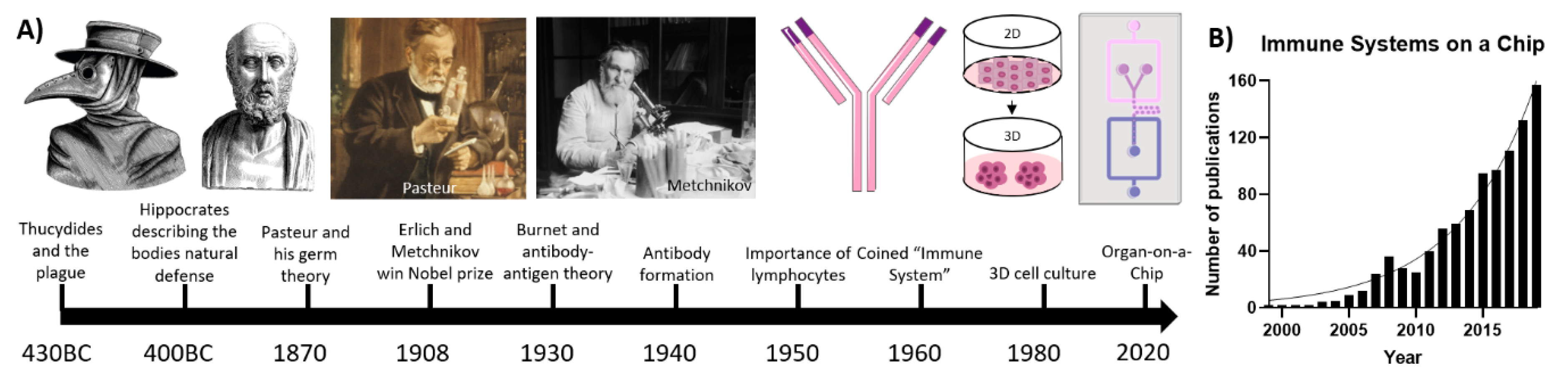
Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications
Abstract
:1. Introduction
2. Physiology of the Immune System
3. State-of-the-Art Immune-System-on-a-Chip Models
3.1. Lymph-Node-on-a-Chip
3.2. Bone-Marrow-on-a-Chip
3.3. Other Immune Organs
4. Integration of Immune Cells and Components for Organs-on-a-Chip
4.1. Inflammation-on-a-Chip
4.2. Skin-on-a-Chip
4.3. Liver-on-a-Chip
4.4. Gut-on-a-Chip
4.5. Tumor-Microenvironment-on-a-Chip
5. Limitations and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ALL | Acute lymphoblastic leukemia |
| BM | Bone marrow |
| BMP-2 | Bone morphogenetic protein 2 |
| CAR-T | Chimeric antigen receptor t |
| CDC | Colorectal cancer cells |
| DC | Dendritic cells |
| eBM | Engineered bone marrow |
| ECM | Extracellular matrix |
| EPCAM | Epithelial cell adhesion molecule |
| GelMA | Gelatin methacryloyl |
| HSC | Hematopoietic stem cell |
| HSPC | Hematopoietic stem cells and precursor cells |
| HuALN | Human artificial lymph node |
| IFN | Interferon |
| IL | Interleukin |
| iPSC | Induced pluripotent stem cell |
| LF | Lymphoid follicle |
| LN | Lymph node |
| LNoC | Lymph-node-on-chip |
| LPS | Lipopolysaccharide |
| MSC | Mesenchymal stem cell |
| NK | Natural killer cells |
| OCC | Ovarian cancer cells |
| OoC | Organ-on-a-chip |
| PDMS | Polydimethylsiloxane |
| PD-L1 | Programmed cell death-ligand 1 |
| pMHC | Peptide-major histocompatibility complex |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| SAC | Staphylococcus aureus Cowan I |
| TMoC | Tumor-microenvironment-on-a-chip |
| TNF-α | Tumor necrosis factor-α |
References
- Pradeu, T. Immunology and individuality. eLife 2019, 8, 47384. [Google Scholar] [CrossRef]
- Greenberg, S. A Concise History of Immunology. 2003. Available online: https://www.semanticscholar.org/paper/A-Concise-History-of-Immunology-Greenberg/9a3a8183f49ca41e83ae107c7cf16b5cb9a61d4a#citing-papers (accessed on 12 September 2020).
