Preparation of Microneedle Array Mold Based on MEMS Lithography Technology
Abstract
1. Introduction
2. Materials and Methods
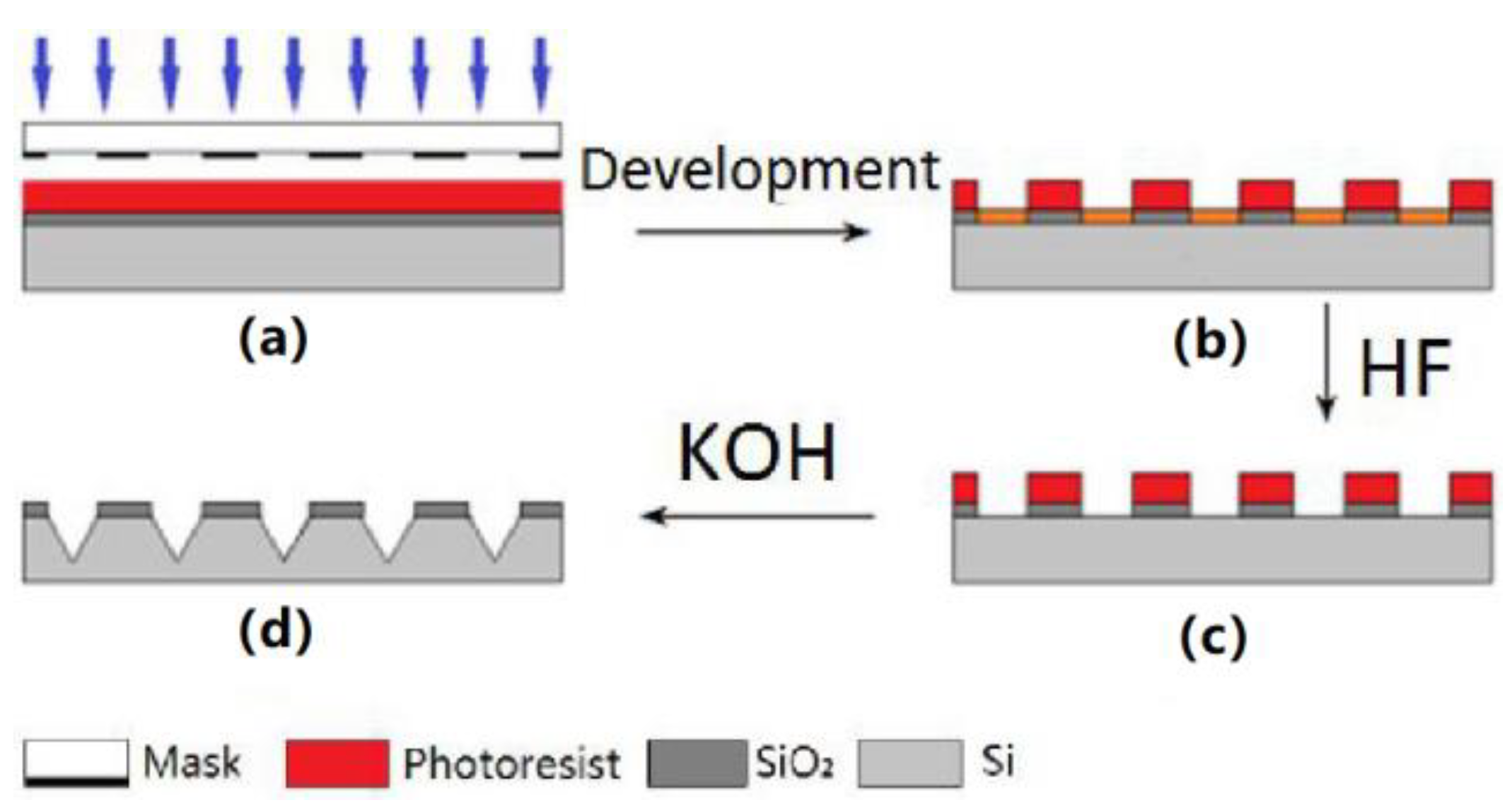
2.1. LIGA (Photolithography, Galvanogormung, Abformung) Processing Technology
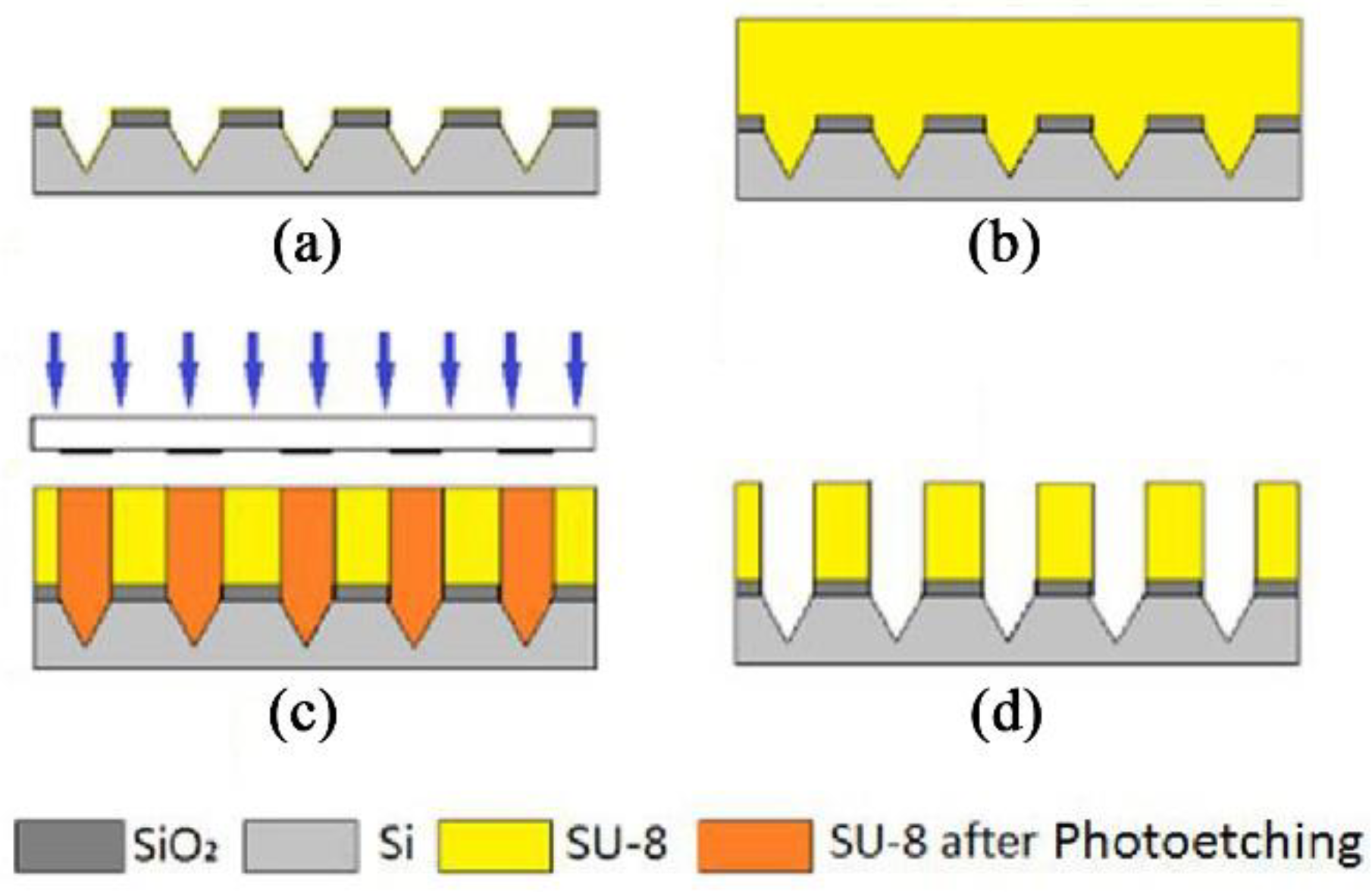
2.2. Engraving Process
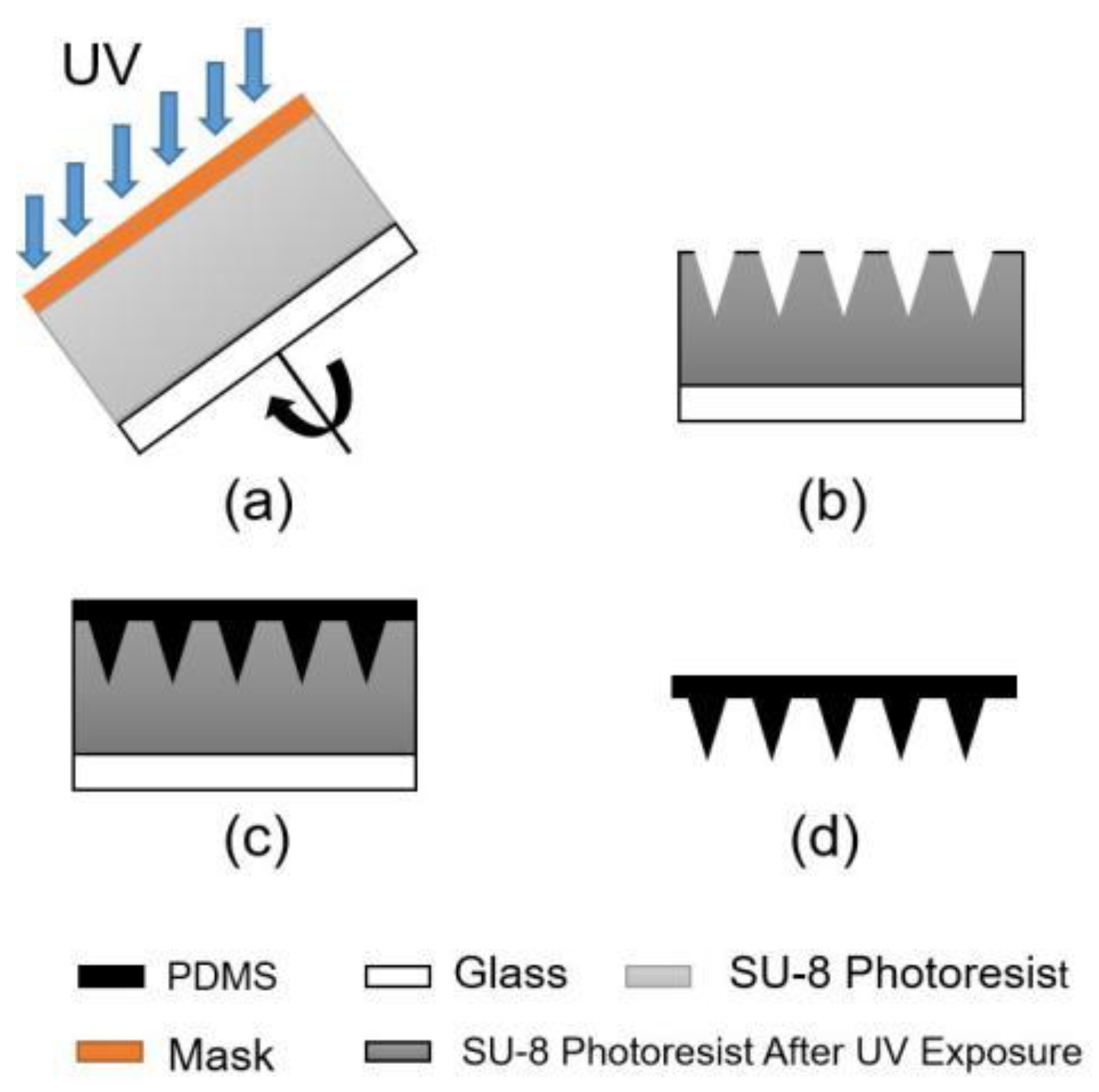
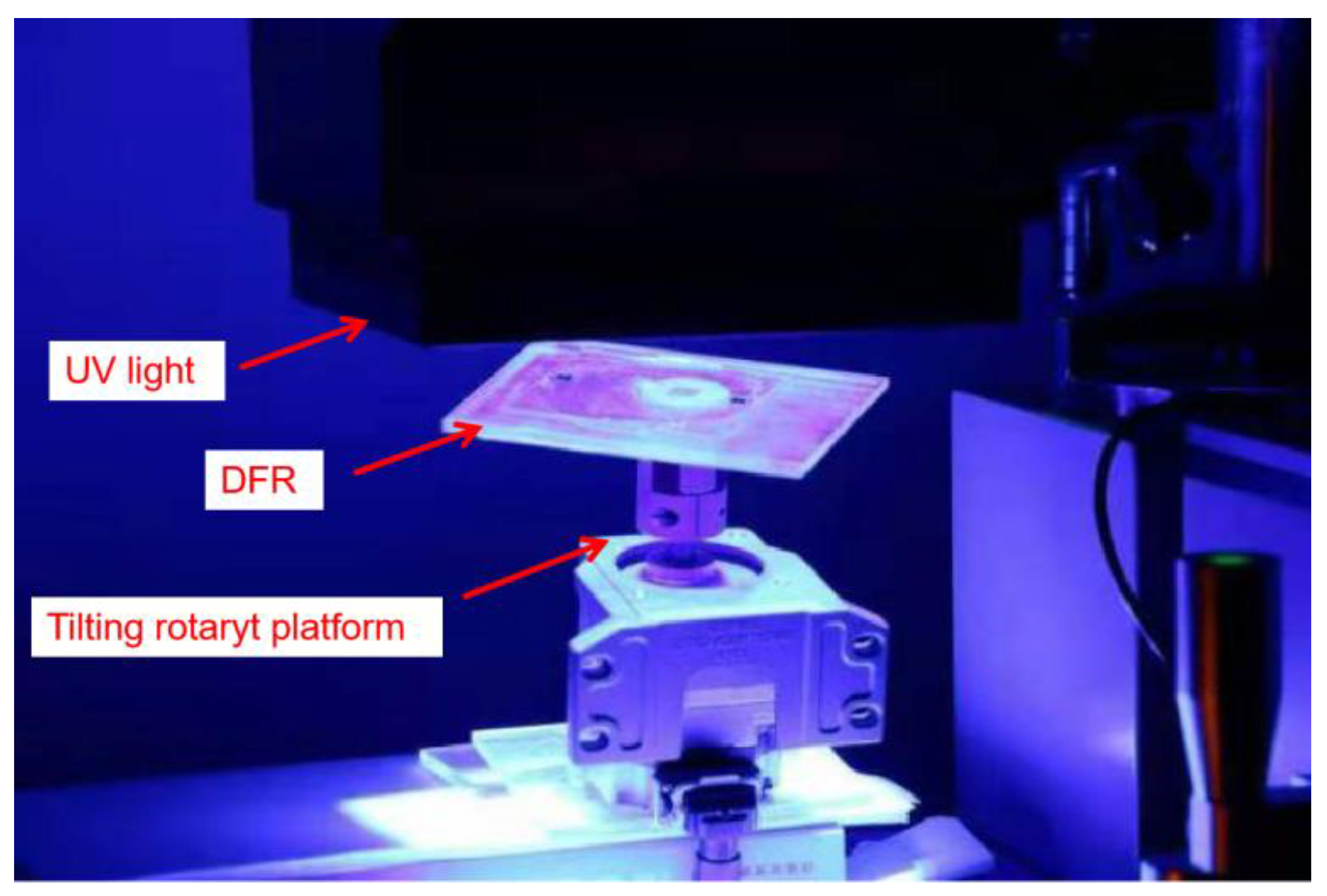
2.3. Tilting Rotary Ultraviolet (UV) Lithography
3. Results
3.1. Fabrication Results of LIGA
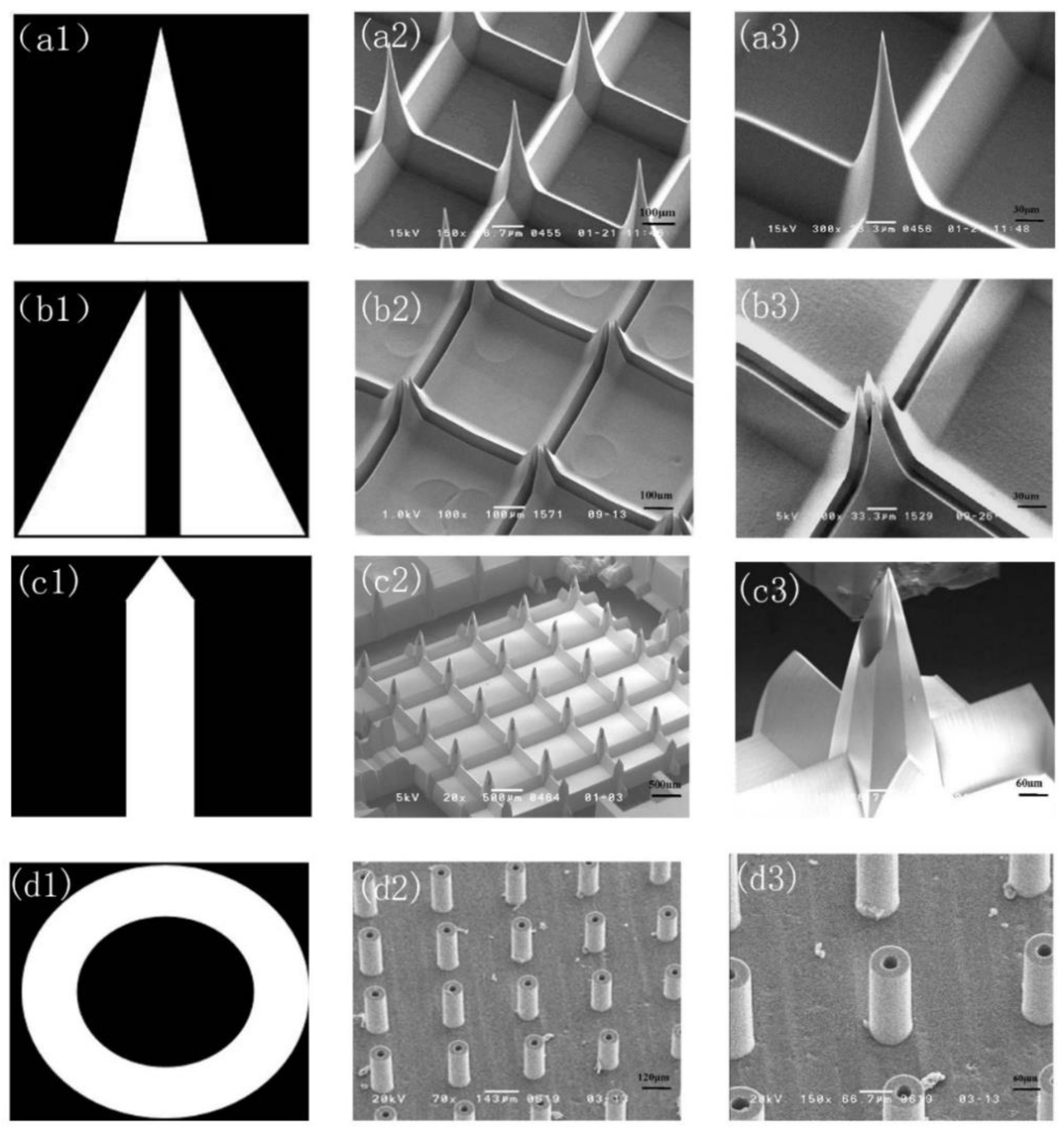
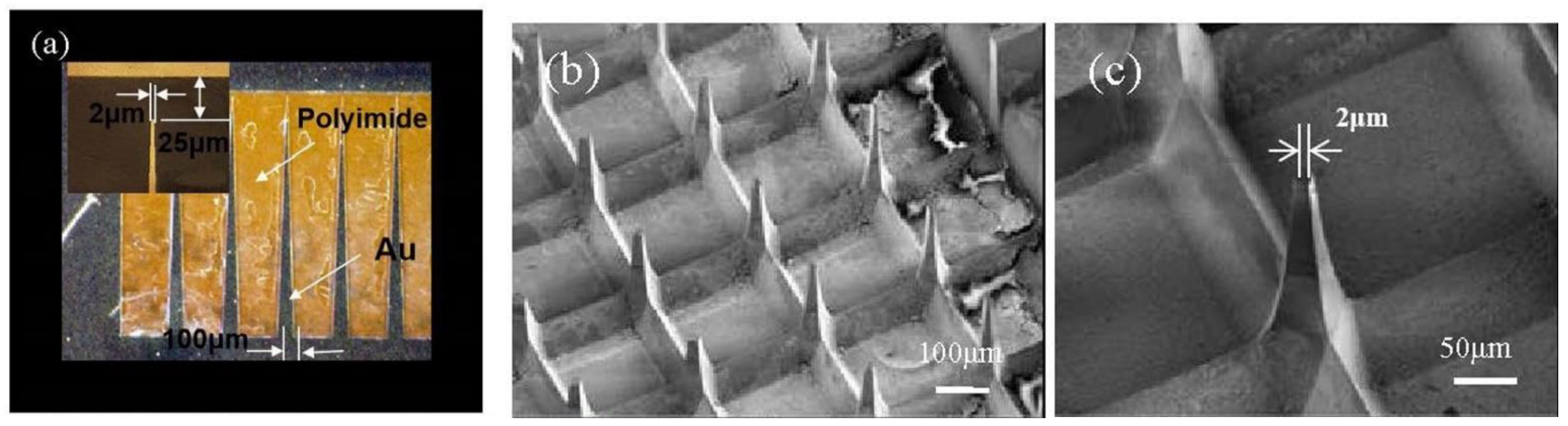
3.1.1. Out-Plane Microneedle Array (MNA)
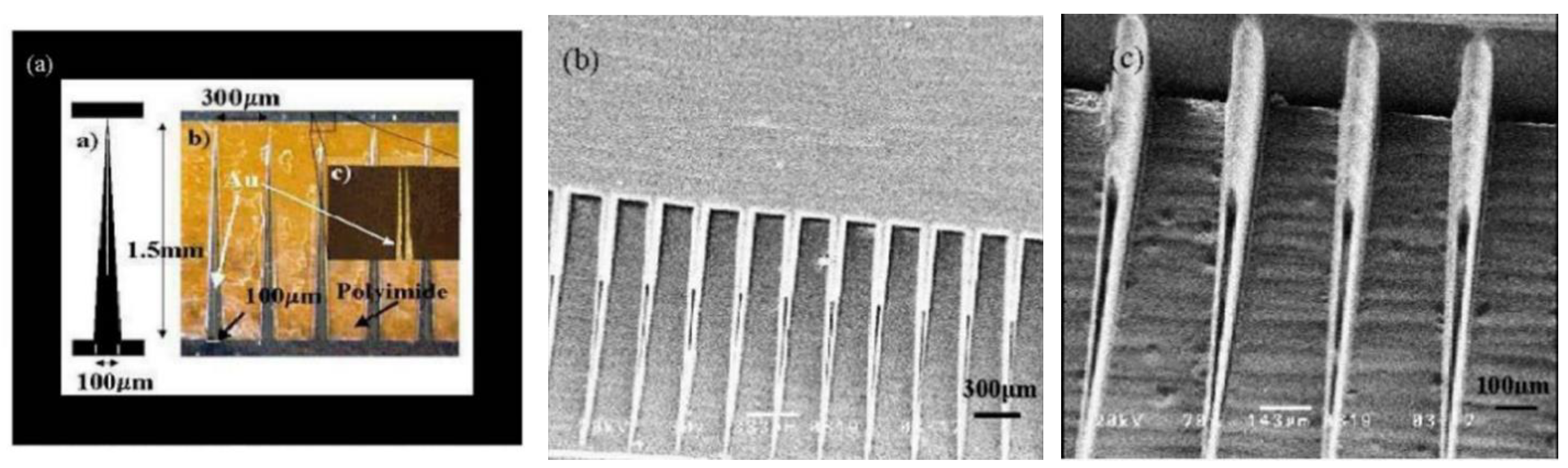
3.1.2. In-Plane MNA
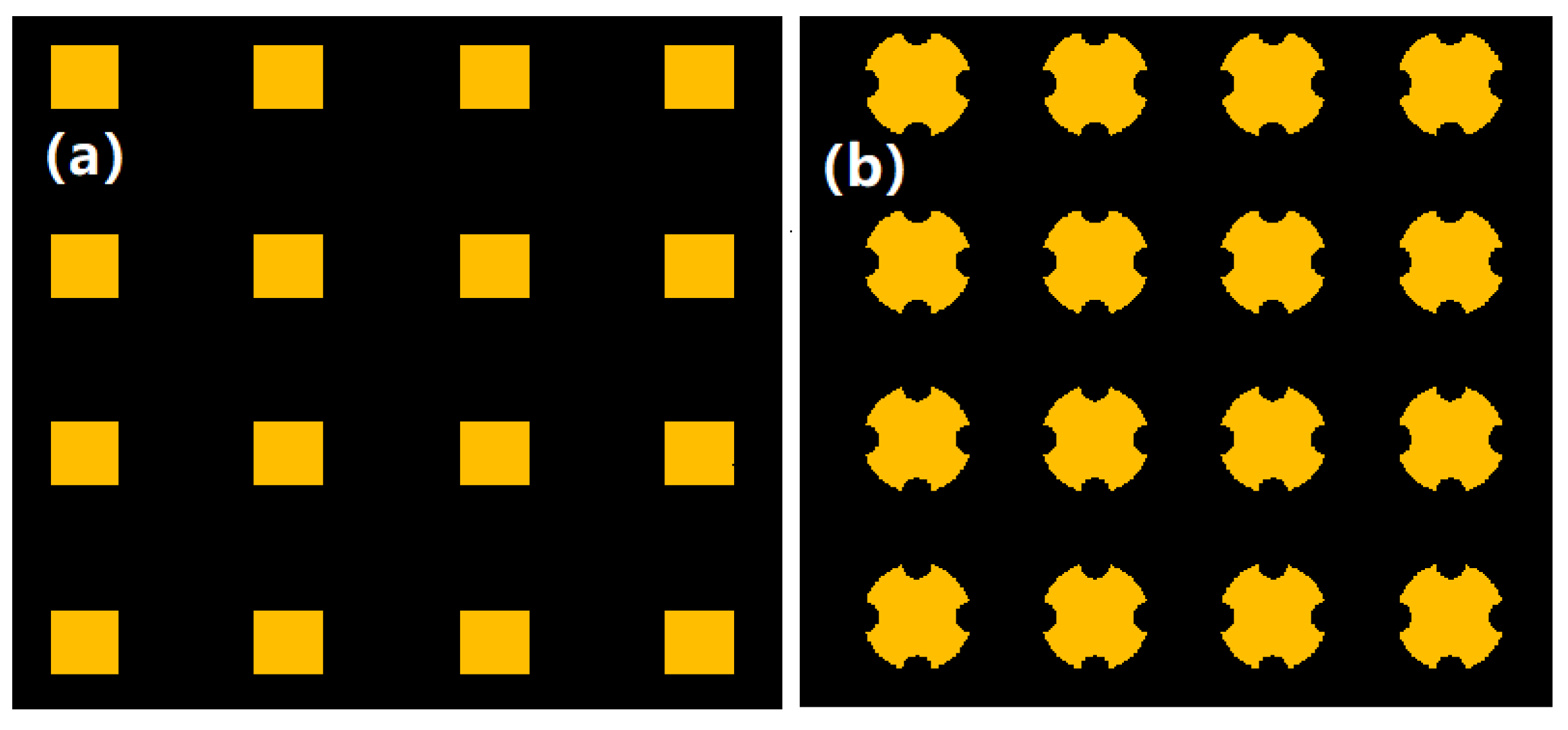
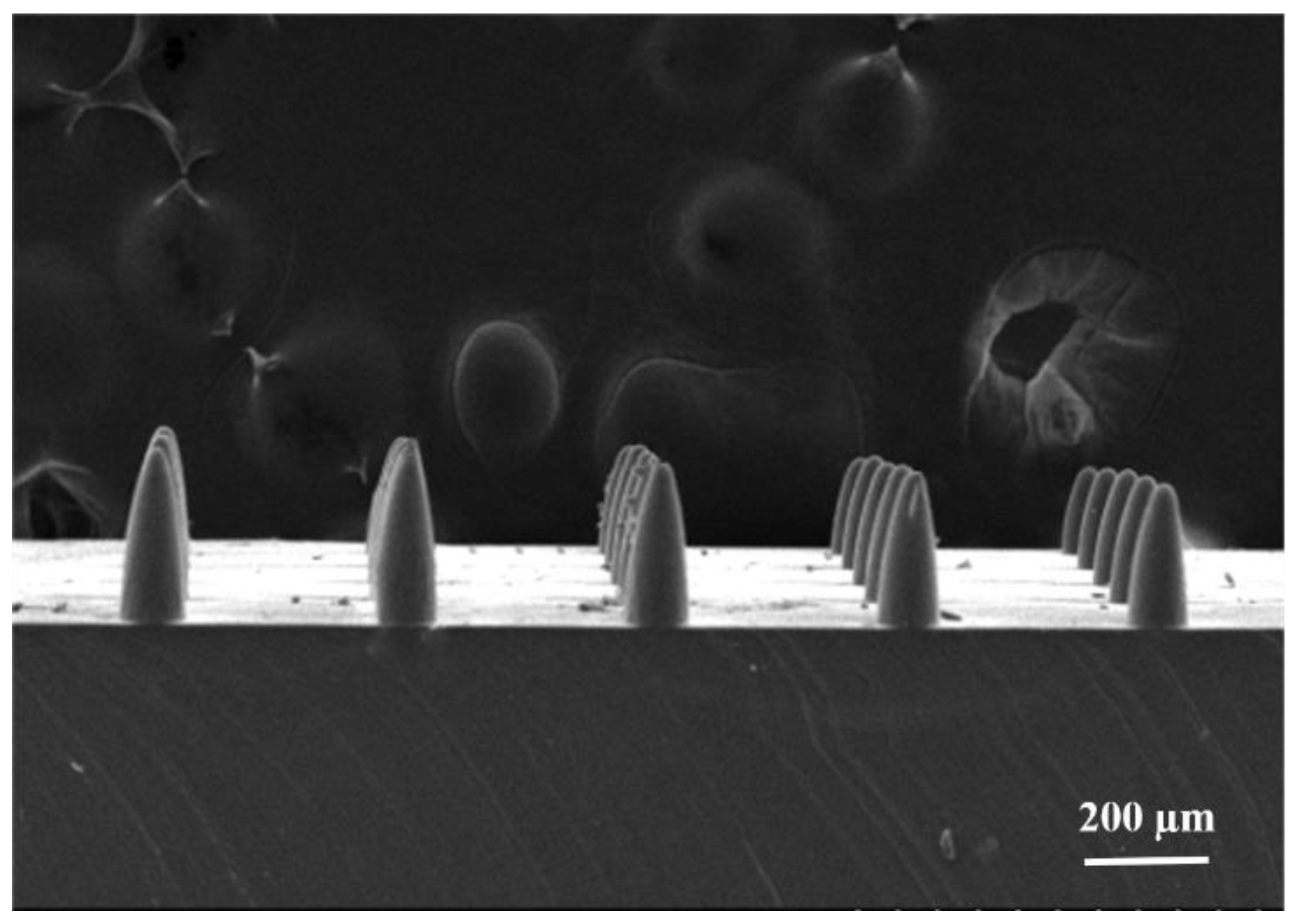
3.2. Fabrication Results of Engraving Process
3.3. Fabrication Results of Tilting Rotary UV Lithography
