Technological Challenges in the Development of Optogenetic Closed-Loop Therapy Approaches in Epilepsy and Related Network Disorders of the Brain
Abstract
:1. Background
2. Biological Constraints of Long-Term Implantation of Optrodes
3. Neural Activity Monitoring
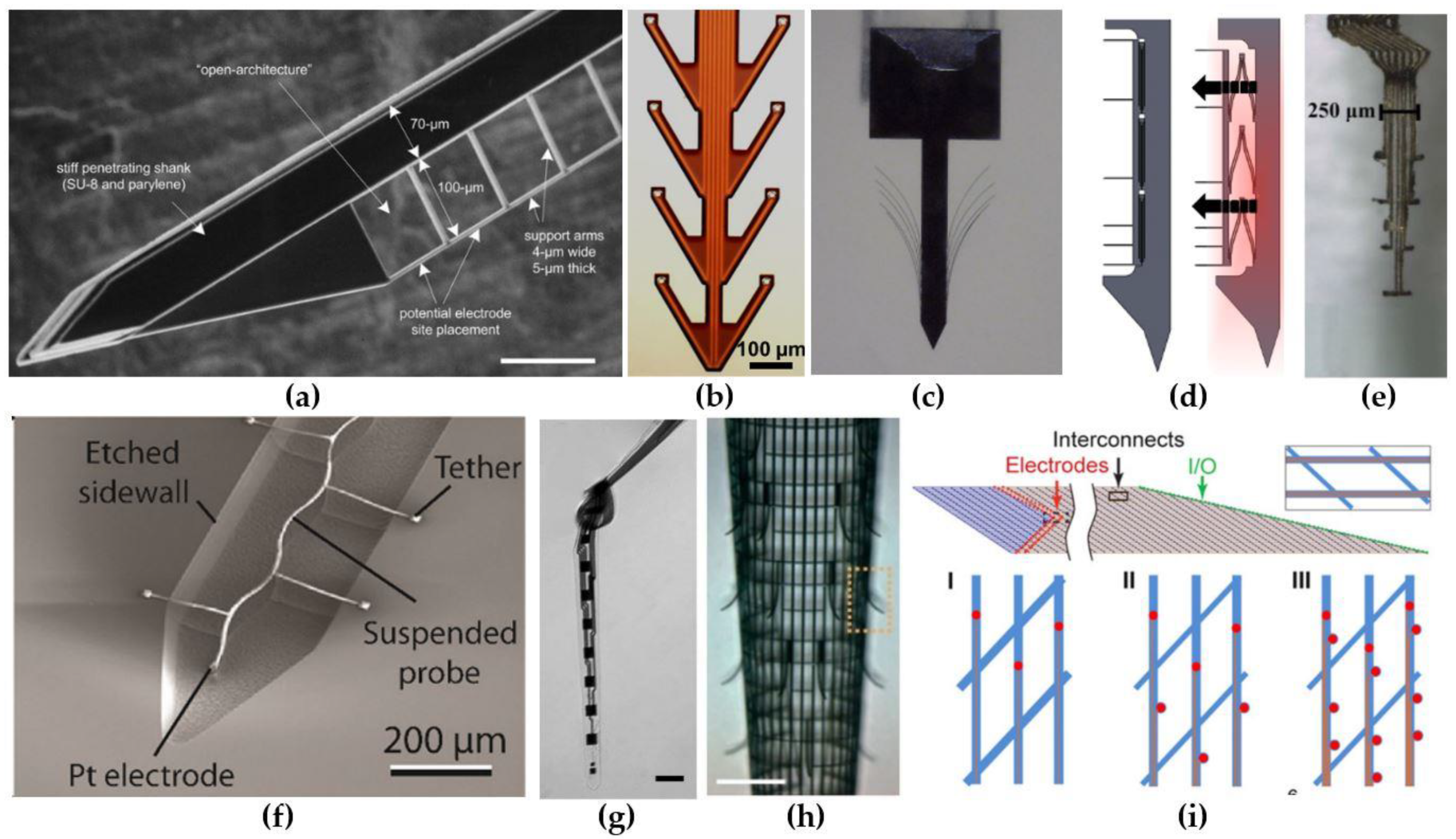
3.1. Electrode Designs That Elicit a Minimal Foreign Body Response
3.2. Reduction of the Electrode-Tissue Impedance
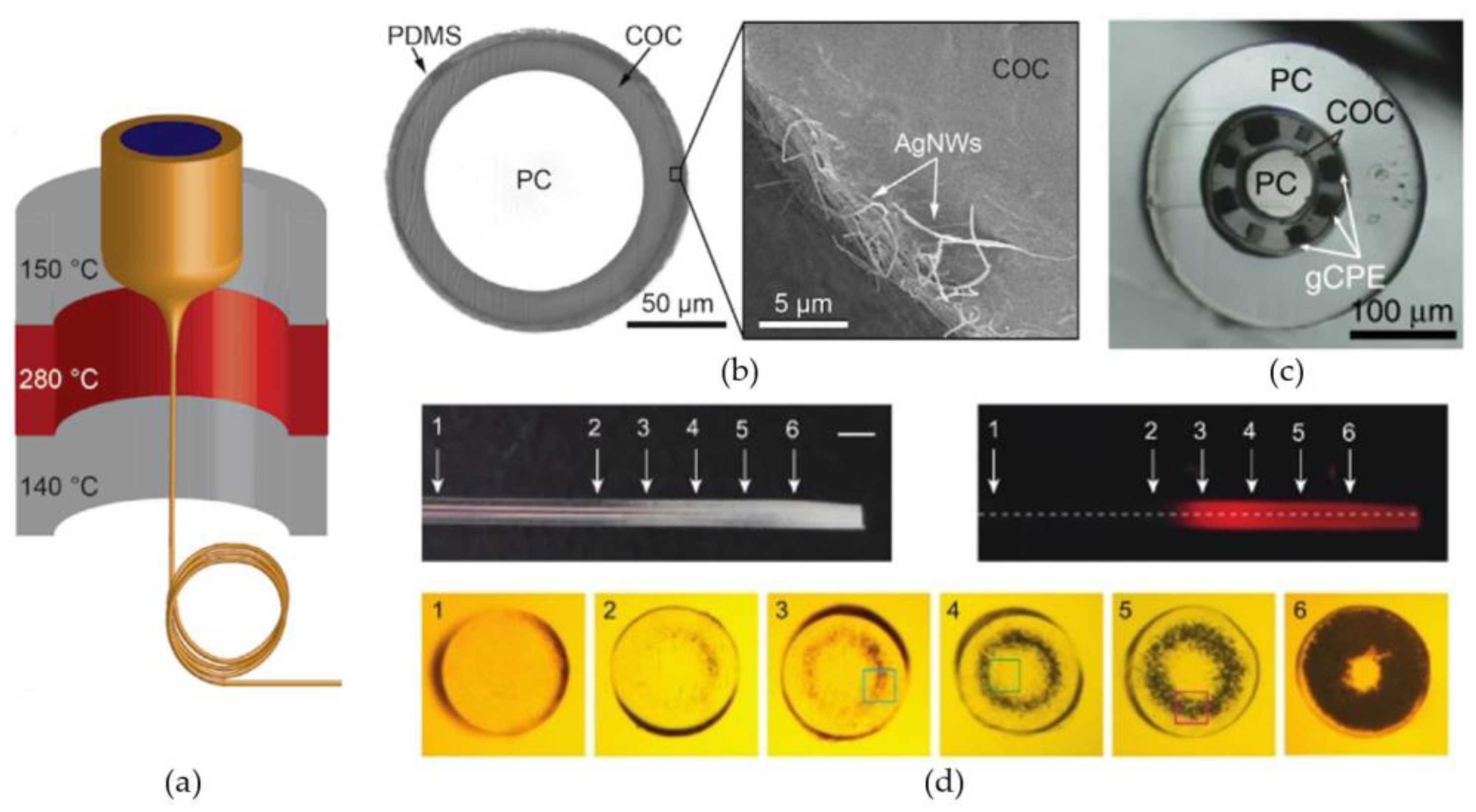
4. Neural Tissue Illumination
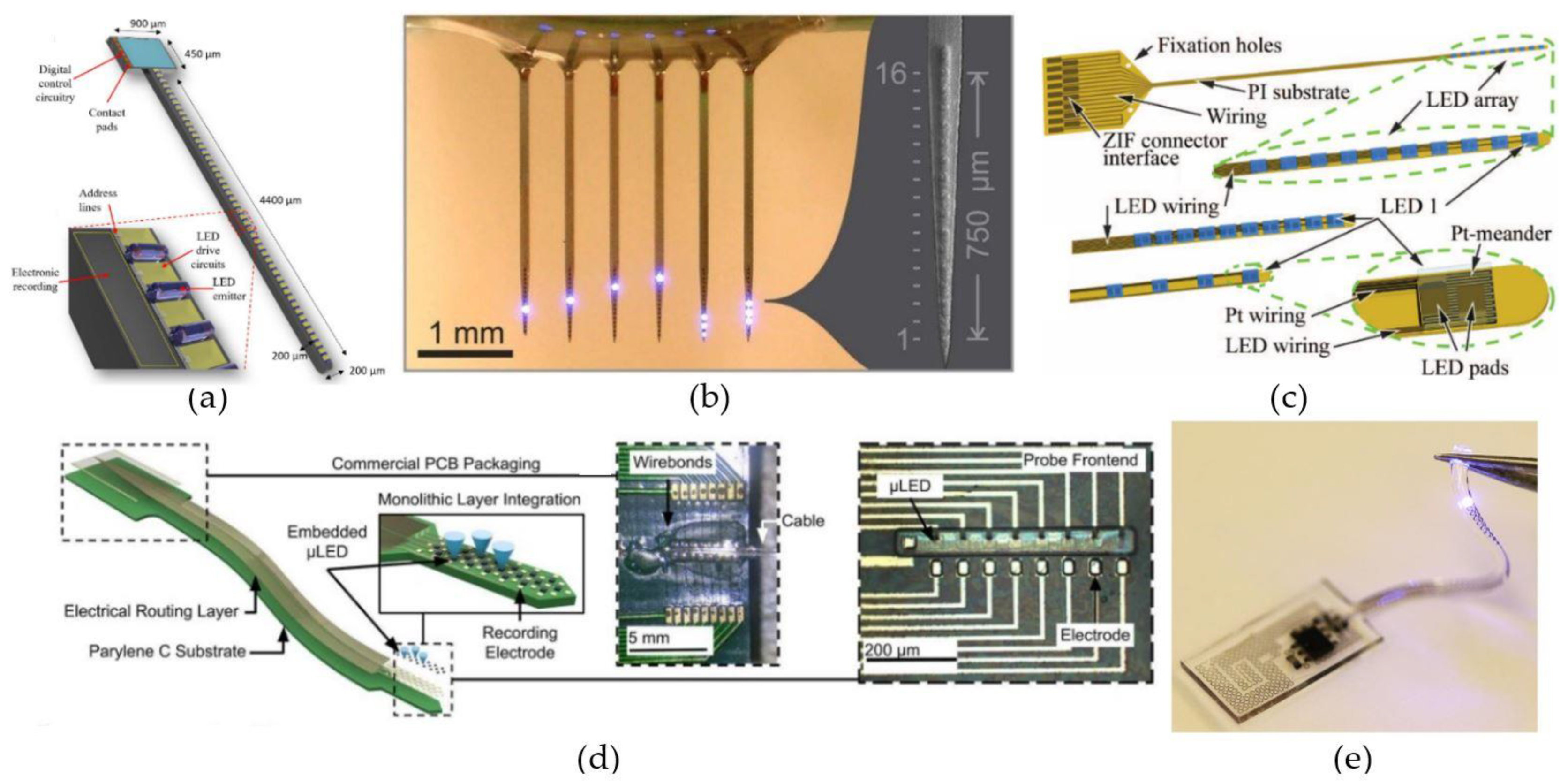
4.1. Implanted µLED-Based Light Delivery