- Bellavite, P.; Conforti, A.; Piasere, V.; Ortolani, R. Immunology and Homeopathy. 1. Historical Background. Evid.-Based Complement. Altern. Med. 2005, 2, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Travis, J. On the Origin of The Immune System. Science 2009, 324, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Lokaj, J.; John, C. [Ilya Ilich Metchnikov and Paul Ehrlich: 1908 Nobel Prize winners for their research on immunity]. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Pro Epidemiol. A Mikrobiol. Ceske Lek. Spol. J.E. Purkyne 2008, 57, 119–124. [Google Scholar]
- Mudd, S. A Hypothetical Mechanism of Antibody Formation. J. Immunol. 1932, 23, 423. [Google Scholar]
- Pauling, L. A Theory of the Structure and Process of Formation of Antibodies*. J. Am. Chem. Soc. 1940, 62, 2643–2657. [Google Scholar] [CrossRef]
- Silverstein, A.M. A history of theories of antibody formation. Cell. Immunol. 1985, 91, 263–283. [Google Scholar] [CrossRef]
- Burnet, F.M. A modification of jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J. Clin. 1976, 26, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Moulin, A.M. The immune system: A key concept for the history of immunology. Hist. Philos. Life Sci. 1989, 11, 221–236. [Google Scholar]
- Pierce, C.W.; Solliday, S.M.; Asofsky, R. Immune responses in vitro: iv. Suppression of primary γm, γg, and γa plaque-forming cell responses in mouse spleen cell cultures by class-specific antibody to mouse immunoglobulins. J. Exp. Med. 1972, 135, 675–697. [Google Scholar] [CrossRef]
- Gordon, S. Elie Metchnikoff: Father of natural immunity. Eur. J. Immunol. 2008, 38, 3257–3264. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Novoa, B.; Figueras, A. Zebrafish: Model for the Study of Inflammation and the Innate Immune Response to Infectious Diseases. Adv. Exp. Med. Biol. 2011, 946, 253–275. [Google Scholar] [CrossRef] [Green Version]
- von Herrath, M.G.; Nepom, G.T. Lost in translation. Barriers to implementing clinical immunotherapeutics for autoimmunity. J. Exp. Med. 2005, 202, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Shanti, A.; Teo, C.; Stefanini, C. In Vitro Immune Organs-on-Chip for Drug Development: A Review. Pharmaceutics 2018, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Boil. 2016, 216, 31–40. [Google Scholar] [CrossRef]
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.J.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater. 2019, 8, 1801363. [Google Scholar] [CrossRef]
- Polini, A.; Del Mercato, L.L.; Barra, A.; Zhang, Y.S.; Calabi, F.; Gigli, G. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today 2019, 24, 517–525. [Google Scholar] [CrossRef]
- Sosa-Hernandez, J.E.; Villalba-Rodriguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldivar, R.; Iqbal, H.M.N. Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, E.; Schmid-Scho¨nbein, G.W. A Model for Mechanics of Primary Lymphatic Valves. J. Biomech. Eng. 2003, 125, 407–414. [Google Scholar] [CrossRef]
- Furdui, V.I.; Harrison, D.J. Immunomagnetic T cell capture from blood for PCR analysis using microfluidic systems. Lab Chip 2004, 4, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.C.; Liepmann, R.; Lee, L.P. A biomimetic method for extracting leukocytes from blood in microfluidic devices. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology Proceedings (Cat No 02EX578) MMB-02, Madison, WI, USA, 2–4 May 2003. [Google Scholar]
- Long, A.; Mitchell, S.; Kashanin, D.; Williams, V.; Prina Mello, A.; Shvets, I.; Kelleher, D.; Volkov, Y. A multidisciplinary approach to the study of T cell migration. Ann. N. Y. Acad. Sci. 2004, 1028, 313–319. [Google Scholar]
- Jeon, N.L.; Baskaran, H.; Dertinger, S.K.W.; Whitesides, G.M.; Van De Water, L.; Toner, M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002, 20, 826–830. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Chaplin, D.D. Development and maturation of secondary Lymphoid tissues. Annu. Rev. Immunol. 1999, 17, 399–433. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2015, 11, 137–154. [Google Scholar] [CrossRef]
- Jonsson, H.; Yokoyama, W.M. Chapter 2 Natural Killer Cell Tolerance. In Advances in Immunology; Elsevier BV: Amsterdam, The Netherlands, 2009; Volume 101, pp. 27–79. [Google Scholar]
- Boni, B.O.O.; Lamboni, L.; Souho, T.; Gauthier, M.; Yang, G. Immunomodulation and cellular response to biomaterials: The overriding role of neutrophils in healing. Mater. Horiz. 2019, 6, 1122–1137. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Culleré, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Schwab, S.R.; Cyster, J.G. Finding a way out: Lymphocyte egress from lymphoid organs. Nat. Immunol. 2007, 8, 1295–1301. [Google Scholar] [CrossRef]
- Allen, C.D.C.; Okada, T.; Cyster, J.G. Germinal-Center Organization and Cellular Dynamics. Immunology 2007, 27, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Safari, H.; Kelley, W.J.; Saito, E.; Kaczorowski, N.; Carethers, L.; Shea, L.D.; Eniola-Adefeso, O. Neutrophils preferentially phagocytose elongated particles—An opportunity for selective targeting in acute inflammatory diseases. Sci. Adv. 2020, 6, eaba1474. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry. ACS Nano 2010, 4, 3073–3086. [Google Scholar] [CrossRef]
- Wen, Y.; Waltman, A.; Han, H.; Collier, J.H. Switching the Immunogenicity of Peptide Assemblies Using Surface Properties. ACS Nano 2016, 10, 9274–9286. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Lee, J.S.; Bell, B.; Fahmy, T.M. Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater. 2017, 57, 136–145. [Google Scholar] [CrossRef]
- Hoehn, E.N.M.A.K. The Lymphatic System and Lymphoid Organs and Tissues. In Human Anatomy & Physiology; Pearson Educated Limited: London, UK, 2006; pp. 7252–7763. [Google Scholar]
- Giese, C.; Lubitz, A.; Demmler, C.D.; Reuschel, J.; Bergner, K.; Marx, U. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 2010, 148, 38–45. [Google Scholar] [CrossRef]
- Giese, C.; Demmler, C.D.; Ammer, R.; Hartmann, S.; Lubitz, A.; Miller, L.; Müller, R.; Marx, U. A Human Lymph Node In Vitro?Challenges and Progress. Artif. Organs 2006, 30, 803–808. [Google Scholar] [CrossRef]
- Giese, C.; Marx, U. Human immunity in vitro—Solving immunogenicity and more. Adv. Drug Deliv. Rev. 2014, 69–70, 103–122. [Google Scholar] [CrossRef]
- Seifert, M.; Lubitz, A.; Trommer, J.; Könnig, D.; Korus, G.; Marx, U.; Volk, H.-D.; Duda, G.; Kasper, G.; Lehmann, K.; et al. Crosstalk between immune cells and mesenchymal stromal cells in a 3D bioreactor system. Int. J. Artif. Organs 2012, 35, 986–995. [Google Scholar] [CrossRef]
- Rosa, P.M.; Gopalakrishnan, N.; Ibrahim, H.; Haug, M.; Sandvig, A. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip 2016, 16, 3728–3740. [Google Scholar] [CrossRef]
- Mitra, B.; Jindal, R.; Lee, S.; Dong, D.X.; Li, L.; Sharma, N.; Maguire, T.; Schloss, R.; Yarmush, M. Microdevice integrating innate and adaptive immune responses associated with antigen presentation by dendritic cells†. RSC Adv. 2013, 3, 16002–16010. [Google Scholar] [CrossRef]
- Shim, S.; Belanger, M.C.; Harris, A.R.; Munson, J.; Pompano, R.R. Two-way communication between ex vivo tissues on a microfluidic chip: Application to tumor–lymph node interaction. Lab Chip 2019, 19, 1013–1026. [Google Scholar] [CrossRef]
- Goyal, G.; Bausk, B.; Prabhala, P.; Xie, L.; Curran, D.; Long, J.; Cohen, L.; Levy, O.; Prantil-Baun, R.; Walt, D.R.; et al. Lymph node follicle formation and vaccination responses reconstituted in vitro in a human Organ Chip. bioRxiv 2019, 806505. [Google Scholar] [CrossRef] [Green Version]
- Torisawa, Y.-S.; Spina, C.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Torisawa, Y.-S.; Mammoto, T.; Jiang, E.; Jiang, A.; Mammoto, A.; Watters, A.L.; Bahinski, A.; Ingber, D.E. Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng. Part C Methods 2016, 22, 509–515. [Google Scholar] [CrossRef]
- Chou, D.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; Macdonald, A.; Bolukbasi, O.V.; Joyce, C.E.; Teixeira, L.M.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Wirth, L.; Cavak, N.; Koenigsmark, M.; Marx, U.; Lauster, R.; Rosowski, M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2017, 12, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Evans, R.; Mezan, R.; Shi, L.; Moses, B.S.; Martin, K.H.; Gibson, L.F.; Yang, Y. Three-Dimensional Microfluidic Tri-Culture Model of the Bone Marrow Microenvironment for Study of Acute Lymphoblastic Leukemia. PLoS ONE 2015, 10, e0140506. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Regionalized immune function of tonsils and adenoids. Immunol. Today 1999, 20, 383–384. [Google Scholar] [CrossRef]
- Wagar, L.E.; DiFazio, R.M.; Davis, M.M. Advanced model systems and tools for basic and translational human immunology. Genome Med. 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Onishi, N.; Takami, H.; Seishima, R.; Inoue, H.; Hirata, Y.; Kameyama, K.; Tsuchihashi, K.; Sugihara, E.; Uchino, S.; et al. Development of a functional thyroid model based on an organoid culture system. Biochem. Biophys. Res. Commun. 2018, 497, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Wyler, D.J. The Spleen in Malaria. Novartis Found. Symp. 2008, 94, 98–116. [Google Scholar] [CrossRef]
- Al-Salem, A.H. Splenic Complications of Sickle Cell Anemia and the Role of Splenectomy. ISRN Hematol. 2010, 2011, 864257. [Google Scholar] [CrossRef] [Green Version]
- Björkholm, M.; Holm, G.; Merk, K. Cyclic autoimmune hemolytic anemia as a presenting manifestation of splenic Hodgkin’s disease. Cancer 1982, 49, 1702–1704. [Google Scholar] [CrossRef]
- Buffet, P.A.; Milon, G.; Brousse, V.; Correas, J.-M.; Dousset, B.; Couvelard, A.; Kianmanesh, R.; Farges, O.; Sauvanet, A.; Paye, F.; et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood 2006, 107, 3745–3752. [Google Scholar] [CrossRef]
- Rigat-Brugarolas, L.G.; Elizalde-Torrent, A.; Bernabeu, M.; De Niz, M.; Martín-Jaular, L.; Fernández-Becerra, C.; Homs-Corbera, A.; Samitier, J.; Del Portillo, H.A. A functional microengineered model of the human splenon-on-a-chip. Lab Chip 2014, 14, 1715–1724. [Google Scholar] [CrossRef]
- Muller, W.A. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Han, S.; Yan, J.-J.; Shin, Y.; Jeon, J.J.; Won, J.; Jeong, H.E.; Kamm, R.D.; Kim, Y.-J.; Chung, S. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab Chip 2012, 12, 3861. [Google Scholar] [CrossRef]
- Ingram, P.N.; Hind, L.E.; Jiminez-Torres, J.A.; Huttenlocher, A.; Beebe, D.J. An Accessible Organotypic Microvessel Model Using iPSC-Derived Endothelium. Adv. Healthc. Mater. 2017, 7, 1700497. [Google Scholar] [CrossRef]
- Jones, C.N.; Dalli, J.; Dimisko, L.; Wong, E.; Serhan, C.N.; Irimia, D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 20560–20565. [Google Scholar] [CrossRef] [Green Version]
- Hamza, B.; Irimia, D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip 2015, 15, 2625–2633. [Google Scholar] [CrossRef]
- Gopalakrishnan, N.; Hannam, R.; Casoni, G.P.; Barriet, D.; Ribe, J.; Haug, M.; Sandvig, A. Infection and immunity on a chip: A compartmentalised microfluidic platform to monitor immune cell behaviour in real time. Lab Chip 2015, 15, 1481–1487. [Google Scholar] [CrossRef]
- Sasserath, T.; Rumsey, J.W.; McAleer, C.W.; Bridges, L.R.; Long, C.J.; Elbrecht, D.; Schuler, F.; Roth, A.; Bertinetti-Lapatki, C.; Shuler, M.L.; et al. Differential Monocyte Actuation in a Three-Organ Functional Innate Immune System-on-a-Chip. Adv. Sci. 2020, 7, 2000323. [Google Scholar] [CrossRef]
- Truskey, G.A.; Kambez, H.B.; Remi, V.; Carolina, L.; Antonio, V.; Cedric, H.; Hyun-Hee, L.; Stephen, E.A.; Michael, S.; Thomas, C.F.; et al. Faculty Opinions recommendation of Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Irimia, D.; Wang, X. Inflammation-on-a-Chip: Probing the Immune System Ex Vivo. Trends Biotechnol. 2018, 36, 923–937. [Google Scholar] [CrossRef]
- Matejuk, A. Skin Immunity. Arch. Immunol. Et Ther. Exp. 2017, 66, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, Q.; Ting, F.C.W. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 2016, 16, 1899–1908. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2015, 113, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Gao, B. Basic liver immunology. Cell. Mol. Immunol. 2016, 13, 265–266. [Google Scholar] [CrossRef] [Green Version]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2007, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X.; Deng, J.; Wei, W.; Chen, Z.; et al. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, S. Miniature liver on a chip could boost US food safety. Nature 2017, 2017, 21818. [Google Scholar] [CrossRef]
- Gröger, M.; Rennert, K.; Giszas, B.; Weiß, E.; Dinger, J.; Funke, H.; Kiehntopf, M.; Peters, F.T.; Lupp, A.; Bauer, M.; et al. Monocyte-induced recovery of inflammation-associated hepatocellular dysfunction in a biochip-based human liver model. Sci. Rep. 2016, 6, 21868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Huh, N.; Hamilton, G.; Ingber, N.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Kämpfer, A.A.; Urban, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. Vitr. 2017, 45, 31–43. [Google Scholar] [CrossRef]
- Min, S.; Kim, S.; Cho, S.-W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Boil. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, Q.; Jing, L. Characterization of tight junction disruption and immune response modulation in a miniaturized Caco-2/U937 coculture-based in vitro model of the human intestinal barrier. Biomed. Microdevices 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-F.; Trubelja, A.; Shen, A.Q.; Bao, G. Tumour-on-a-chip: Microfluidic models of tumour morphology, growth and microenvironment. J. R. Soc. Interface 2017, 14, 20170137. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Parlato, S.; De Ninno, A.; Molfetta, R.; Toschi, E.; Salerno, D.; Mencattini, A.; Romagnoli, G.; Fragale, A.; Roccazzello, L.; Buoncervello, M.; et al. 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells. Sci. Rep. 2017, 7, 1093. [Google Scholar] [CrossRef]