3.4. Comparison of Three Processes and Special Processing Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, L.; Wu, Y.; Sun, Y.; Yang, Z.; Zhao, X. Enhanced adhesive strength between SU-8 photoresist and titanium substrate by improved anodic oxidation method for high aspect-ratio microstructure. J. Micromech. Microeng. 2019, 29, 047002. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Technol. 2017, 93, 407–422. [Google Scholar] [CrossRef]
- Daugimont, L.; Baron, N.; Vandermeulen, G.; Pavselj, N.; Miklavcic, D.; Jullien, M.C.; Cabodevila, G.; Mir, L.M.; Préat, V. Hollow MNA Arrays for Intradermal Drug Delivery and DNA Electroporation. J. Membr. Biol. 2010, 236, 117–125. [Google Scholar] [CrossRef]
- Larraneta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R. Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Hong, X.; Wu, Z.; Chen, L. Hydrogel MNA Arrays for Transdermal Drug Delivery. Nano Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of drugs with MNAs: Strategies and outcomes. J. Drug Deliv. Sci. Technol. 2015, 29, 16–23. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, J.U.; Kim, S.H.; Ji, Y.N. Successful transdermal allergen delivery and allergen-specific immunotherapy using biodegradable MNA patches. Biomaterials 2017, 150, 38–48. [Google Scholar] [CrossRef]
- Himanshu, K.; Kochhar, J.S.; Lifeng, K. Micro and nanoneedles for drug delivery and biosensing. Ther. Deliv. 2018, 9, 489–492. [Google Scholar]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. MNAs for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.H.; Chen, M.C. Dissolving polymer MNA patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952. [Google Scholar] [CrossRef] [PubMed]
- Demuth, P.C.; Min, Y.; Irvine, D.J. Implantable Silk Composite Microneedles for Programmable Vaccine Release Kinetics and Enhanced Immunogenicity in Transcutaneous Immunization. Adv. Healthc. Mater. 