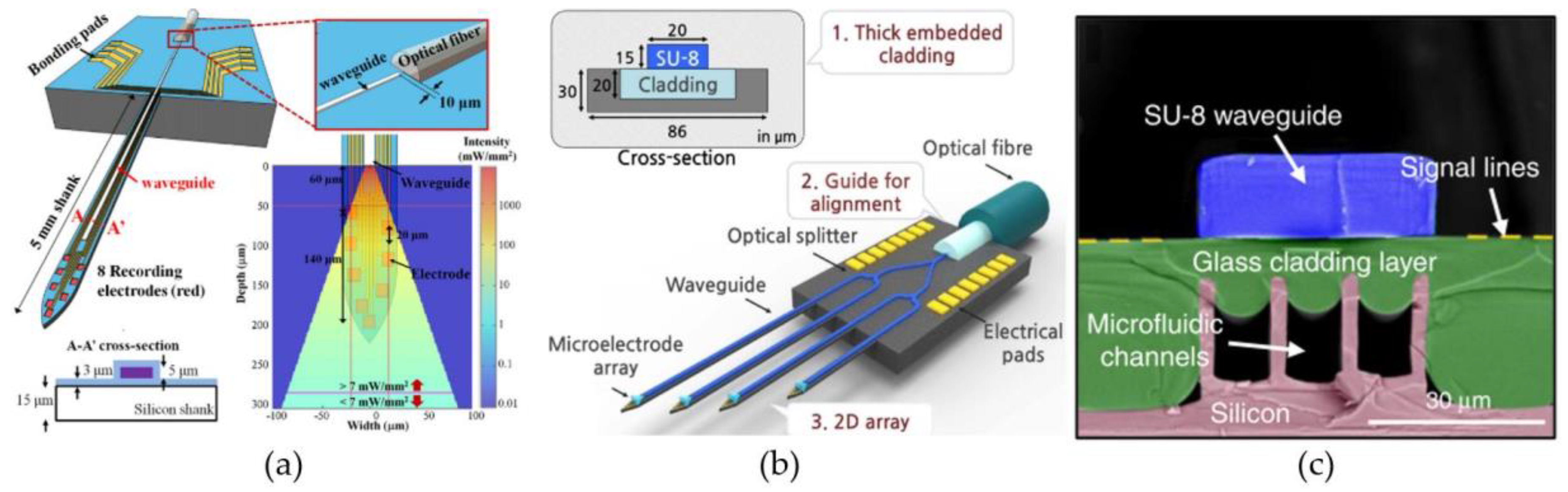
4.2. Waveguide-Based Light Delivery
4.2.1. Waveguide-on-Carrier
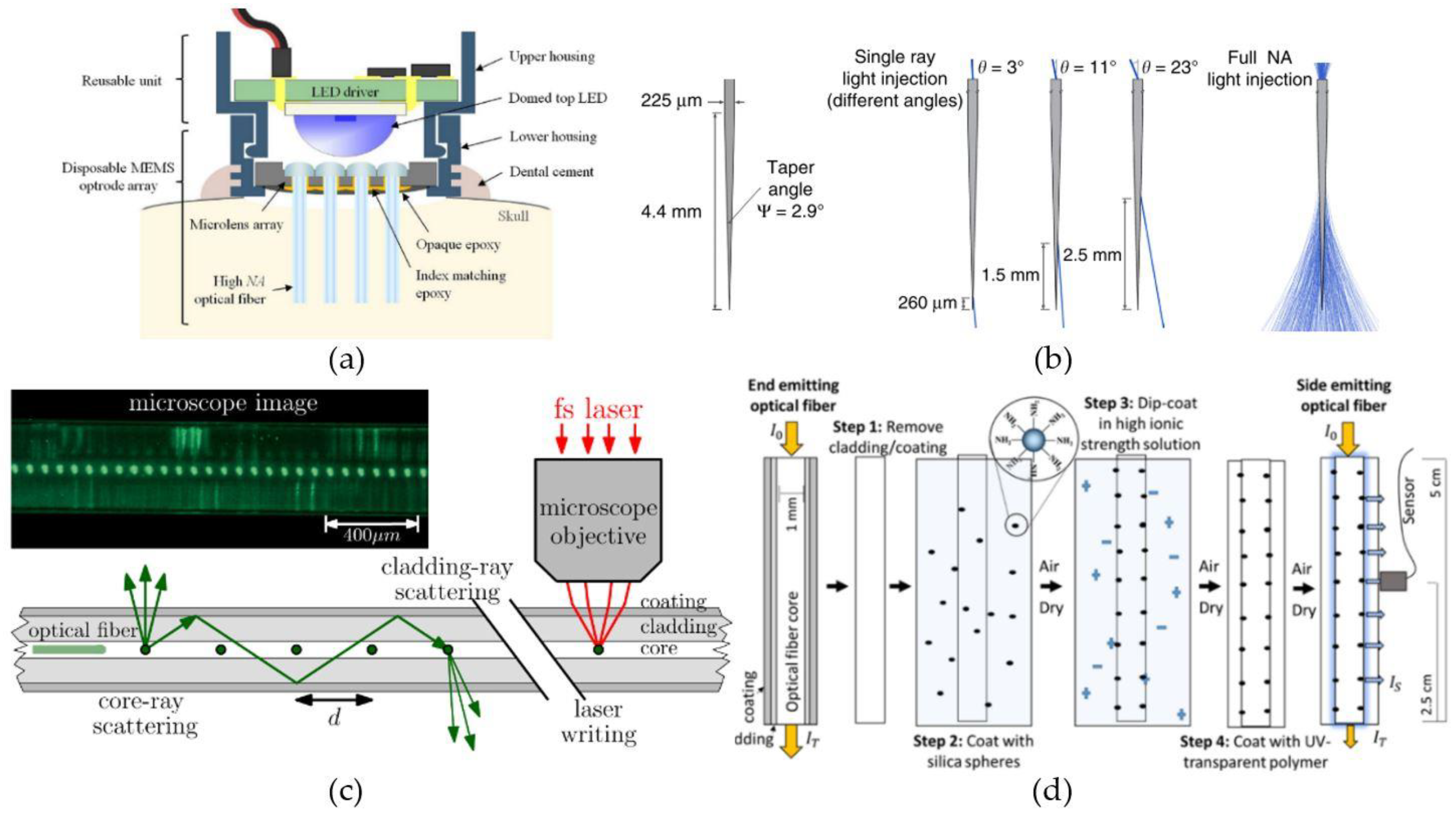
4.2.2. Optical Fibers
4.2.3. Low-Modulus Waveguides and Optical Fibers
Thermoplastic Polymers
Hydrogels
Elastomers
5. Implantation Methods for Soft/Flexible Neural Implants
5.1. External Measures for Eased Probe Implantation
5.2. Temporary Stiffeners
5.2.1. Stiff Probes That Soften after Implantation
5.2.2. Insertion Shuttles
5.2.3. Dissolvable Coatings
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 16 June 2020).