- Ando, Y.; Siegler, E.L.; Ta, H.P.; Cinay, G.E.; Zhou, H.; Gorrell, K.A.; Au, H.; Jarvis, B.M.; Wang, P.; Shen, K. Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model. Adv. Healthc. Mater. 2019, 8, 1900001. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Truttschel, R.; Gong, M.M.; Humayun, M.; Virumbrales-Munoz, M.; Vitek, R.; Felder, M.; Gillies, S.D.; Sondel, P.M.; Wisinski, K.B.; et al. Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. OncoImmunology 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.S.; Zervantonakis, I.K.; Chung, S.; Kamm, R.D.; Charest, J.L. In Vitro Model of Tumor Cell Extravasation. PLoS ONE 2013, 8, e56910. [Google Scholar] [CrossRef] [Green Version]
- Ayuso, J.M.; Gong, M.M.; Skala, M.C.; Harari, P.M.; Beebe, D.J. Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, e1900925. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, A.; Tan, A.T.; Koh, S.; Chia, A.; Colombo, M.; Antonecchia, E.; Miccolis, C.; Ceccarello, E.; Adriani, G.; Raimondi, M.T.; et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Doty, D.; Zielstorff, M.; Kariv, I.; Moy, L.Y.; Gimbel, A.; Chevillet, J.R.; Lowry, N.; Santos, J.; Mott, V.; et al. A multiplexed microfluidic system for evaluation of dynamics of immune–tumor interactions. Lab Chip 2018, 18, 1844–1858. [Google Scholar] [CrossRef]
- Kwak, B.; Ozcelikkale, A.; Shin, C.S.; Park, K.; Han, B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J. Control. Release 2014, 194, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Agliari, E.; Biselli, E.; De Ninno, A.; Schiavoni, G.; Gabriele, L.; Gerardino, A.; Mattei, F.; Barra, A.; Businaro, L. Cancer-driven dynamics of immune cells in a microfluidic environment. Sci. Rep. 2014, 4, 6639. [Google Scholar] [CrossRef]
- Mattei, F.; Schiavoni, G.; De Ninno, A.; Lucarini, V.; Sestili, P.; Sistigu, A.; Fragale, A.; Sanchez, M.; Spada, M.; Gerardino, A.; et al. A multidisciplinary study usingin vivotumor models and microfluidic cell-on-chip approach to explore the cross-talk between cancer and immune cells. J. Immunotoxicol. 2014, 11, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Kajahn, J.; Franz, S.; Rueckert, E.; Forstreuter, I.; Hintze, V.; Moeller, S.; Simon, J.C. Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatterials 2013, 2, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Kou, P.M.; Pallassana, N.; Bowden, R.; Cunningham, B.; Joy, A.; Kohn, J.; Babensee, J.E. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 2012, 33, 1699–1713. [Google Scholar] [CrossRef] [Green Version]
- Dohle, E.; Bischoff, I.; Böse, T.; Marsano, A.; Banfi, A.; Unger, R.E.; Kirkpatrick, C.J. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur. Cell Mater. 2014, 27, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Swartzlander, M.D.; Blakney, A.K.; Amer, L.D.; Hankenson, K.D.; Kyriakides, T.R.; Bryant, S.J. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials 2014, 41, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, B.; De Vries, H.; Firth, K.; Van Weerd, J.; Tertoolen, L.; Karperien, H.; Jonkheijm, P.; Denning, C.; Ijzerman, A.P.; Mummery, C. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Ghaemmaghami, A.M.; Hancock, M.J.; Harrington, H.; Kaji, H.; Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 2012, 17, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Imura, Y.; Sato, K.; Yoshimura, E. Micro Total Bioassay System for Ingested Substances: Assessment of Intestinal Absorption, Hepatic Metabolism, and Bioactivity. Anal. Chem. 2010, 82, 9983–9988. [Google Scholar] [CrossRef]
- Tatosian, D.A.; Shuler, M.L. A novel system for evaluation of drug mixtures for potential efficacy in treating multidrug resistant cancers. Biotechnol. Bioeng. 2009, 103, 187–198. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Curtis, E.L.; Eagleton, Z.E.; Evans, B.C.; Kole, A.; Hofmeister, L.H.; Matloff, W.J. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013, 13, 3496–3511. [Google Scholar] [CrossRef]

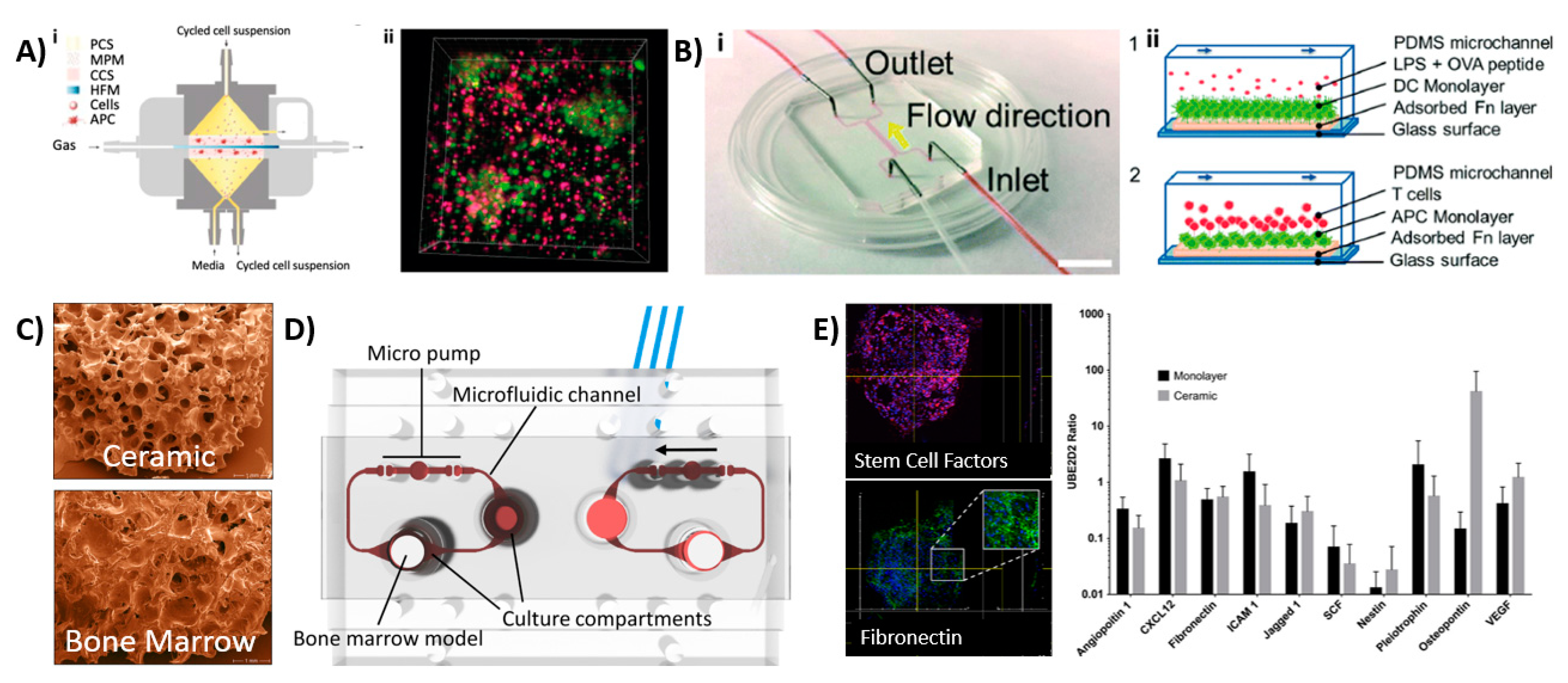

| Design of Microfluidic Device | Key Cell Types | Findings | Ref. |
|---|---|---|---|
| Lymph-node-on-a-chip | |||
| Membrane-based perfusion bioreactor system containing multiple chambers for antigen-induced B-cell activation, DC–T-cell crosstalk, peripheral space to mimic lymphatic drainage and a DC-loaded hydrogel (Figure 3Ai). | B and T-lymphocytes from healthy donors Monocyte-derived human DCs | Migration of B- and T-cells from peripheral fluidic space towards DCs. LF structure formation upon immunization and activation. Controlled IgM release post-activation. | [44] |
| Membrane-based perfusion bioreactor system containing culture compartment with LN cells and MSCs -laden agarose gel discs. | Rat-derived MSCs LN cells derived from rat lymph nodes | Concanavalin A-stimulated LN cells showed reduced proliferation in MSC co-culture. MSC co-culture suppressed levels of proinflammatory molecules (TNFα and IFNγ) and induced IL-1a and IL-6 secretion. | [47] |
| Two chamber microfluidic system with recirculating flow to transport secreted signals between tumor and lymph-node tissue. | BALb/c-derived tumor and lymph node tissue slices | Real-time monitoring of tissue interactions, fluid flow and shear stress. Decreased IFNγ secretion within lymph nodes cultured with immunosuppressed T-cell containing tumor tissue | [50] |
| PDMS chip with one flow channel connected to two inlets and two outlets. | LPS-activated DCs CD8+ and CD4+ T-cells | Duration and strength of immune cell response depended upon shear stress. Stronger DC interaction with CD4+ T-cells. | [48] |
| Microdevice with chemotaxis compartment filled with DCs linked to a T-cell compartment. Separate media and chemokine channels. | MUTZ-3-derived DCs, T-lymphocytes | Design allowed chemotaxis of DCs under non-adherent conditions. CCR7-induced mature DC migration towards T-cells. Mature DCs showed stronger T-cell activation than immature DCs. Showed chemotaxis is critical in T-cell activation. | [49] |
| Two-channel device with media in upper channel and B- and T-cells laden Matrigel in bottom channel. | B-lymphocytes, T-lymphocytes | Perfusion stimulated the formation of LFs inside the chip. Formation of plasma B-cell clusters 7 days post-stimulation. Class-switching of B-cells was induced with specific cytokines and antibodies. Similar cytokine profiles were observed to human volunteers when exposed to Fluzone. | [51] |
| Bone-marrow-on-a-chip | |||
| Cylindrical PDMS device suitable for implantation. | HSCs Hematopoietic progenitor cells Osteoblasts Endothelial cells Perivascular cells Nestin+ MSCs | Formation and characterization of BM within device 8 weeks post-implantation. Presence of nestin+ cells indicate support of HSC and hematopoietic function. No expensive cytokines were needed to maintain cellular function. | [52] |
| Microfluidic chip device with central chamber containing BM tissue with underlying microfluidic channel, separated by a porous PDMS membrane. | In vivo-derived BM tissue | BM tissue produced and released blood cells into microfluidic circulation. Able to maintain viability and function of HSCs, which could differentiate into mature blood cells on-chip. Organ-level response to radiation toxicity. Showed that the hematopoietic microenvironment is crucial for modeling radiation toxicity. | [54] |
| Two-channel device with BM stem cell- and CD34+ progenitor cell-loaded hydrogel in top channel and endothelial cell lining in bottom vascular channel. | BM stem cells CD34+ progenitor cells Endothelial cells | Differentiation and maturation of different blood cell lineages, including neutrophils, erythroids and megakaryocytes. Maintain CD34+ viability up to 4 weeks Successful modeling of BM dysfunction using diseased CD34+ cells. | [55] |
| Microfluidic device consisting of a BM compartment and a compartment for other organs. | hMSCs HSPCs | Preculture of MSC on ceramic scaffold-induced ECM, which allowed maintenance of HSPC phenotype. Range of genes which are involved in multiple hematopoietic niche functions were observed. | [56] |
| Four-channel microfluidic platform filled with tumor cell, BMSC and HOB-laden collagen I. | Human Philadelphia chromosome positive B lineage ALL cell line BMSCs HOBs | Cell-matrix interactions influenced cell migration and invasion and led to cellular responses not observed in 2D. No BMSC spreading was observed in 3D dynamic condition. Decreased chemotherapeutic drug sensitivity was observed compared to 2D cultures. | [57] |
| Splenon-on-a-chip | |||
| Two-layered microengineered device which mimicked the closed-fast and open-slow microcirculation. | Uninfected and infected red blood cells | Microfluidic device accurately mimicked the red pulp and thus the filtering function of the spleen with accurate recognition of different RBC types. | [68] |
| Inflammation-on-a-chip | |||
| Multichannel device incorporating a co-culture of neutrophils and endothelial cells, ECM and concentration gradients of various inflammatory proteins. | Neutrophils Endothelial cells | The system showed transendothelial migration of neutrophils. N-formyl-methionyl-leucyl-phenylalanine showed higher attraction than IL-8. Strong correlation between matrix stiffness and migration was found. | [71] |
| Microfluidic culture platform with lumen channel inside a protein matrix. | Neutrophils iPSC-derived endothelial cells | Precise control over lumen size, structure and configuration. Composition of the ECM influences the barrier function of endothelial cells. Secretion of angiogenic and inflammatory factors. Neutrophil chemotaxis towards IL-8 improved in presence of endothelial cells. | [72] |
| Microfluidic device containing a central cell loading chamber and a chemoattractant gradient along migration channel. | Primary human neutrophils Human monocytes | Maintains chemotactic gradients up to 48 h but does change over time. Allows single-cell resolution of chemotaxis of neutrophils. Indication of bidirectional communication between monocytes and neutrophils. | [73] |
| PDMS device with central loading inlet, leading to eight channels connected to the chemoattractant chambers. | Human whole blood | Assay allowed passaging of neutrophils only. Chemoattractant gradients were maintained up to 8 h. Showed that neutrophils could regulate their traffic in absence of monocytes. | [74] |
| Multichannel PDMS device which allowed migration of cells through migration channels towards cytokine-laden channels. | MF2.2D9 T0 cell hybridomas IC-21 macrophages Immortalized B6 macrophages LPS-activated DCs | Successful migration of cells by chemoattractant gradient. Phagocytosis stopped macrophages from migrating further. Little cell proliferation observed. CCL19-induced mature DC chemotaxis. | [75] |
| A three-organ device with a liver module, cardiac cantilevers and stimulation electrodes, skeletal muscle cantilevers and recirculating THP-1 monocytes in medium. | THP-1 monocytes Primary human hepatocytes Human cardiomyocytes Human skeletal muscle myoblasts | Non-selective damage to cells in three different organs. Increased proinflammatory molecule release. Amiodarone-induced M2 polarization indicated by increased IL-6 release. | [76] |
| Lung-on-a-chip | |||
| Two-channel device with a polyester membrane. Primary human airway epithelial cells cultured on membrane in upper channel, with medium flowing in bottom channel. | Primary human airway epithelial cells Epithelial cells derived from COPD patients. | Inflammatory response was induced by an IL-13 insult, resulting in a proinflammatory response with hyperplasia of mucus secreting goblet cells. Showed neutrophil recruitment to diseased epithelial cells. | [77] |
| Skin-on-a-chip | |||
| Multilayer device with layer of HaCaT cultured on top of a porous membrane and an immune cell layer positioned beneath the KC layer. | HaCaTs U937 cell line | U937 monoculture showed highest expression of inflammation after LPS treatment. Perfusion induced the formation of tighter junctions. | [80] |
| Multilayer chip consisting of a HaCaT layer, a fibroblast layer and an endothelial cell layer, separated by porous membranes. | HaCaTs HS27 fibroblasts HUVECs | Successful design of skin model to mimic epidermis, dermis and vessels of the skin. Dexamethasone prevented tight junction damage and lowered IL-1β, IL-6 and IL-8 expression, thereby showing recovery of skin with edema. | [81] |
| Multichambered microfluidic device with interchangeable lids and insets for developing a full-thickness skin-on-a-chip model. | Human primary foreskin-derived dermal fibroblasts Immortalized human N/TERT keratinocytes | Developed a flexible bioreactor for tissue culture, with the ability to perform TEER measurements, permeation assays and assessing the skin’s integrity. Potential to culture multiple organs in parallel or addition of immune system. Dynamic perfusion improved morphogenesis, differentiation and maturation. | [83] |
| Liver-on-a-chip | |||
| Multilayer biochip containing a HUVEC/macrophage layer with monocytes freely flowing in the media and a hepatocyte/hepatic stellate cell layer at the bottom. | HepaRG hepatocytes HUVECs LX-2 stellate cells Peripheral blood mononuclear cell-derived macrophages Primary monocytes THP-1 monocytes | Migration and M1 polarization of monocytes upon LPS treatment. IL-10 production upon monocyte invasion, inducing M2 polarization. Monocyte invasion inhibited inflammation-related cell death and induced the recovery of metabolic functions. | [89] |
| Gut-on-a-chip | |||
| Two-channel device with porous membrane coated with ECM, with one side of the membrane coated with intestinal epithelial cells and the other with endothelial cells. Incorporation of vacuum chambers allowed recapitulation of peristaltic movements. | Caco-2 intestinal epithelial cells Human capillary endothelial cells. Human lymphatic microvascular endothelial cells E. coli strain | Formation of intestinal villi in 5 days. Inflammation was induced by co-culture with the E. coli strain, leading to secretion of TNF-α, IL-1β, IL-6 and IL-8 Growth of bacteria also resulted in epithelial deformation and disturbance of peristaltic movements. | [82,90,94] |
| Multichambered chip with separately controlled microbial and epithelial cell microchambers. | Caco-2 intestinal epithelial cells Noncancerous colonic cell line Primary CD4+ T-cells Lactobacillus rhamnosus GG | Successful incorporation of co-culture of human and microbial cells. Independently controlled chambers allowed for anaerobic culture conditions for the microbial cells. Slight inflammatory response after addition of microbial cells. Showed crosstalk between microbial and human cells, depicted by alteration of several genes and miRNAs. | [95] |
| Microfluidic device with apical and basolateral compartments separated by a porous membrane. | U937 cells Caco-2 intestinal epithelial cells | Full, confluent layers formed 5 days after Caco-2 cell seeding. Dynamic cell culture conditions improved viability. LPS and cytokine addition increased permeability of the epithelial cell layer. | [96] |
| Tumor-microenvironment-on-a-chip | |||
| Central immune chamber with floating IFN-DCs connected to two side tumor chambers with treated and untreated cancer cells in type I collagen. | IFN-DCs RI+ and RI- SW620 CRCs | IFN-DCs migrated towards RI-treated cancer cells. Increased antigen take up resulting in increased phagocytosis and antitumor function. | [99] |
| CAR-T cells delivered through microfluidic channels. Tumor cells in GelMA between two oxygen diffusion barriers. | HER2+ SKOV3 human OCCs Anti HER2 CAR-T cells | Hypoxia alters PD-L1 expression. Limited CAR-T infiltration due to matrix stiffness and oxygen concentration. Hypoxia promotes immunosuppression. | [100] |
| Channel containing tumor spheroids embedded with NK cells in collagen. Two endothelial vascular lamina on lateral sides. | MCF7 breast tumor spheroids NK-92CD16V NK cells HUVECs | Delayed anti-EpCAM-IL-2 antibody penetration by endothelial barrier and cell–cell interactions. NK cell cytotoxicity and ADCC was enhanced by anti-EpCAM-IL-2. | [101] |
| Tubular lymphatic vessel adjacent to lumen filled with breast cancer cells, co-cultured in collagen hydrogel. | Estrogen-positive MCF-7 cells MDA-MB-231 breast cancer cells Human lymphatic endothelial cells (HLECs) | Co-culture with MCF-7 led to alteration of multiple HLEC genes, which correlated to functional changes in endothelial barrier capacity. | [105] |
| One channel filled with liver tumor cells in type I collagen. Second channel containing tumor specific T-cells. Control over oxygen levels and inflammatory cytokines. | TCR engineered T-cells HBV+ HepG2 cells | T-cells are dependent on tumor cells for migration and induction of apoptosis. Level of oxygen and cytokines important factor in their optimal activity. | [106] |
| Multiplexed microfluidic device laden with tumor tissue. Infusion of tumor infiltrating lymphocytes. | MC38 tumors and cells PD38+ T-cells Human tumor tissue CD45+ tumor infiltrating lymphocytes | Presence of anti-PD-1 inhibitor led to higher cell death and infiltration into the tumor tissue. | [107] |
| Breast cancer cells seeded into type I collagen. Separate microchannels mimicking the lymphatic and blood vessels. | MCF-7 breast cancer cells Microvascular endothelial cells | Research on cutoff pore size, ECM structure and lymphatic drainage showed that extravasation and interstitial diffusion was significantly decreased with particles of 100 to 200 nm (smaller than EPR window). | [108] |
| Two culture chambers (melanoma and splenocytes compartment) connected via narrow capillary migration channels. | B16.F10 murine melanoma cells Murine splenocytes | Absence of IFN regulatory factor 8 (IRF-8) led to poor splenocyte migration towards and interaction with cancer cells. | [109,110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsink, M.A.J.; Willemen, N.G.A.; Leijten, J.; Bansal, R.; Shin, S.R. Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications. Micromachines 2020, 11, 849. https://doi.org/10.3390/mi11090849
Morsink MAJ, Willemen NGA, Leijten J, Bansal R, Shin SR. Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications. Micromachines. 2020; 11(9):849. https://doi.org/10.3390/mi11090849
Chicago/Turabian StyleMorsink, Margaretha A. J., Niels G. A. Willemen, Jeroen Leijten, Ruchi Bansal, and Su Ryon Shin. 2020. "Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications" Micromachines 11, no. 9: 849. https://doi.org/10.3390/mi11090849
APA StyleMorsink, M. A. J., Willemen, N. G. A., Leijten, J., Bansal, R., & Shin, S. R. (2020). Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications. Micromachines, 11(9), 849. https://doi.org/10.3390/mi11090849