2014, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jung, H. Drawing lithography for MNAs: A review of fundamentals and biomedical applications. Biomaterials 2012, 33, 7309–7326. [Google Scholar] [CrossRef]
- Trautmann, A.; Roth, G.L.; Nujiqi, B. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst. Nanoeng. 2019, 5, 0190046. [Google Scholar] [CrossRef] [PubMed]
- Pere, C.P.P.; Economidou, S.N.; Lall, G. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Heo, Y.J.; Shimoyama, I. Scalable fabrication of PEGDA microneedles using UV exposure via a rotating prism. J. Microelectromech. Syst. 2017, 26, 990–992. [Google Scholar] [CrossRef]
- Huang, D.; Wang, H.C.; Li, J.S. A wireless flexible wearable bopotential acquisition system utilizing parylenebased microneedle array. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII, Berlin, Germany, 23–27 June 2019; Volume 42, pp. 298–301. [Google Scholar]
- Wang, J.; Wang, H.; Wu, W.Y.; LI, Y.G. PMMA microchannels with isosceles triangle structure prepared by moving X-ray lithography. Micronanoelectron. Technol. 2020, 57, 292–297. [Google Scholar]
- Schröter, T.J.; Koch, F.; Meyer, P. Large area gratings by X-ray LIGA dynamic exposure for X-ray phase-contrast imaging. J. Micro/Nanolithogr. MEMS MOEMS 2017, 16, 013501. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhu, D.; Li, H.S. Fabrication of ultra-high metal micro electrode array using UV-LIGA technology. Opt. Precis. Eng. 2010, 18, 670–676. [Google Scholar]
- Li, Y.; Zhang, H.; Yang, R. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Tomono, T. A new way to control the internal structure of microneedles: A case of chitosan lactate. Mater. Today Chem. 2019, 13, 79–87. [Google Scholar] [CrossRef]
- Du, L.Q.; Wang, Z.Z.; Runa, X.P. Fabrication of SU-8 microneedle based on backside exposure technology. Key Eng. Mater. 2015, 645, 853–858. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Battisti, M.; Vecchione, R.; Casale, C. Non-invasive Production of Multi-Compartmental Biodegradable Polymer Microneedles for Controlled Intradermal Drug Release of Labile Molecules. Front. Bioeng. Biotechnol. 2019, 7, 00296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, R.; Yang, J. Rapidly Fabricated Microneedle Arrays Using Magnetorheological Drawing Lithography for Transdermal Drug Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5506–5513. [Google Scholar] [CrossRef]


| Process Comparison Items | LIGA (A) | Engraving Process (B) | Tilting Rotary UV Lithography (C) | Special Processing Technology (D) |
|---|---|---|---|---|
| Type of MNA | in-plane MNA and out-plane MNA | out-plane MNA | out-plane MNA | out-plane MNA |
| Shape of MNA | The shape of the MNA changes according to the shape of the mask | quadrangular pyramid shaped MNAs | Conical MNA | Layered MNA |
| Hollow MNA | Yes | No | No | Yes |
| Experimental steps | Subtractive manufacturing | Subtractive manufacturing | Subtractive manufacturing | Additive manufacturing |
| aspect ratios | Up to 30:1 | ~2:1 | ~2:1 | Up to 5:1 |
| Fabrication costs | expensive | Cheap | cheap | cheap |
| Fabrication time | save time | save time | save time | Time consuming |
| Material | PMMA | Si + SU-8 | SU-8 | Resin/polymer |
| Geometrical capability | Cutting-edge accuracy reaches the nano-level | the tip is anisotropically etched at 54.7° | Cutting-edge accuracy reaches micron-level | Cutting-edge accuracy reaches micron-level |
| Accuracy | Up to 0.1 μm | Up to 10 μm | Up to 10 μm | Up to 10 μm |
| Reproducibility | coincident | Coincident | coincident | erroneous |
| Scalability | nm-μm | Μm | μm | μm |
| Processing methods | Parallel manufacturing | Parallel manufacturing | Parallel manufacturing | Serial manufacturing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, H.; Lai, L.; Li, Y. Preparation of Microneedle Array Mold Based on MEMS Lithography Technology. Micromachines 2021, 12, 23. https://doi.org/10.3390/mi12010023
Wang J, Wang H, Lai L, Li Y. Preparation of Microneedle Array Mold Based on MEMS Lithography Technology. Micromachines. 2021; 12(1):23. https://doi.org/10.3390/mi12010023
Chicago/Turabian StyleWang, Jie, Huan Wang, Liyan Lai, and Yigui Li. 2021. "Preparation of Microneedle Array Mold Based on MEMS Lithography Technology" Micromachines 12, no. 1: 23. https://doi.org/10.3390/mi12010023
APA StyleWang, J., Wang, H., Lai, L., & Li, Y. (2021). Preparation of Microneedle Array Mold Based on MEMS Lithography Technology. Micromachines, 12(1), 23. https://doi.org/10.3390/mi12010023