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs a 30-year longitudinal cohort study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Martin-Mcgill, K.J.; Jackson, C.F.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.; Raedt, R.; Gadeyne, S.; Carrette, E.; Boon, P.; Vonck, K. Recent advances in devices for vagus nerve stimulation. Expert Rev. Med. Devices 2018, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, M.; Vonck, K.; Carrette, E.; Marson, A.G.; Boon, P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobst, B.C.; Cascino, G.D. Resective epilepsy surgery for drug-resistant focal epilepsy: A review. JAM -J. Am. Med. Assoc. 2015, 313, 285–293. [Google Scholar] [CrossRef]
- Krook-Magnuson, E.; Soltesz, I. Beyond the hammer and the scalpel: Selective circuit control for the epilepsies. Nat. Neurosci. 2015, 18, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Pnas 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A.; et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in Neural Systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The Development and Application of Optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Krook-Magnuson, E.; Armstrong, C.; Oijala, M.; Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, J.T.; Davidson, T.J.; Frechette, E.S.; Delord, B.; Parada, I.; Peng, K.; Deisseroth, K.; Huguenard, J.R. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013, 16, 64–70. [Google Scholar] [CrossRef]
- Krook-Magnuson, E.; Armstrong, C.; Bui, A.; Lew, S.; Oijala, M.; Soltesz, I. In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 2015, 593, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Alleva, R.; Ma, H.; