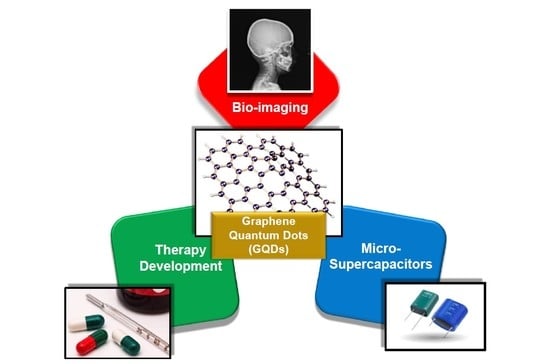
Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors
Abstract
:1. Introduction
1.1. Synthesis of GQDs
1.2. Biofunctionalization/ Conjugation of GQDs
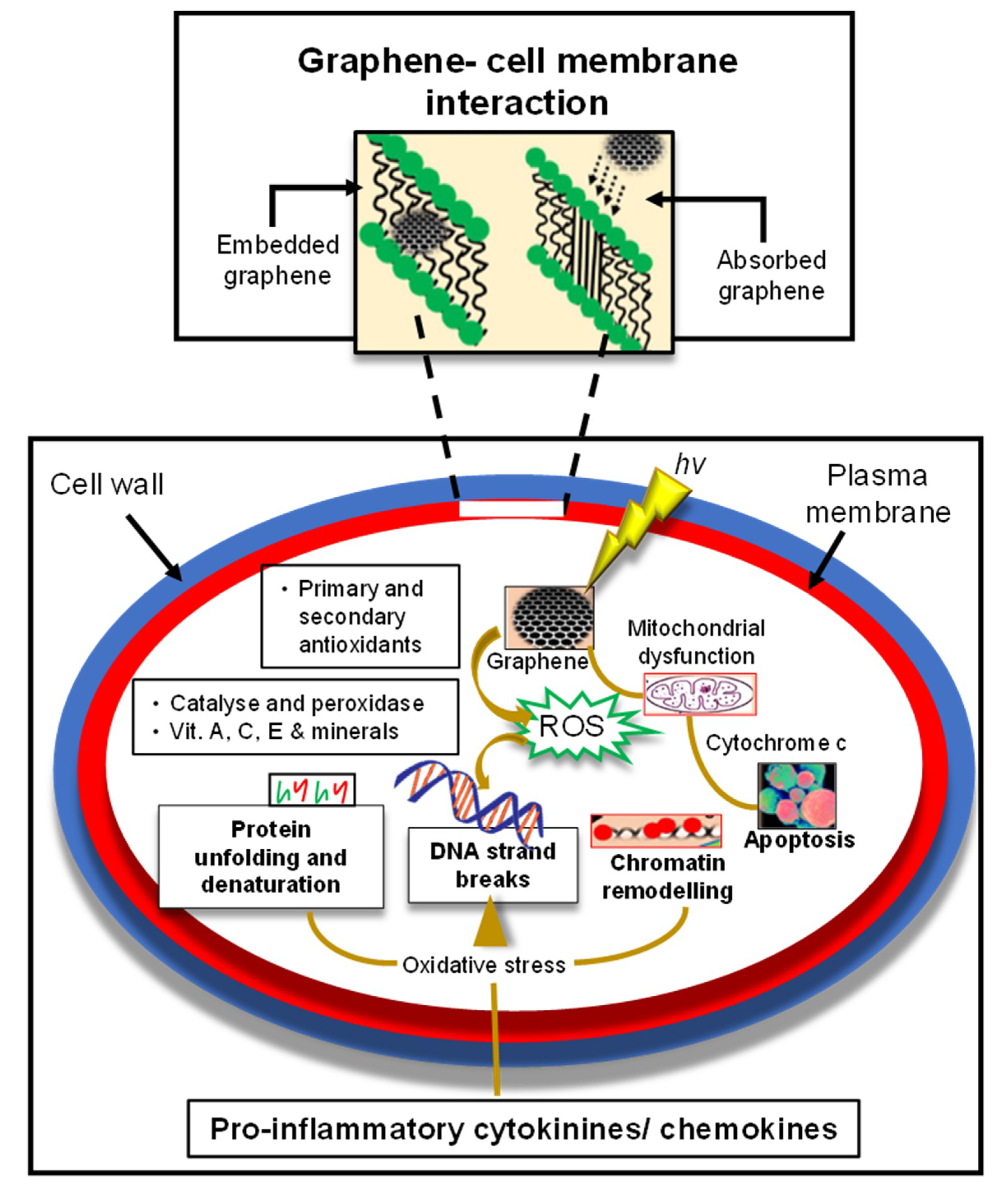
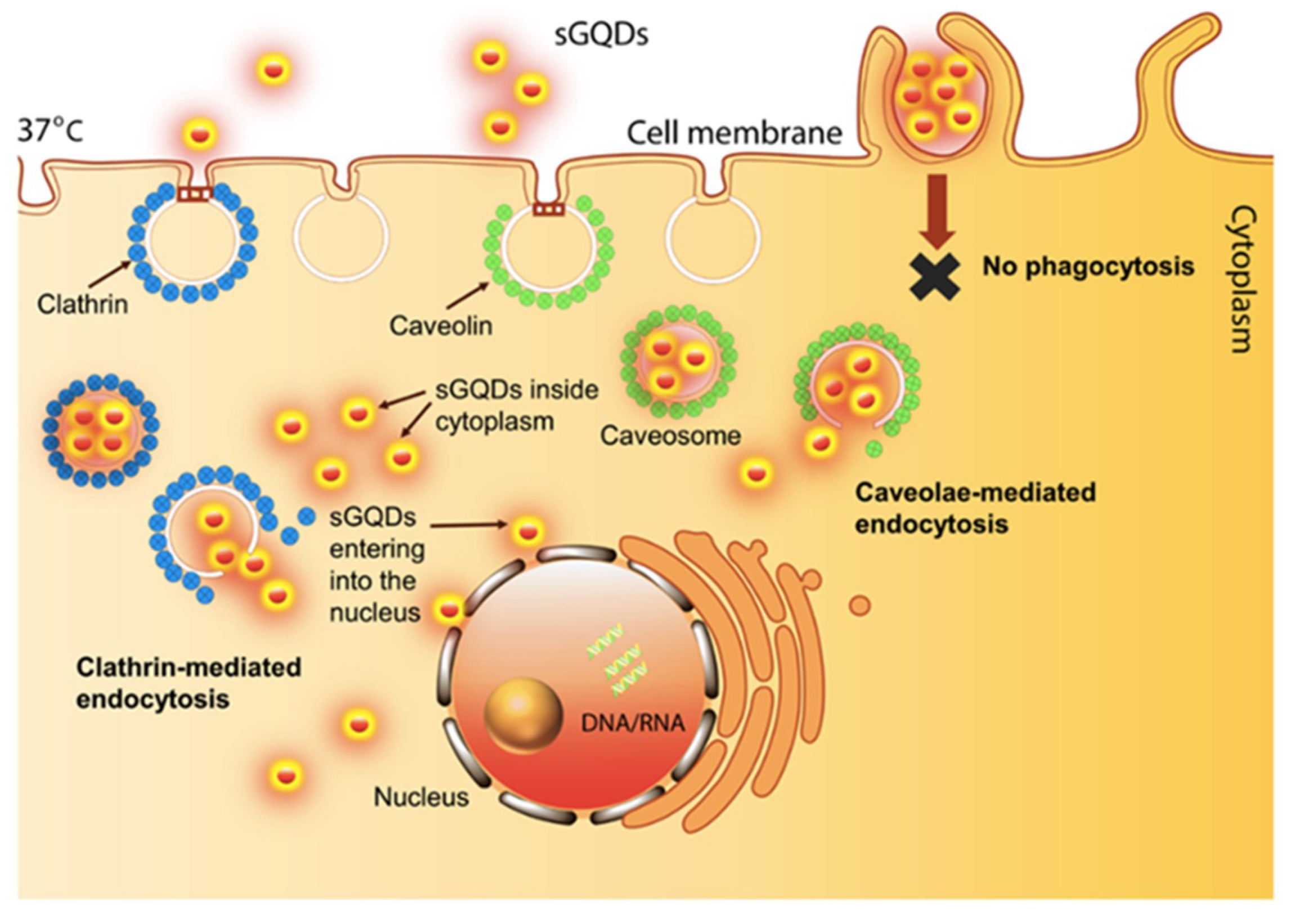
1.3. Impact of Toxicity Potential and Biocompatibility of GQDs
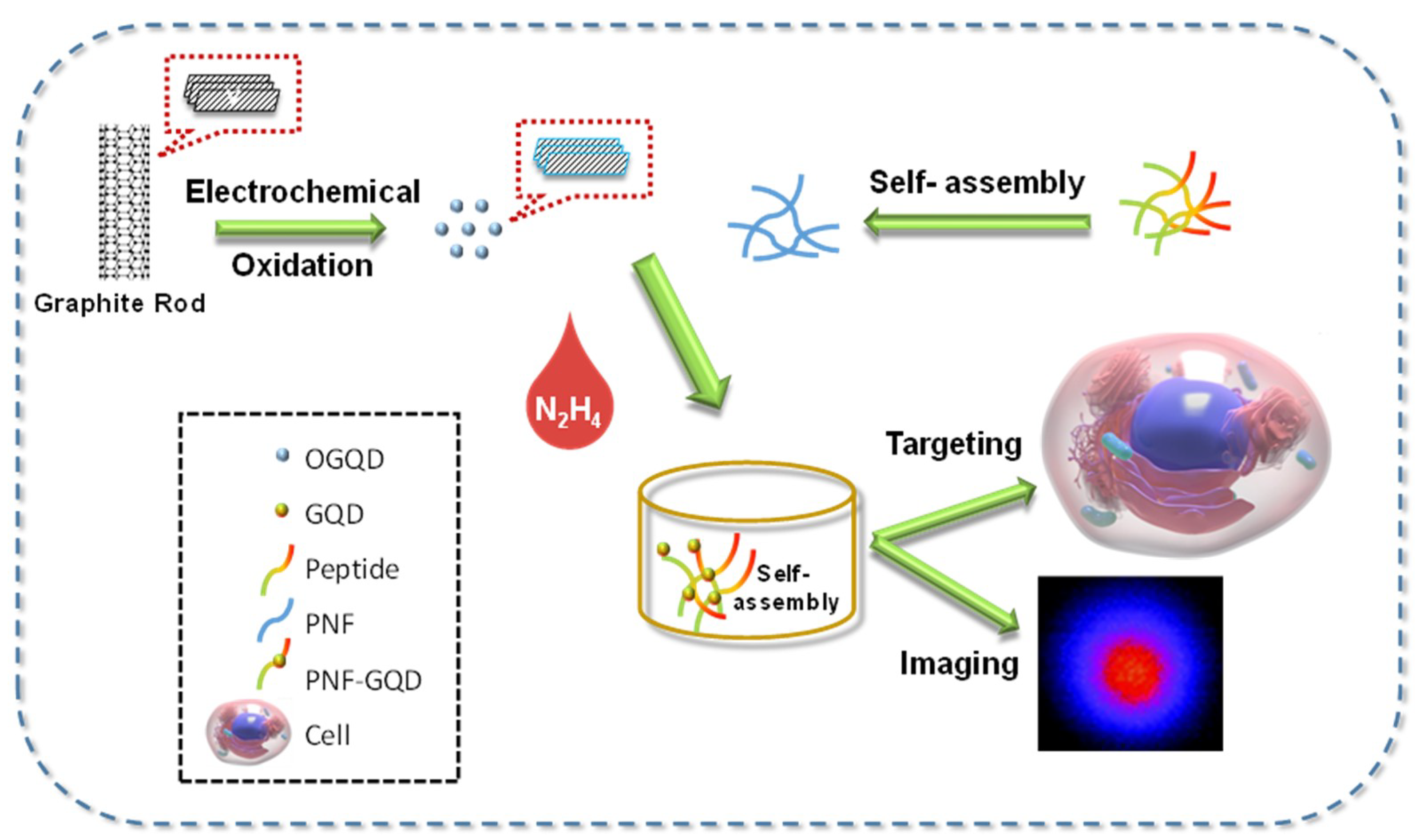
2. Bio-Imaging Applications of GQDs
3. Role of GQDs in Therapy Development
4. GQDs as Potent Electrode Material for the Development of Micro-Supercapacitors (MSCs)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Ali, N.; Milne, W.; Ozkan, C.; Mitura, S.; Gervasoni, J. Graphene science handbook: Applications and industrialization. In Graphene Science Handbook: Applications and Industrialization; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781466591332. [Google Scholar]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, A.L.; Roberts, M.A.J.; Kiappes, J.L.; Scott, K.A. Essential chemistry for biochemists. Essays Biochem. 2017, 61, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Hong, G.L.; Zhao, H.L.; Deng, H.H.; Yang, H.J.; Peng, H.P.; Liu, Y.H.; Chen, W. Fabrication of ultra-small monolayer graphene quantum dots by pyrolysis of trisodium citrate for fluorescent cell imaging. Int. J. Nanomed. 2018, 13, 4807–4815. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Hu, X.; Guan, P.; Hou, T.; Chen, P.; Wan, D.; Zhang, X.; Wang, J.; Wang, C. Highly biocompatible graphene quantum dots: Green synthesis, toxicity comparison and fluorescence imaging. J. Mater. Sci. 2020, 55, 1198–1215. [Google Scholar] [CrossRef]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Wei, G. Motif-designed peptide nanofibers decorated with graphene quantum dots for simultaneous targeting and imaging of tumor cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Abbas, A.; Tuti Mariana, L.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Cao, Q.; Li, M.; Liu, F.; Tang, N.; Du, Y. Synthesis and photoluminescence of fluorinated graphene quantum dots. Appl. Phys. Lett. 2013, 102, 013111. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Qi, S.; Jia, J.; Torre, B.; Zeng, H.; Wu, H.; Xu, X. Size and refinement edge-shape effects of graphene quantum dots on UV-visible absorption. J. Alloys Compd. 2015, 623, 186–191. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Qi, B.P.; Hu, H.; Bao, L.; Zhang, Z.L.; Tang, B.; Peng, Y.; Wang, B.S.; Pang, D.W. An efficient edge-functionalization method to tune the photoluminescence of graphene quantum dots. Nanoscale 2015, 7, 5969–5973. [Google Scholar] [CrossRef]
- Savas, S.; Altintas, Z. Graphene quantum dots as nanozymes for electrochemical sensing of yersinia enterocolitica in milk and human serum. Materials 2019, 12, 2189. [Google Scholar] [CrossRef] [Green Version]
- Tashkhourian, J.; Nami-Ana, S.F.; Shamsipur, M. Designing a modified electrode based on graphene quantum dot-chitosan application to electrochemical detection of epinephrine. J. Mol. Liq. 2018, 266, 548–556. [Google Scholar] [CrossRef]
- Wang, H.; Revia, R.; Wang, K.; Kant, R.J.; Mu, Q.; Gai, Z.; Hong, K.; Zhang, M. Paramagnetic properties of metal-free boron-doped graphene quantum dots and their application for safe magnetic resonance imaging. Adv. Mater. 2017, 29, 1605416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.I.M.; Rodríguez-Pérez, L.; Gonçalves, G.; Pinto, S.N.; Melle-Franco, M.; Marques, P.A.A.P.; Faustino, M.A.F.; Herranz, M.Á.; Martin, N.; Neves, M.G.P.M.S.; et al. Novel hybrids based on graphene quantum dots covalently linked to glycol corroles for multiphoton bioimaging. Carbon N. Y. 2020, 166, 164–174. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Q.; Geng, L.; Shu, X.; Wang, Y. A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 2019, 197, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, B.; Zhang, Q.; Tang, Y.; Liu, X.; Li, J. The influence of combination mode on the structure and properties of graphene quantum dot-porphyrin composites. Colloids Surf. B Biointerfaces 2018, 172, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, Y.J.; Bao, T.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Li, W.; Li, Z.; Zhan, J.; Geng, B.; Wang, S.; Pan, D.; Wu, M. Industrial production of ultra-stable sulfonated graphene quantum dots for Golgi apparatus imaging. J. Mater. Chem. B 2017, 5, 5355–5361. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Han, T.; Zhou, X.; Guo, S.; Zhang, J. Insight into the cellular internalization and cytotoxicity of graphene quantum dots. Adv. Healthc. Mater. 2013, 2, 1613–1619. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Şenel, B.; Demir, N.; Büyükköroğlu, G.; Yıldız, M. Graphene quantum dots: Synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharm. J. 2019, 27, 846–858. [Google Scholar] [CrossRef]
- Liu, X.; Na, W.; Liu, Q.; Su, X. A novel label-free fluorescent sensor for highly sensitive detection of bleomycin based on nitrogen-doped graphene quantum dots. Anal. Chim. Acta 2018, 1028, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Applications of graphene quantum dots in biomedical sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansuriya, B.D.; Altintas, Z. Graphene quantum dot-based electrochemical immunosensors for biomedical applications. Materials 2019, 13, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Seo, S.W.; Kim, J.M.; Lee, H.S.; Choi, S.H. Graphene transparent conductive electrodes doped with graphene quantum dots-mixed silver nanowires for highly-flexible organic solar cells. J. Alloys Compd. 2018, 744, 1–6. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, W.; Xie, F.; Li, Y.; Abate, A.; Wei, M. Graphene quantum dots decorated TiO2 mesoporous film as an efficient electron transport layer for high-performance perovskite solar cells. J. Power Sources 2018, 402, 320–326. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.; Kim, Y.J.; Hwang, S.W. Enhancement of dye-sensitized solar cells efficiency using graphene quantum dots as photoanode. Bull. Korean Chem. Soc. 2019, 40, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Bu, F.; Wei, J.; Yao, W.; Wang, L.; Chen, Z.; Pan, D.; Wu, M. Boosting the energy storage densities of supercapacitors by incorporating N-doped graphene quantum dots into cubic porous carbon. Nanoscale 2018, 10, 22871–22883. [Google Scholar] [CrossRef]
