978-0-444-64279-0. [Google Scholar]
- Angelaalincy, M.J.; Navanietha Krishnaraj, R.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm Engineering Approaches for Improving the Performance of Microbial Fuel Cells and Bioelectrochemical Systems. Front. Energy Res. 2018, 6, 63. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Saxena, A.K. Chapter 18—Microbial Biofilms: Functional Annotation and Potential Applications in Agriculture and Allied Sectors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–301. [Google Scholar]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- El Moustaid, F.; Eladdadi, A.; Uys, L. Modeling bacterial attachment to surfaces as an early stage of biofilm development. Math. Biosci. Eng. 2013, 10, 821–842. [Google Scholar]
- Krasteva, P.V.; Jiunn, J.C.; Shikuma, N.J.; Beyhan, S.; Navarro, M.V.; Yildiz, F.H.; Sondermann, H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 2010, 327, 866–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elimelech, M. Particle deposition on ideal collectors from dilute flowing suspensions: Mathematical formulation, numerical solution, and simulations. Sep. Technol. 1994, 4, 186–212. [Google Scholar] [CrossRef]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by Gram--negative bacteria. Microb. Biofilms 2015, 163–199. [Google Scholar] [CrossRef] [Green Version]
- Bixler, G.D.; Bhushan, B. Rice and butterfly wing effect inspired low drag and antifouling surfaces: A review. Crit. Rev. Solid State Mater. Sci. 2015, 40, 1–37. [Google Scholar] [CrossRef]
- Li, G.; Tam, L.K.; Tang, J.X. Amplified effect of Brownian motion in bacterial near-surface swimming. Proc. Natl. Acad. Sci. USA 2008, 105, 18355–18359. [Google Scholar] [CrossRef] [Green Version]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Bispo, P.J.M.; Haas, W.; Gilmore, M.S. Biofilms in infections of the eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936. [Google Scholar] [CrossRef]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef] [Green Version]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A brief recap of microbial adhesion and biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef] [Green Version]
- Van Oss, C.J. Acid—Base interfacial interactions in aqueous media. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Chen, Y.; Harapanahalli, A.K.; Busscher, H.J.; Norde, W.; van der Mei, H.C. Nanoscale cell wall deformation impacts long-range bacterial adhesion forces on surfaces. Appl. Environ. Microbiol. 2014, 80, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surf. B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Hanson, L.; Xie, W.; Lin, Z.; Cui, B.; Cui, Y. Noninvasive Neuron Pinning with Nanopillar Arrays. Nano Lett. 2010, 10, 4020–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, F.; Liu, J.; Guo, L.; Zhang, L.; Li, Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015, 385, 1–7. [Google Scholar] [CrossRef]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2015, 18, 1311–1316. [Google Scholar] [CrossRef]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 052419. [Google Scholar] [CrossRef]