- Liu, W.-W.; Feng, Y.-Q.; Yan, X.-B.; Chen, J.-T.; Xue, Q.-J. Superior micro-supercapacitors based on graphene quantum dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.; Liu, S.; Wu, W.; Jia, T.; Yang, Z.; Mu, S.; Huang, Y. Graphene quantum dots encapsulated tremella-like NiCo2O4 for advanced asymmetric supercapacitors. Carbon N. Y. 2019, 146, 1–8. [Google Scholar] [CrossRef]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anticancer photodynamic therapy properties of sulfur-doped graphene quantum dot and methylene blue preparations in MCF-7 breast cancer cell culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.X. Graphene quantum dots (GQDs) and its derivatives for multifarious photocatalysis and photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Yue, S.; Tong, H.; Lu, L.; Tang, W.; Bai, W.; Jin, F.; Han, Q.; He, J.; Liu, J.; Zhang, X. Hierarchical NiCo2O4 nanosheets/nitrogen doped graphene/carbon nanotube film with ultrahigh capacitance and long cycle stability as a flexible binder-free electrode for supercapacitors. J. Mater. Chem. A 2017, 5, 689–698. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ziccarelli, I.; Pistone, A. Graphene quantum dots: Multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 2017, 5, 6471–6489. [Google Scholar] [CrossRef]
- Agarwal, S.; Sadeghi, N.; Tyagi, I.; Gupta, V.K.; Fakhri, A. Adsorption of toxic carbamate pesticide oxamyl from liquid phase by newly synthesized and characterized graphene quantum dots nanomaterials. J. Colloid Interface Sci. 2016, 478, 430–438. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Tong, X.; Wang, X.; Yang, P.; Yao, F.; Guo, R.; Yuan, C. Preparation of graphene quantum dots with high quantum yield by a facile one-step method and applications for cell imaging. Mater. Lett. 2020, 271, 127806. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Zhao, M. Direct synthesis of graphene quantum dots with different fluorescence properties by oxidation of graphene oxide using nitric acid. Appl. Sci. 2018, 8, 1303. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.B.; Zhu, Z.T.; Wang, H.X.; Huang, X.; Zhang, X.; Qi, X.; Zhang, H.L.; Zhu, Y.; Deng, X.; Peng, Y.; et al. A general solid-state synthesis of chemically-doped fluorescent graphene quantum dots for bioimaging and optoelectronic applications. Nanoscale 2015, 7, 10162–10169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Z.; Dervishi, E.; Doorn, S.K.; Sykora, M. Size-dependent electronic properties of uniform ensembles of strongly confined graphene quantum dots. J. Phys. Chem. Lett. 2019, 10, 953–959. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-dependent structural and optical characteristics of glucose-derived graphene quantum dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.; et al. Coal as an abundant source of graphene quantum dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Li, C.; Luo, P.; Tao, L.; Wei, Y.; Shi, G. Large scale preparation of graphene quantum dots from graphite with tunable fluorescence properties. Phys. Chem. Chem. Phys. 2013, 15, 9907–9913. [Google Scholar] [CrossRef]
- Nilewski, L.; Mendoza, K.; Jalilov, A.S.; Berka, V.; Wu, G.; Sikkema, W.K.A.; Metzger, A.; Ye, R.; Zhang, R.; Luong, D.X.; et al. Highly oxidized graphene quantum dots from coal as efficient antioxidants. ACS Appl. Mater. Interfaces 2019, 11, 16815–16821. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Dourandish, Z.; Beitollahi, H.; Tajik, S.; Hajiaghababaei, L.; Larijani, B. Highly sensitive determination of theophylline based on graphene quantum dots modified electrode. Int. J. Electrochem. Sci. 2018, 13, 2448–2461. [Google Scholar] [CrossRef]
- Kellici, S.; Acord, J.; Moore, K.E.; Power, N.P.; Middelkoop, V.; Morgan, D.J.; Heil, T.; Coppo, P.; Baragau, I.A.; Raston, C.L. Continuous hydrothermal flow synthesis of graphene quantum dots. React. Chem. Eng. 2018, 3, 949–958. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, D.; Yang, C.; Wu, X.; Hu, Y.; Zhang, Y.; Yuwen, L.; Yeow, E.K.L.; Weng, L.; Huang, W.; et al. Graphene quantum dots modified with adenine for efficient two-photon bioimaging and white light-activated antibacteria. Appl. Surf. Sci. 2018, 434, 155–162. [Google Scholar] [CrossRef]
- Ping, Y.; Ruiyi, L.; Yongqiang, Y.; Zaijun, L.; Zhiguo, G.; Guangli, W.; Junkang, L. Pentaethylenehexamine and D-penicillamine co-functionalized graphene quantum dots for fluorescent detection of mercury(II) and glutathione and bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Y.; Li, X.; Zhou, S.; Fan, L.; Yang, S. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem. Commun. 2015, 51, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Fan, L.; Fan, H. Synthesis of red fluorescent graphene quantum dot-europium complex composites as a viable bioimaging platform. Microchim. Acta 2016, 183, 2605–2613. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Sun, L.; Sun, B.; Yang, M.; Chen, H.; Wang, Y.; Sun, J.; Dong, L. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens. Actuators B Chem. 2018, 256, 616–623. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Srivastava, R.; Thakur, M.; Gurung, R.B. Graphene quantum dots from mangifera indica: Application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Y.; Shi, C.; Pei, Y.T. Large-scale synthesis of defect-selective graphene quantum dots by ultrasonic-assisted liquid-phase exfoliation. Carbon N. Y. 2016, 109, 373–383. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Photodynamic therapy using graphene quantum dot derivatives. J. Solid State Chem. 2020, 282, 121107. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Xu, M.; Wang, J.; Hu, X.; Anwar, S.; Tedesco, A.C.; Morais, P.C.; Bi, H. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mater. Chem. B 2020, 8, 2598–2606. [Google Scholar] [CrossRef]
- Zhou, L.; Geng, J.; Liu, B. Graphene quantum dots from polycyclic aromatic hydrocarbon for bioimaging and sensing of Fe3+ and hydrogen peroxide. Part. Part. Syst. Charact. 2013, 30, 1086–1092. [Google Scholar] [CrossRef]
- Lu, J.; Yan, M.; Ge, L.; Ge, S.; Wang, S.; Yan, J.; Yu, J. Electrochemiluminescence of blue-luminescent graphene quantum dots and its application in ultrasensitive aptasensor for adenosine triphosphate detection. Biosens. Bioelectron. 2013, 47, 271–277. [Google Scholar] [CrossRef]
- Mehrdad-Vahdati, B.; Pourhashem, S.; Sedghi, M.; Vaezi, Z.; Shojaedin-Givi, B.; Rashidi, A.; Naderi-Manesh, H. A novel aspect of functionalized graphene quantum dots in cytotoxicity studies. Toxicol. In Vitro 2019, 61, 104649. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Deshpande, S.; Shinde, D.B.; Pillai, V.K.; Singh, N. Mitigating the cytotoxicity of graphene quantum dots and enhancing their applications in bioimaging and drug delivery. ACS Macro Lett. 2014, 3, 1064–1068. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Z.; Guo, Z.; Ji, Y.; Jin, M.; Wang, X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Kong, Z.; Kang, Z.; Wang, H.; Zhang, L.; Shen, J.W. Theoretical evaluation on potential cytotoxicity of graphene quantum dots. ACS Biomater. Sci. Eng. 2016, 2, 1983–1991. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, B.; Yang, Y.; Cui, X.; Xin, Y.; Guo, L.H. Cytotoxicity and autophagy induction by graphene quantum dots with different functional groups. J. Environ. Sci. (China) 2019, 77, 198–209. [Google Scholar] [CrossRef]
- Xu, S.; Li, F.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Novel graphene quantum dots (GQDs)-incorporated thin film composite (TFC) membranes for forward osmosis (FO) desalination. Desalination 2019, 451, 219–230. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Yazdian, F. Application of green synthesized TiO2 /Sb2S3 /GQDs nanocomposite as high efficient antibacterial agent against E. coli and Staphylococcus aureus. Mater. Sci. Eng. C 2019, 99, 296–303. [Google Scholar] [CrossRef]
- Mondal, M.K.; Mukherjee, S.; Joardar, N.; Roy, D.; Chowdhury, P.; Sinha Babu, S.P. Synthesis of smart graphene quantum dots: A benign biomaterial for prominent intracellular imaging and improvement of drug efficacy. Appl. Surf. Sci. 2019, 495, 143562. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. What are the reasons for low use of graphene quantum dots in immunosensing of cancer biomarkers? Mater. Sci. Eng. C 2017, 71, 1313–1326. [Google Scholar] [CrossRef]
- Vázquez-Nakagawa, M.; Rodríguez-Pérez, L.; Herranz, M.A.; Martín, N. Chirality transfer from graphene quantum dot. Chem. Commun. 2016, 52, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Gao, J.; Chen, L.; Dong, S.; Li, H.; Qiu, H.; Zhao, L. Graphene quantum dots functionalized β-cyclodextrin and cellulose chiral stationary phases with enhanced enantioseparation performance. J. Chromatogr. A 2019, 1600, 209–218. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, Y.; Kong, Y.; Ma, J. Graphene quantum dots/β-cyclodextrin nanocomposites: A novel electrochemical chiral interface for tryptophan isomer recognition. Electrochem. Commun. 2015, 60, 60–63. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, N.; Gui, W.; Ma, Q. Nitrogen-doped graphene quantum dots-based fluorescence molecularly imprinted sensor for thiacloprid detection. Talanta 2018, 183, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; He, J.; Bian, S.; Zhou, C.; Li, Z.; Xi, F.; Liu, J.; Dong, X. S-doped graphene quantum dots as nanophotocatalyst for visible light degradation. Chin. Chem. Lett. 2018, 29, 1698–1701. [Google Scholar] [CrossRef]
- Bian, S.; Shen, C.; Hua, H.; Zhou, L.; Zhu, H.; Xi, F.; Liu, J.; Dong, X. One-pot synthesis of sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of lead(II). RSC Adv. 2016, 6, 69977–69983. [Google Scholar] [CrossRef]
- Xia, C.; Hai, X.; Chen, X.W.; Wang, J.H. Simultaneously fabrication of free and solidified N, S-doped graphene quantum dots via a facile solvent-free synthesis route for fluorescent detection. Talanta 2017, 168, 269–278. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.Y.; Liang, R.P.; Li, Y.H.; Qiu, J.D. Boron-doped graphene quantum dots for selective glucose sensing based on the ‘abnormal’ aggregation-induced photoluminescence enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef]
- Kang, G.S.; Lee, S.; Yeo, J.S.; Choi, E.S.; Lee, D.C.; Na, S.I.; Joh, H.I. Graphene quantum dots with nitrogen and oxygen derived from simultaneous reaction of solvent as exfoliant and dopant. Chem. Eng. J. 2019, 372, 624–630. [Google Scholar] [CrossRef]
- Xue, Z.; Gao, H.; Li, X. A Green and lower-temperature synthesis of two-color fluorescent nitrogen doped graphene quantum dots. Dye. Pigment. 2018, 156, 379–385. [Google Scholar] [CrossRef]
- Chen, J.; Than, A.; Li, N.; Ananthanarayanan, A.; Zheng, X.; Xi, F.; Liu, J.; Tian, J.; Chen, P. Sweet graphene quantum dots for imaging carbohydrate receptors in live cells. FlatChem 2017, 5, 25–32. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Gu, B.; Gao, B.; Wang, T.; Zheng, X.; Wang, G.; Guo, Q.; Chen, D. Ultraviolet light-driven controllable doping of graphene quantum dots with tunable emission wavelength for fluorescence bio-imaging. Mater. Lett. 2020, 266, 127468. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Sun, L.; Xu, Y.; Li, M.; Hu, G.; Tang, T.; Wen, J.; Li, X.; Zhang, J.; et al. High fluorescent sulfur regulating graphene quantum dots with tunable photoluminescence properties. J. Colloid Interface Sci. 2018, 529, 205–213. [Google Scholar] [CrossRef]
- Mandal, B.; Sarkar, S. Exploring the electronic structure of graphene quantum dots. J. Nanopart. Res. 2012. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile preparation and upconversion luminescence of graphene quantum dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Yang, L.; Li, R.S.; Bin Chen, B.; Liu, H.; Huang, C.Z. Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chem. 2017, 19, 3611–3617. [Google Scholar] [CrossRef]