- Liu, T.; Cui, Q.; Wu, Q.; Li, X.; Song, K.; Ge, D.; Guan, S. Mechanism Study of Bacteria Killed on Nanostructures. J. Phys. Chem. B 2019, 123, 8686–8696. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Mainwaring, D.E.; Nguyen, S.H.; Webb, H.; Jakubov, T.; Tobin, M.; Lamb, R.N.; Wu, A.H.F.; Marchant, R.; Crawford, R.J.; Ivanova, E.P. The nature of inherent bactericidal activity: Insights from the nanotopology of three species of dragonfly. Nanoscale 2016, 8, 6527–6534. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Truong, V.K.; Geeganagamage, N.M.; Baulin, V.A.; Vongsvivut, J.; Tobin, M.J.; Luque, P.; Crawford, R.J.; Ivanova, E.P. The susceptibility of Staphylococcus aureus CIP 65.8 and Pseudomonas aeruginosa ATCC 9721 cells to the bactericidal action of nanostructured Calopteryx haemorrhoidalis damselfly wing surfaces. Appl. Microbiol. Biotechnol. 2017, 101, 4683–4690. [Google Scholar] [CrossRef]
- Hasan, J.; Raj, S.; Yadav, L.; Chatterjee, K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv. 2015, 5, 44953–44959. [Google Scholar] [CrossRef] [Green Version]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Nakade, K.; Asai, N.; Shimizu, T.; Shingubara, S. Antibacterial Property of Si Nanopillar Array Fabricated Using Metal Assisted Etching; Mimic a Cicada Wing. ECS Trans. 2017, 75, 1–5. [Google Scholar] [CrossRef]
- Han, S.; Ji, S.; Abdullah, A.; Kim, D.; Lim, H.; Lee, D. Superhydrophilic nanopillar-structured quartz surfaces for the prevention of biofilm formation in optical devices. Appl. Surf. Sci. 2018, 429, 244–252. [Google Scholar] [CrossRef]
- Viela, F.; Navarro-Baena, I.; Hernández, J.J.; Osorio, M.R.; Rodriguez, I. Moth-eye mimetic cytocompatible bactericidal nanotopography: A convergent design. Bioinspir. Biomim. 2018, 13, 026011. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuber, F.; Weber, K.M.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef]
- Maldonado-Codina, C. Soft Lens Materials, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Li, Q.; Fang, F. Advances and challenges of soft contact lens design for myopia control. Appl. Opt. 2019, 58, 1639. [Google Scholar] [CrossRef]
- Efron, N. Microbial keratitis. Contact Lens Complicat. 2012, 6, 245–258. [Google Scholar]
- Sankaridurg, P.R.; Sharma, S.; Willcox, M.; Sweeney, D.F.; Naduvilath, T.J.; Holden, B.A.; Rao, G.N. Colonization of hydrogel lenses with Streptococcus pneumoniae: Risk of development of corneal infiltrates. Cornea 1999, 18, 289–295. [Google Scholar] [CrossRef]
- Sankaridurg, P.R.; Sharma, S.; Willcox, M.; Naduvilath, T.J.; Sweeney, D.F.; Holden, B.A.; Rao, G.N. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J. Clin. Microbiol. 2000, 38, 4420–4424. [Google Scholar] [CrossRef] [Green Version]
- Holden, B.A.; Reddy, M.K.; Sankaridurg, P.R.; Buddi, R.; Sharma, S.; Willcox, M.D.P.; Sweeney, D.F.; Rao, G.N. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: Histopathologic observations on the nature and type of corneal infiltrate. Cornea 1999, 18, 538–543. [Google Scholar] [CrossRef]
- Mowrey-McKee, M.F.; Sampson, H.J.; Proskin, H.M. Microbial Contamination of Hydrophilic Contact Lenses. Part II: Quantitation of Microbes After Patient Handling and After Aseptic Removal from the Eye. Eye Contact Lens 1992, 18, 240–244. [Google Scholar]
- Wu, Y.T.; Zhu, H.; Willcox, M.; Stapleton, F. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5287–5292. [Google Scholar] [CrossRef]
- Cano-Parra, J.; Bueno-Gimeno, I.; Lainez, B.; Córdoba, J.; Montés-Micó, R. Antibacterial and antifungal effects of soft contact lens disinfection solutions. Contact Lens Anterior Eye 1999, 22, 83–86. [Google Scholar] [CrossRef]
- Dantam, J.; Zhu, H.; Stapleton, F. Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Investig. Ophthalmol. Vis. Sci. 2011, 52, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.; Stapleton, F.; Carnt, N. Microbiology, Lens Care and Maintenance, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Dart, J.K.G.; Radford, C.F.; Minassian, D.; Verma, S.; Stapleton, F. Risk Factors for Microbial Keratitis with Contemporary Contact Lenses. A Case-Control Study. Ophthalmology 2008, 115, 1647–1654. [Google Scholar] [CrossRef]
- Dutta, D.; Vijay, A.K.; Kumar, N.; Willcox, M.D.P. Melimine-coated antimicrobial contact lenses reduce microbial keratitis in an animal model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5616–5624. [Google Scholar] [CrossRef] [Green Version]
- Malakooti, N.; Alexander, C.; Alvarez-lorenzo, C. Imprinted Contact Lenses for Sustained Release of Polymyxin B and Related Antimicrobial Peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S.; Dehghan, M.M.; Chaleshtori, S.S.; Shafiee, A. Preparation and Evaluation of Contact Lenses Embedded with Polycaprolactone-Based Nanoparticles for Ocular Drug Delivery. Biomacromolecules 2016, 17, 485–495. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Khameneh, B.; Jalili-Behabadi, M.M.; Malaekeh-Nikouei, B.; Mohajeri, S.A. Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles. Contact Lens Anterior Eye 2014, 37, 149–152. [Google Scholar] [CrossRef]
- Kharaghani, D.; Dutta, D.; Gitigard, P.; Tamada, Y.; Katagiri, A.; Phan, D.N.; Willcox, M.D.P.; Kim, I.S. Development of antibacterial contact lenses containing metallic nanoparticles. Polym. Test. 2019, 79, 106034. [Google Scholar] [CrossRef]
- Ramli, N.A.H.; Zaaba, S.K.; Mustaffa, M.T.; Zakaria, A.; Shahriman, A.B. Review on prevention of bacterial adhesion on contact lens using plasma treatment. AIP Conf. Proc. 2017, 1824, 030014. [Google Scholar]
- Schornack, M.M.; Faia, L.J.; Griepentrog, G.J. Pseudomonas keratitis associated with daily wear of silicone hydrogel contact lenses. Eye Contact Lens 2008, 34, 124–128. [Google Scholar] [CrossRef]
- Lee, K.Y.C.; Lim, L. Pseudomonas keratitis associated with continuous wear silicone-hydrogel soft contact lens: A case report. Eye Contact Lens 2003, 29, 255–257. [Google Scholar] [CrossRef]
- Tran, V.B. Pseudomonas Aeruginosa Adhesion to Soft Contact Lenses: Effects of Cell Surface and Substrate Chemistry. Ph.D. Thesis, UC Berkeley, Berkeley, CA, USA, 2011. [Google Scholar]
- Nguyen, V.; Lee, G.A. Management of microbial keratitis in general practice. Aust. J. Gen. Pract. 2019, 48, 516–519. [Google Scholar] [CrossRef]
- Epstein, A.B.; Freedman, J.M. Keratitis associated with hydrogen peroxide disinfection in soft contact lens wearers. Int. Contact Lens Clin. 1990, 17, 74–82. [Google Scholar] [CrossRef]
- Giraldez, M.J.; Yebra-Pimentel, E. Hydrogel Contact Lenses Surface Roughness and Bacterial Adhesion. In Ocular Diseases; IntechOpen: London, UK, 2016; p. 13. [Google Scholar] [CrossRef]
- Dutta, D.; Cole, N.; Willcox, M. Factors influencing bacterial adhesion to contact lenses. Mol. Vis. 2012, 18, 14–21. [Google Scholar] [PubMed]
- Lin, A.; Rhee, M.K.; Akpek, E.K.; Amescua, G.; Farid, M.; Garcia-Ferrer, F.J.; Varu, D.M.; Musch, D.C.; Dunn, S.P.; Mah, F.S. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology 2019, 126, P1–P55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipener, C.; Nagoya, F.R.; Zamboni, F.J.; Lewinski, R.; Kwitko, S.; Uras, R. Bacterial contamination in soft contact lens wearers. CLAO J. 1995, 21, 122–124. [Google Scholar]
- Bruinsma, G.M.; Van Der Mei, H.C.; Busscher, H.J. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Emina, M.O.; Idu, F.K. Bacteria and parasites in contact lenses of asymptomatic wearers in Nigeria. J. Optom. 2011, 4, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Shellenberger, K.; Logan, B.E. Effect of molecular scale roughness of glass beads on colloidal and bacterial deposition. Environ. Sci. Technol. 2002, 36, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, G.M.; Rustema-Abbing, M.; De Vries, J.; Stegenga, B.; Van der Mei, H.C.; Van der Linden, M.L.; Hooymans, J.M.M.; Busscher, H.J. Influence of wear and overwear on surface properties of etafilcon a contact lenses and adhesion of Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3646–3653. [Google Scholar]
- Musgrave, C.S.A.; Fang, F. Contact lens materials: A materials science perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, E.L.; Fleiszig, S.M.J.; Brennan, N.A. Lipopolysaccharide in adherence of Pseudomonas aeruginosa to the cornea and contact lenses. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1930–1936. [Google Scholar]
- Miller, M.J.; Wilson, L.A.; Ahearn, D.G. Adherence of Pseudomonas aeruginosa to Rigid Gas-Permeable Contact Lenses. Arch. Ophthalmol. 1991, 109, 1447–1448. [Google Scholar] [CrossRef]
- Holden, B.A.; Sweeney, D.F.; Seger, R.G. Epithelial erosions caused by thin high water content lenses. Clin. Exp. Optom. 2015, 3, 54–67. [Google Scholar] [CrossRef]
- Tighe, B. Silicone hydrogels: What are they and how should they be used in everyday practice? Optician 1999, 218, 31–32. [Google Scholar]
- Andrasko, G. Hydrogel dehydration in various environments. Int. Contact Lens Clin. 1983, 10, 22–28. [Google Scholar]
- Lever, O.W.; Groemminger, S.F.; Allen, M.E.; Bornemann, R.H.; Dey, D.R.; Barna, B.J. Evaluation of the relationship between total lens protein deposition and patient-rated comfort of hydrophilic (soft) contact lenses. Int. Contact Lens Clin. 1995, 22, 5–13. [Google Scholar] [CrossRef]
- Salerno, M.B.; Logan, B.E.; Velegol, D. Importance of molecular details in predicting bacterial adhesion to hydrophobie surfaces. Langmuir 2004, 20, 10625–10629. [Google Scholar] [CrossRef]
- Giraldez, M.J.; Resua, C.G.; Lira, M.; Oliveira, M.E.C.D.R.; Magariños, B.; Toranzo, A.E.; Yebra-Pimentel, E. Contact lens hydrophobicity and roughness effects on bacterial adhesion. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2010, 87, E426–E431. [Google Scholar] [CrossRef] [PubMed]
- Efron, N.; Maldonado-Codina, C. Development of Contact Lenses from a Biomaterial Point of View—Materials, Manufacture, and Clinical Application; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 6. [Google Scholar]
- Yin, S.; Ruffin, P.; Brantley, C.; Edwards, E.; Luo, C. Fabrication of nanostructures on curved surfaces. Photonic Fiber Cryst. Devices Adv. Mater. Innov. Device Appl. VII 2013, 8847, 88470T. [Google Scholar]
- Fang, F.Z.; Zhang, X.; Gao, W.; Guo, Y.; Byrne, G.; Hansen, H.N. Nanomanufacturing—Perspective and applications. CIRP Ann. Manuf. Technol. 2017, 66, 683–705. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Lv, S.; Nie, J.; Gao, Q.; Xie, C.; Zhou, L.; Qiu, J.; Fu, J.; Zhao, X.; He, Y. Micro/nanofabrication of brittle hydrogels using 3D printed soft ultrafine fiber molds for damage-free demolding. Biofabrication 2020, 12, 025015. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.T.; Kim, S.K.; Lee, J.H.; Choi, H.S.; Kim, C.S.; Jeong, M.Y. Nanostructured multifunctional surface with antireflective and antimicrobial characteristics. ACS Appl. Mater. Interfaces 2015, 7, 326–331. [Google Scholar] [CrossRef]

| Bacteria | Size | Morphology | Source | Infections |
|---|---|---|---|---|
| Gram negative | ||||
| Escherichia coli | 2 μm long, 0.25–1 μm diameter | Rods | Contaminated food, personal contact | Watery diarrhea, abdominal cramping, nausea, vomiting, urinary tract infection |
| Pseudomonas aeruginosa | 1.5–3 μm long, 0.5–0.8 μm diameter | Rods | Water, soil | Dermatitis, cystic fibrosis, most bacterial cause of microbial keratitis in contact lenses wearers |
| Pseudomonas fluorescens | 1–3 μm long, 0.5–0.7 μm diameter | Rods | Plants, soil, water surfaces | Blood transfusion-related septicemia, catheter-related bacteremia, peritonitis |
| Klebsiella pneumoniae | 2 μm long, 0.5 μm diameter | Rods | Personal contact, indwelling catheters | Respiratory tract infections, urinary tract infections, endophthalmitis, skin and soft tissue infections, meningitis |
| Gram positive | ||||
| Staphylococcus aureus | 0.6 μm cell diameter | Coccal | Nose, respiratory tract, direct personal contact | Bloodstream infections, endocarditis, osteomyelitis |
| Bacillus subtilis | 4–10 μm long, 0.25–1 μm in diameter | Rods | Soil | Food contamination |
| Enterococcus faecalis | 0.6–2 μm by 0.6–2.5 μm | Coccal | Gastrointestinal tract | Urinary tract infection, endocarditis, abdominal and pelvic infection, septicemia |
| Natural Resource | Surface Features | Wettability | Bactericidal Efficacy | Lethality | Reference |
|---|---|---|---|---|---|
| Cicada wing (Psaltoda claripennis) | Nanopillar (200 nm height, diameter 100 nm at the base, diameter 60 nm at the cap, and spaced 170 nm apart from center to center) | Superhydrophobic Average water contact angle: 158.8° (147°–172°) | Individual cells were killed within approximately 3 min | Pseudomonas aeruginosa | [13] |
| Cicada wing (Psaltoda claripennis) | Nanopillar (200 nm height, base diameter 100 nm, cap diameter 60 nm, space 170 nm) | Hydrophobic Water contact angle: 158.8° | P. aeruginosa– No remarkable effect on the viability of gram-positive cells | Branhamella catarrhalis Escherichia coli Pseudomonas aeruginosa Pseudomonas fluorescens Planococcus maritimus | [12] |
| Dog day annual cicada (Tibicen tibicen) Brood II periodical cicada (Magicicada septendecim) | Spherically capped cone (183 nm height, 104 nm base diameter, 57 nm cap diameter, spacing 175 nm) Hemisphere (83.5 nm height, 167 nm width, 252 nm spacing) | Hydrophobic Water contact angle: 132°, 80.1° | Dog day annual cicada–25% contamination comparing to control sample Brood II periodical cicada–54% contamination comparing to control sample | Saccharomyces cerevisiae | [9] |
| Cicada wing (Megapomponia intermedia, Cryptotympana aguila, Ayuthia spectabile) | Nanopillar (241 nm height, 165 nm pitch, 156 nm diameter, 9 nm spacing) Nanopillar (182 nm height, 187 pitch, 159 nm diameter, 28 nm spacing) Nanopillar (182 nm height, 251 nm pitch, 207 nm diameter, 44 nm spacing) | Hydrophobic Water contact angle: 135.5°, 113.2°, 95.65° | The bacterial live ratio for M. intermedia, C. aguila, A. spectabile, respectively is 0.222, 0.123 and 0.067 | P. fluorescens | [7] |
| Dragonfly (Diplacodes bipunctata, Hemianax papuensis, Austroaeschna multipunctata) | Height 200–300 nm, top diameter 80 ± 20 nm, interpillar spacing 180 ± 30 nm | Hydrophobic Contact angle: ~(152°–162°) | 13.0 × 104 to 47 × 104 cell killed per cm2 per min | P. aeruginosa S. aureus B. subtilis B. subtilis spores | [63] |