- Jovanović, S.P.; Syrgiannis, Z.; Budimir, M.D.; Milivojević, D.D.; Jovanovic, D.J.; Pavlović, V.B.; Papan, J.M.; Bartenwerfer, M.; Mojsin, M.M.; Stevanović, M.J.; et al. Graphene quantum dots as singlet oxygen producer or radical quencher-The matter of functionalization with urea/thiourea. Mater. Sci. Eng. C 2020, 109, 110539. [Google Scholar] [CrossRef] [PubMed]
- Drissi, L.B.; Ouarrad, H.; Ramadan, F.Z.; Fritzsche, W. Graphene and silicene quantum dots for nanomedical diagnostics. RSC Adv. 2019, 10, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.A.; Lin, L.; Ali, M.; Jabeen, F.; Ali, M.; Iqbal, R.; Horsell, D.W.; Winyard, P.G.; Zhang, S. Investigating the bioavailability of graphene quantum dots in lung tissues via fourier transform infrared spectroscopy. Interface Focus 2018, 8, 20170054. [Google Scholar] [CrossRef]
- Tabish, T.A.; Zhang, S. Graphene Quantum Dots: Syntheses, Properties, and Biological Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 1–5, ISBN 9780128122952. [Google Scholar]
- Wang, T.; Zhu, S.; Jiang, X. Toxicity mechanism of graphene oxide and nitrogen-doped graphene quantum dots in RBCs revealed by surface-enhanced infrared absorption spectroscopy. Toxicol. Res. 2015, 4, 885–894. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; Ferguson, D.C.J.; Lin, L.; Van Der Veen, A.; Lowry, S.; Ali, M.; Jabeen, F.; Winyard, P.G.; Zhang, S. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.C.; Lee, J.Y.; Kim, J.; Yoo, J.M.; Kang, I.; Kim, J.J.; Shin, N.; Kim, D.J.; Choi, S.W.; Kim, D.; et al. Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 2020, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Jun, G.; Schurhammer, R.; Reina, G.; Chen, P.; Bianco, A.; Ménard-Moyon, C. Enzymatic degradation of graphene quantum dots by human peroxidases. Small 2019, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef]
- Fasbender, S.; Zimmermann, L.; Cadeddu, R.-P.; Luysberg, M.; Moll, B.; Janiak, C.; Heinzel, T.; Haas, R. The low toxicity of graphene quantum dots is reflected by marginal gene expression changes of primary human hematopoietic stem cells. Sci. Rep. 2019, 9, 12028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; He, H.; Yang, H.; Lao, J.; Song, Y.; Xia, Y.; Xu, H.; Zhang, X.; Huang, F. A two-component active targeting theranostic agent based on graphene quantum dots. J. Mater. Chem. B 2015, 3, 3583–3590. [Google Scholar] [CrossRef]
- Fan, Z.; Nie, Y.; Wei, Y.; Zhao, J.; Liao, X.; Zhang, J. Facile and large-scale synthesis of graphene quantum dots for selective targeting and imaging of cell nucleus and mitochondria. Mater. Sci. Eng. C 2019, 103, 109824. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.K. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Song, L.; Liu, X.; Hu, Z. Microwave assisted one-pot synthesis of graphene quantum dots as highly sensitive fluorescent probes for detection of iron ions and pH value. Talanta 2016, 150, 54–60. [Google Scholar] [CrossRef]
- Malik, N.; Arfin, T.; Khan, A.U. Graphene Nanomaterials: Chemistry and Pharmaceutical Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128165058. [Google Scholar]
- Kikuchi, K. Design, synthesis and biological application of chemical probes for bio-imaging. Chem. Soc. Rev. 2010, 39, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Glasser, O.; Boveri, M. Rontgen, and the Discovery of X-rays. Nature 1890, 32, 511–512. [Google Scholar]
- Rizzo, P.F.; Gould, E.S.; Lyden, J.P.; Asnis, S.E. Diagnosis of occult fractures about the hip. Magnetic resonance imaging compared with bone-scanning. J. Bone Jt. Surg. Ser. A 1993, 75, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Bieling, H.B.; Wardelmann, E.; Leutner, C.C.; Koenig, R.; Kuhn, W.; Schild, H.H. MRI for diagnosis of pure ductal carcinoma in situ: A prospective observational study. Lancet 2007, 370, 485–492. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Hotter, A.; Hussl, A.; Esterhammer, R.; Schocke, M.; Seppi, K. Significance of MRI in diagnosis and differential diagnosis of parkinson’s disease. Neurodegener. Dis. 2010, 7, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.T.; Pitchaimani, A.; Ferrel, C.; Thakkar, R.; Aryal, S. Nano-confinement-driven enhanced magnetic relaxivity of SPIONs for targeted tumor bioimaging. Nanoscale 2018, 10, 284–294. [Google Scholar] [CrossRef]
- Van Tam, T.; Choi, W.M. One-pot synthesis of highly fluorescent amino-functionalized graphene quantum dots for effectivae detection of copper ions. Curr. Appl. Phys. 2018, 18, 1255–1260. [Google Scholar]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep ultraviolet to near-infrared emission and photoresponse in layered n-doped graphene quantum dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for bioimaging. Adv. Colloid Interface Sci. 2006, 123–126, 471–485. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–72. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Lin, Y. Graphene and graphene-like 2D materials for optical biosensing and bioimaging: A review. 2D Mater. 2015, 2, 32004. [Google Scholar] [CrossRef]
- Nair, R.V.; Thomas, R.T.; Sankar, V.; Muhammad, H.; Dong, M.; Pillai, S. Rapid, Acid-Free Synthesis of High-Quality Graphene Quantum Dots for Aggregation Induced Sensing of Metal Ions and Bioimaging. ACS Omega 2017, 2, 8051–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Gao, N.; Ren, J.; Qu, X. Improvement of photoluminescence of graphene quantum dots with a biocompatible photochemical reduction pathway and its bioimaging application. ACS Appl. Mater. Interfaces 2013, 5, 1174–1179. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Shao, L.; Zhou, J.; Chen, J.; Feng, H. Surface functionalization of graphene quantum dots with small organic molecules from photoluminescence modulation to bioimaging applications: An experimental and theoretical investigation. RSC Adv. 2013, 3, 14571–14579. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Yang, Y.; Cui, J.; Huang, Z.; Wang, Y.; Yang, L.; Wang, H.; Xiao, Y.; Rong, J. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. B 2013, 1, 39–42. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Umrao, S.; Parashar, V.; Abraham, S.; Singh, A.K.; Nath, G.; Saxena, P.S.; Srivastava, A. Facile, rapid and upscaled synthesis of green luminescent functional graphene quantum dots for bioimaging. RSC Adv. 2014, 4, 21101–21107. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Pu, K. Biosenors and Bioimaging. ChemBioChem 2019, 20, 420–421. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.H.; Bae, K.; Huang, Z.W.; Xue, J.M. Two-photon graphene quantum dot modified Gd2O3 nanocomposites as a dual-mode MRI contrast agent and cell labelling agent. Nanoscale 2018, 10, 5642–5649. [Google Scholar] [CrossRef] [PubMed]