| Gecko skin (Lucasium steindachneri) | Length 2–4 μm Base thickness and spacing ~ 500 nm | Hydrophobic Contact angle: 150° ± 5° | 88% P. gingivalis killed 66% S. mutans killed | Porphyromonas gingivalis Streptococcus mutans | [64] |
| Dragonfly wing (Orthetrum villosovittatum) | Height (short pillar 189 ± 67 nm, tall pillar 311 ± 52 nm) Pillar diameter (short pillar 37 ± 6 nm, tall pillar 57 ± 8 nm) | N/A | Escherichia coli | [8] | |
| Damselfly (Calopteryx haemorrhoidalis) | Height 433.4 ± 71.2 nm Tip diameter 47.7 ± 11.1 nm Interspacing distance 116.1 ± 39.6 nm | Contact angle: 157.0° ± 4.9° | P. aeruginosa S. aureus | P. aeruginosa S. aureus | [65] |
| Dragonfly wing (Austrothemis nigrescens; Trithemis annulata) | A. nigrescens (height 307 ± 34 nm, diameter 45 ± 7 nm) T. annulate (height 292 ± 34 nm, diameter 45 ± 7 nm) | Contact angle (A. nigrescens: 162° ± 8°, T. annulate: 167° ± 6°) | Cell was ruptured within 3–5 min | Giant unilamellar vesicle | [10] |
| Substratum Material | Natural Templates | Fabrication Method | Geometrical Features | Wettability | Bactericidal Efficacy | Lethality | Reference |
|---|---|---|---|---|---|---|---|
| Silicon | Dragonfly | Reactive-ion beam etching | Height 500 nm | Hydrophilic Contact angle 80° | Killing rate P. aeruginosa: 4.3 × 105 per cm−2min−1 S. aureus: 4.5 × 105 per cm−2min−1 B. subtilis: 1.4 × 105 per cm−2min−1 | Gram-negative bacteria Gram-positive bacteria Spores | [62] |
| Silicon | Dragonfly | Deep reactive ion etching | Height 4 μm Diameter 220 nm | Hydrophobic Contact angle 154° | 86% of S. aureus and 83% of E. coli were non-viable after 3 h incubation | Gram-positive bacteria Gram-negative bacteria Mammalian cell | [66] |
| PMMA | Cicada | Soft lithography | Height 210–300 nm Spacing 100–380 nm Width 70–215 nm | N/A | E. coli: 16–141% higher dead fraction than a flat film | Gram-negative bacteria | [67] |
| Silicon | Cicada | Metal assisted etching | Height 200 nm Pitch 200 nm Width 150 nm | E. coli: 24 h from 3.9 × 106 CFU/mL to 1 CFU/mL | Gram-negative bacteria | [68] | |
| Quartz | N/A | Nanosphere lithography | Height 300 nm Apex diameter 10 nm | Hydrophilic Contact angle ~ 0° | Kill ~38,000 P. aeruginosa and ~27,000 E. coli cm−2min−1 | Gram-negative bacteria | [69] |
| PMMA | Moth-eye | Thermal polymer nanoimprint | Height 350 nm Width 80 nm Pitch 250 nm Aspect ratio 4.3 | Hydrophobic 135 ± 4° | Percentage of non-viable bacteria are 55%, 45%, 30% for S. aureus, E. coli, and P. aeruginosa respectively | Gram-positive bacteria Gram-negative bacteria | [70] |
| Silicon | Cicada | Deep ultraviolet immersion lithography Plasma etching | Diameter 35 nm Periodicity 90 nm Increasing height 220, 360, 420 nm | N/A | 360 nm-height 95 ± 5% P. aeruginosa and 83 ± 12% S. aureus cell death | Gram-negative bacteria Gram-positive bacteria | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Fang, F. Biomimetic Functional Surfaces towards Bactericidal Soft Contact Lenses. Micromachines 2020, 11, 835. https://doi.org/10.3390/mi11090835
Mao T, Fang F. Biomimetic Functional Surfaces towards Bactericidal Soft Contact Lenses. Micromachines. 2020; 11(9):835. https://doi.org/10.3390/mi11090835
Chicago/Turabian StyleMao, Tianyu, and Fengzhou Fang. 2020. "Biomimetic Functional Surfaces towards Bactericidal Soft Contact Lenses" Micromachines 11, no. 9: 835. https://doi.org/10.3390/mi11090835
APA StyleMao, T., & Fang, F. (2020). Biomimetic Functional Surfaces towards Bactericidal Soft Contact Lenses. Micromachines, 11(9), 835. https://doi.org/10.3390/mi11090835