- Theer, P.; Hasan, M.T.; Denk, W. Two-photon imaging to a depth of 1000 µm in living brains by use of a TiAl2O3 regenerative amplifier. Opt. Lett. 2003, 28, 1022. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Belfield, K.D. Two-photon fluorescent probes for bioimaging. Eur. J. Org. Chem. 2012, 2012, 3199–3217. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-molecule two-photon probes for bioimaging applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Sun, S.; Zhang, L.; Jiang, K.; Lin, H. Near-infrared emissive carbon dots for two-photon fluorescence bioimaging. Nanoscale 2016, 8, 17350–17356. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, S.; Li, Y.; Li, X.; Fan, L.; Voelcker, N.H. Rhodamine-functionalized graphene quantum dots for detection of Fe3+ in cancer stem cells. ACS Appl. Mater. Interfaces 2015, 7, 23958–23966. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Xue, Z.; Huang, C.; Shan, Y.; Liu, C.; Qin, X.; Yang, W.; Chen, X.; Wang, T. Understanding the selective detection of Fe3+ based on graphene quantum dots as fluorescent probes: The Ksp of a metal hydroxide-assisted mechanism. Anal. Chem. 2017, 89, 12054–12058. [Google Scholar] [CrossRef]
- Sheng, L.; Huangfu, B.; Xu, Q.; Tian, W.; Li, Z.; Meng, A.; Tan, S. A highly selective and sensitive fluorescent probe for detecting Cr(VI) and cell imaging based on nitrogen-doped graphene quantum dots. J. Alloys Compd. 2020, 820, 153191. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Gu, B.; Gao, B.; Wang, T.; Guo, Q.; Wang, G. Biomass-derived nitrogen doped graphene quantum dots with color-tunable emission for sensing, fluorescence ink and multicolor cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117671. [Google Scholar] [CrossRef]
- Bilan, R.; Fleury, F.; Nabiev, I.; Sukhanova, A. Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjug. Chem. 2015, 26, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, P.; He, R.; Lin, J.; Yang, S.; Zhang, X.; Ren, Q.; Cui, D. Aptamer-conjugated dendrimer-modified quantum dots for cancer cell targeting and imaging. Mater. Lett. 2010, 64, 375–378. [Google Scholar] [CrossRef]
- Park, J.-H.; von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Wang, X.; Yang, L.Y.; He, H.; Ba, X.X.; Zhao, J.; Jiang, F.L.; Liu, Y. Red, Yellow, and Blue Luminescence by Graphene Quantum Dots: Syntheses, Mechanism, and Cellular Imaging. ACS Appl. Mater. Interfaces 2017, 9, 24846–24856. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Xian, M.; Dong, C.; Shuang, S. Folic acid-conjugated green luminescent carbon dots as a nanoprobe for identifying folate receptor-positive cancer cells. Talanta 2018, 183, 39–47. [Google Scholar] [CrossRef]
- John, A.A.; Kumar Jaganathan, S.; Ayyar, M.; Krishnasamy, N.P.; Rajasekar, R.; Supriyanto, E. Folic acid decorated chitosan nanoparticles and its derivatives for the delivery of drugs and genes to cancer cells. Curr. Sci. 2017, 113. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Shi, L.; Xian, M.; Dong, C.; Shuang, S. Folic acid-conjugated carbon dots as green fluorescent probes based on cellular targeting imaging for recognizing cancer cells. RSC Adv. 2017, 7, 42159–42167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, H.; Wang, G.; Yang, X.; Wang, J.; Wei, H. 131I-labeled PEG and folic acid co-functionalized graphene quantum dots for tumor-targeted imaging. J. Radioanal. Nucl. Chem. 2019, 319, 1119–1125. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag+ ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Yang, J.; Park, J.; Lee, K.; Kim, S.; Park, Y.; Lee, H. Mass production of graphene quantum dots by one-pot synthesis directly from graphite in high yield. Small 2014, 10, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Li, H.; Zhang, Y.; Yao, S. Large scale preparation of graphene quantum dots from graphite oxide in pure water via one-step electrochemical tailoring. RSC Adv. 2015, 5, 29704–29707. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Singh, J.; Kumari, U.; Kaur, I.P.; Barnwal, R.P.; Kumar, R.; Singh, S.; Singh, G.; Chatterjee, M. Development of biosurfactant-based graphene quantum dot conjugate as a novel and fluorescent theranostic tool for cancer. Int. J. Nanomed. 2019, 14, 809–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.J. Crossover between anti- and pro-oxidant activities of graphene quantum dots in the absence or presence of light. ACS Nano 2016, 10, 8690–8699. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patané, S.; Giofré, S.V.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A smart nanovector for cancer targeted drug delivery based on graphene quantum dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Ud-Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Palazzolo, S.; Bayda, S.; Corona, G.; Toffoli, G.; Rizzolio, F. DNA nanotechnology for cancer therapy. Theranostics 2016, 6, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.R.; Liu, H. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int. J. Nanomed. 2015, 10, 6709–6724. [Google Scholar]
- Wang, C.; Wu, C.; Zhou, X.; Han, T.; Xin, X.; Wu, J.; Zhang, J.; Guo, S. Enhancing cell nucleus accumulation and dna cleavage activity of anti-cancer drug via graphene quantum dots. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhang, Y.; Wang, C.; Wu, X.; Yang, Y.; Zheng, B.; Wu, H.; Guo, S.; Zhang, J. Photo-Fenton reaction of graphene oxide: A new strategy to prepare graphene quantum dots for DNA cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef]
- Finder, V.H.; Glockshuber, R. Amyloid-β aggregation. Neurodegener. Dis. 2007, 4, 13–27. [Google Scholar] [CrossRef]
- Fink, A.L. The aggregation and fibrillation of alfa synuclein. Acc. Chem. Res. 2006, 39, 628–634. [Google Scholar] [CrossRef]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid-From bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kadiyala, U.; Qu, Z.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; Vanepps, J.S. Anti-biofilm activity of graphene quantum dots via self-assembly with bacterial amyloid proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Mao, X.; Yu, Y.; Wang, C.X.; Yang, Y.L. Nanomaterials for reducing amyloid cytotoxicity. Adv. Mater. 2013, 25, 3780–3801. [Google Scholar]
- Ke, P.C.; Pilkington, E.H.; Sun, Y.; Javed, I.; Kakinen, A.; Peng, G.; Ding, F.; Davis, T.P. Mitigation of Amyloidosis with Nanomaterials. Adv. Mater. 2020, 32, 1901690. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.; Selokoe, D. The amyloid hypothesis of Alzheimer’s disease. Amyloid Int. J. Exp. Clin. Investig. 2002, 297, 353–357. [Google Scholar] [CrossRef]
- Takahashi, T.; Mihara, H. Peptide and protein mimetics inhibiting amyloid β-peptide aggregation. Acc. Chem. Res. 2008, 41, 1309–1318. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.J.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.P.; Dai, W.; Dong, H.; Wen, Y.; Zhang, X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale 2015, 7, 19060–19065. [Google Scholar] [CrossRef]
- Faridi, A.; Sun, Y.; Mortimer, M.; Aranha, R.R.; Nandakumar, A.; Li, Y.; Javed, I.; Kakinen, A.; Fan, Q.; Purcell, A.W.; et al. Graphene quantum dots rescue protein dysregulation of pancreatic β-cells exposed to human islet amyloid polypeptide. Nano Res. 2019, 12, 2827–2834. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Cao, X.; Peng, G.; Javed, I.; Kakinen, A.; Davis, T.P.; Lin, S.; Liu, J.; Ding, F.; et al. Graphene quantum dots against human IAPP aggregation and toxicity: In vivo. Nanoscale 2018, 10, 19995–20006. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Yang, K.; Li, Y.; Zeng, X.; Shao, M.; Lee, S.T.; Liu, Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials 2012, 33, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally high payload of the IR780 iodide on folic acid-functionalized graphene quantum dots for targeted photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.S.; Chen, H.H.; Chen, S.Y.; Chang, C.Y.; Chen, P.C.; Hou, Y.I.; Shao, Y.T.; Kao, H.F.; Lilian Hsu, C.L.; Chen, Y.C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.L.; Goreham, R.V.; Nann, T. Graphene quantum dots for theranostics and bioimaging. Pharm. Res. 2016, 33, 2337–2357. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent advances on graphene quantum dots for bioimaging applications. Front. Chem. 2020, 8, 1–25. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How functional groups to influence ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef]
- Mei, L.; Cao, F.; Zhang, L.; Xu, J.; Xu, Z.; Yu, Y.; Zhang, X.; Shi, Y.; Li, X.; Cheng, K.; et al. Ag-conjugated graphene quantum dots with blue light-enhanced singlet oxygen generation for ternary-mode highly-efficient antimicrobial therapy. J. Mater. Chem. B 2020, 8, 1371–1382. [Google Scholar]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagnosis Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhai, T.; Zhang, X.; Shen, Y.; Yuan, L.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.; Zhou, J.; et al. WO3-x@Au@MnO2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv. Mater. 2012, 24, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.L.; Wen, Y.; Wu, C.; Van Aken, P.A.; Maier, J.; Yu, Y. 3D V6O13 nanotextiles assembled from interconnected nanogrooves as cathode materials for high-energy lithium ion batteries. Nano Lett. 2015, 15, 1388–1394. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Flexible graphene-polyaniline composite paper for high-performance supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef] [PubMed]
- Khaleed, A.A.; Bello, A.; Dangbegnon, J.K.; Madito, M.J.; Olaniyan, O.; Barzegar, F.; Makgopa, K.; Oyedotun, K.O.; Mwakikunga, B.W.; Ray, S.C.; et al. Solvothermal synthesis of surfactant free spherical nickel hydroxide/graphene oxide composite for supercapacitor application. J. Alloys Compd. 2017, 721, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yang, W.; Kong, L.; Song, A.; Qin, X. Facile synthesis of nitrogen-doped porous carbon for high-performance supercapacitors. RSC Adv. 2017, 7, 55257–55263. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; He, D.; Wang, Y.; Zhao, X.; Zhao, W.; Wu, H. High performance asymmetric supercapacitors with ultrahigh energy density based on hierarchical carbon nanotubes@NiO core-shell nanosheets and defect-introduced graphene sheets with hole structure. RSC Adv. 2017, 7, 7843–7856. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Gan, L.; Xiong, W.; Xu, Z.; Zhu, D.; Chen, L. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 2555–2562. [Google Scholar] [CrossRef]
- Li, Z.; Wei, J.; Ren, J.; Wu, X.; Wang, L.; Pan, D.; Wu, M. Hierarchical construction of high-performance all-carbon flexible fiber supercapacitors with graphene hydrogel and nitrogen-doped graphene quantum dots. Carbon N. Y. 2019, 154, 410–419. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Shin, Y.; Yoon, Y.; Kim, D.; Lee, H. Highly transparent and flexible supercapacitors using graphene-graphene quantum dots chelate. Nano Energy 2016, 26, 746–754. [Google Scholar] [CrossRef]
- Tjandra, R.; Liu, W.; Zhang, M.; Yu, A. All-carbon flexible supercapacitors based on electrophoretic deposition of graphene quantum dots on carbon cloth. J. Power Sources 2019, 438, 227009. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Huang, L.; Li, C.; Shi, G. Three-dimensional porous graphene/polyaniline composites for high-rate electrochemical capacitors. J. Mater. Chem. A 2014, 2, 17489–17494. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Lei, Y.; Gu, L.; Xiao, D. Microwave-assisted chemical-vapor-induced in situ polymerization of polyaniline nanofibers on graphite electrode for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 19978–19989. [Google Scholar] [CrossRef]
- Zang, X.; Li, X.; Zhu, M.; Li, X.; Zhen, Z.; He, Y.; Wang, K.; Wei, J.; Kang, F.; Zhu, H. Graphene/polyaniline woven fabric composite films as flexible supercapacitor electrodes. Nanoscale 2015, 7, 7318–7322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, T.; Li, M.; Yu, M.; Luo, Y.; Tong, Y.; Li, Y. Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Lett. 2017, 17, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhi, C.; Wang, X.; Tang, D.; Xu, Y.; Weng, Q.; Jiang, X.; Mitome, M.; Golberg, D.; et al. Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Z.; Shen, P.K. Simultaneous formation of ultrahigh surface area and three-dimensional hierarchical porous graphene-like networks for fast and highly stable supercapacitors. Adv. Mater. 2013, 25, 2474–2480. [Google Scholar] [CrossRef]
- Feng, J.X.; Xu, H.; Ye, S.H.; Ouyang, G.; Tong, Y.X.; Li, G.R. Silica–polypyrrole hybrids as high-performance metal-free electrocatalysts for the hydrogen evolution reaction in neutral media. Angew. Chem. Int. Ed. 2017, 56, 8120–8124. [Google Scholar] [CrossRef]
- Jeon, J.W.; Han, J.H.; Kim, S.K.; Kim, D.G.; Kim, Y.S.; Suh, D.H.; Hong, Y.T.; Kim, T.H.; Kim, B.G. Intrinsically microporous polymer-based hierarchical nanostructuring of electrodes: Via nonsolvent-induced phase separation for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 8909–8915. [Google Scholar] [CrossRef]
- Hsieh, W.; Horng, T.L.A.; Huang, H.C.; Teng, H. Facile simulation of carbon with wide pore size distribution for electric double-layer capacitance based on Helmholtz models. J. Mater. Chem. A 2015, 3, 16535–16543. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.; Zeng, Y.; Balogun, M.S.; Mai, K.; Zhang, Z.; Lu, X.; Tong, Y. Water surface assisted synthesis of large-scale carbon nanotube film for high-performance and stretchable supercapacitors. Adv. Mater. 2014, 26, 4724–4729. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Qu, T.; Xiang, K.; Zhang, Y.; Tian, Z.; Xie, M.; Guo, X. Porous carbon nanosheets with abundant oxygen functionalities derived from phoenix seeds for high-performance supercapacitor. ChemistrySelect 2017, 2, 10704–10708. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and future challenges of functional nanocarbon hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.A.; Tao, Y.; Song, X.Z.; Bao, M.; Tan, Z. A three dimensional N-doped graphene/CNTs/AC hybrid material for high-performance supercapacitors. RSC Adv. 2017, 7, 6664–6670. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, S.; Garcia, C.; Fan, L.; Zhou, J. PH-Responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale 2017, 9, 4928–4933. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.W.; Yan, X.; Xue, Q.J. Multilayer hybrid films consisting of alternating graphene and titanium dioxide for high-performance supercapacitors. J. Mater. Chem. C 2013, 1, 1413–1422. [Google Scholar] [CrossRef]
- Liu, W.W.; Yan, X.; Lang, J.W.; Peng, C.; Xue, Q.J. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012, 22, 17245–17253. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Lu, G.; Chen, N.; Zhang, Z.; Li, H.; Shao, H.; Qu, L. Graphene quantum dots-carbon nanotube hybrid arrays for supercapacitors. Nanotechnology 2013, 24, 195401. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Hu, C.; Cheng, H.; Zhang, Z.; Shao, H.; Qu, L. Graphene quantum dots-three-dimensional graphene composites for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 19307–19313. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Wang, L.; Bu, F.; Wei, J.; Pan, D.; Wu, M. Hierarchical 3D all-carbon composite structure modified with n-doped graphene quantum dots for high-performance flexible supercapacitors. Small 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Guan, X.; Yu, Y.; Li, X.; Yan, F. Hydrothermal synthesis of graphene quantum dots supported on three-dimensional graphene for supercapacitors. Nanomaterials 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Z.; Lei, Y.; Chen, Y.; Zhang, Z.; Jiang, Z.; Hu, J.; Lin, Y. Preparation and specific capacitance properties of sulfur, nitrogen co-doped graphene quantum dots. Nanoscale Res. Lett. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Rana, U.; Malik, S. Graphene quantum dot-doped polyaniline nanofiber as high performance supercapacitor electrode materials. Chem. Commun. 2015, 51, 12365–12368. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.; Dong, H.; He, W.; Dong, L.; Yu, L. High-performance supercapacitor of graphene quantum dots with uniform sizes. ACS Appl. Mater. Interfaces 2018, 10, 12983–12991. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Qin, P.; Liu, X.; Chen, Z.; Wang, L.; Pan, D.; Wu, M. Nitrogen and oxygen co-doped graphene quantum dots with high capacitance performance for micro-supercapacitors. Carbon N. Y. 2018, 139, 67–75. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Dutta Chowdhury, A.; Doong, R.A. New avenue for appendage of graphene quantum dots on halloysite nanotubes as anode materials for high performance supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4930–4940. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Doong, R. An Nano assembly of N-doped graphene quantum dots anchored Fe3O4/halloysite nanotubes for high performance supercapacitor. Electrochim. Acta 2017, 245, 912–923. [Google Scholar] [CrossRef]
- Li, Z.; Qin, P.; Wang, L.; Yang, C.; Li, Y.; Chen, Z.; Pan, D.; Wu, M. Amine-enriched graphene quantum dots for high-pseudocapacitance supercapacitors. Electrochim. Acta 2016, 208, 260–266. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, L.; Cao, L.; Liu, X.; Chen, Z.; Pan, D.; Wu, M. Assembling nitrogen and oxygen co-doped graphene quantum dots onto hierarchical carbon networks for all-solid-state flexible supercapacitors. Electrochim. Acta 2017, 235, 561–569. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, T.; Zhong, Y.; Cheng, S.; Jiang, S.; Chen, C.; Liao, G.; Tang, Z. Graphene-quantum-dots induced NiCo2S4 with hierarchical-like hollow nanostructure for supercapacitors with enhanced electrochemical performance. Electrochim. Acta 2018, 269, 45–54. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W.D. Heterostructural Graphene Quantum Dot/MnO2 Nanosheets toward High-Potential Window Electrodes for High-Performance Supercapacitors. Adv. Sci. 2018, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhu, J.; Qing, Y.; Wang, L.; Zhao, J.; Li, J.; Tian, W.; Jia, D.; Fan, Z. Ultramicroporous carbons puzzled by graphene quantum dots: Integrated high gravimetric, volumetric, and areal capacitances for Supercapacitors. Adv. Funct. Mater. 2018, 28, 1805898. [Google Scholar] [CrossRef]
- Wu, K.; Xu, S.; Zhou, X.; Wu, H. Graphene quantum dots enhanced electrochemical performance of polypyrrole as supercapacitor electrode. Dianhuaxue 2013, 19, 361–370. [Google Scholar]
- Kharangarh, P.R.; Umapathy, S.; Singh, G.; Sharma, R.K.; Kumar, A. High-performance pseudocapacitor electrode materials: Cobalt (II) chloride–GQDs electrodes. Emerg. Mater. Res. 2017, 6, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Kharangarh, P.R.; Gupta, V.; Singh, A.; Bhardwaj, P.; Grace, A.N. An efficient pseudocapacitor electrode material with co-doping of iron (II) and sulfur in luminescent graphene quantum dots. Diam. Relat. Mater. 2020, 107, 107913. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Qing, Y.; Fan, C.; Wang, L.; Huang, Y.; Sheng, R.; Guo, Y.; Wang, T.; Pan, Y.; et al. Construction of hierarchical porous carbon nanosheets from template-assisted assembly of coal-based graphene quantum dots for high performance supercapacitor electrodes. Mater. Today Energy 2017, 6, 36–45. [Google Scholar] [CrossRef]










| Modification | Source/Precursor | Synthesis Method | Size | QY | Reference |
|---|---|---|---|---|---|
| A-GQDs | Graphite and adenine precursors | Two-step microwave-assisted method | 3–5 nm | 21.63% | [61] |
| Hg-PEHA-GQD-DPA | Citric acid | Two-step continuous thermal pyrolysis | 3.16 nm | 90.91% | [62] |
| RF-GQDs | Graphite in K2S2O8 solution | Electrochemical exfoliation | 3 nm | 1.8% | [63] |
| B-GQDs | 4-vinylphenylboronic acid and boric acid | - | 5.8 nm | 11.2% | [23] |
| (GQD/DBM)3EuPhen/GQD | Graphite rod | Electrochemical exfoliation | 5.5 ± 0.4 nm | 15.5% | [64] |
| sGQDs | Ethanolic extract of grape seed extract powder | One-pot microwave-assisted synthesis | ~50–60 nm | 31.79% | [30] |
| DOX-GQD-RGD | Thermally exfoliated graphite oxide | Refluxing with concentrated nitric acid | 3.7 nm | - | [65] |
| GQDs | Trisodium citrate | Pyrolytic carbonization route | 1.3 ± 0.5 nm | 3.6% | [9] |
| mGQDs | Mango leaves | One-pot microwave-assisted green-synthesis route | 2–8 nm | - | [66] |
| HD-GQDs, LD-GQDs | Acetylene black, nano-graphite | Ultrasonic-assisted liquid-phase exfoliation technique | 2–6 nm, 2–9 nm | 1.8%, 2.4% | [67] |
| GQDs | Graphite rod | Electrochemical exfoliation method | 1.5–5.5 nm | - | [68] |
| F-GQDs | Fluorinated graphite | Oxidative cutting method | 2.1 nm | 13.72% | [69] |
| GQDs | Pyrene precursor | Wet chemistry treatment of commercially available polycyclic aromatic hydrocarbon | 5–10 nm | 11.7% | [70] |
| GQD-Type | Cytotoxicity | Media | Notes | References |
|---|---|---|---|---|
| GQDs | GQDs have minimal dark toxicity | In vivo toxicity in rats | Some minor changes were particularly noted in the liver and lungs at the 10 and 15 mg/kg doses of GQDs | [103] |
| Carboxylated GQD | No acute cytotoxicity between the range of 5 and 10 mg/kg | Liver, spleen, kidney, and tumor/In vivo and in vitro | Accumulation in mice liver, spleen, kidney, and tumor at 24 h after intravenous injection of GQDs | [111] |
| GQDs | No severe toxicity in mice with 300 micrograms of GQDs (per head, 15 mg/kg) | Intraperitoneal infusion to mice | GQDs could be excreted out of the body | [104] |
| GQDs | Low toxicity | Blood derived CD34+ cells from leukapheresis | SEPW1 is downregulated with a fold change of −5 | [107] |
| GQDs | Very low cytotoxicity | B16F10 cells and MCF-7 cells | [68] | |
| GQDs | Low cytotoxicity to 0–400 µg mL−1 GQDs for 24 h | HeLa | More than 80% cell survival rate | [9] |
| GQDs | No overt acute toxicity | Lung tissues of rats | In high dose group alveolar septa thickening during inflammation | [100] |
| Electrode Material | Specific Capacitance | References |
|---|---|---|
| Amine-functionalized single-crystalline GQDs | 400–595 F g−1 | [243] |
| Nitrogen and oxygen co-doped GQDs/carbon nanotubes/carbon cloth | 212 F g−1 | [244] |
| GQDs/NiCo2S4 with hierarchical-like hollow nanostructure | 678.22 F g−1 | [245] |
| GQDs/MnO2 | 1170 F g−1 | [246] |
| Ultra-microporous carbons integrated GQDs | 270 F g−1 | [247] |
| GQDs/polypyrrole | 485 F g−1 | [248] |
| Cobalt (II) chloride–GQDs | ~300 F g−1 | [249] |
| Fe (II)S–GQDs | 476.2 F g−1 | [250] |
| Porous carbon nanosheets/GQDs | 230 F g−1 | [251] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortel, M.; Mansuriya, B.D.; Vargas Santana, N.; Altintas, Z. Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors. Micromachines 2020, 11, 866. https://doi.org/10.3390/mi11090866
Kortel M, Mansuriya BD, Vargas Santana N, Altintas Z. Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors. Micromachines. 2020; 11(9):866. https://doi.org/10.3390/mi11090866
Chicago/Turabian StyleKortel, Merve, Bhargav D. Mansuriya, Nicole Vargas Santana, and Zeynep Altintas. 2020. "Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors" Micromachines 11, no. 9: 866. https://doi.org/10.3390/mi11090866
APA StyleKortel, M., Mansuriya, B. D., Vargas Santana, N., & Altintas, Z. (2020). Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors. Micromachines, 11(9), 866. https://doi.org/10.3390/mi11090866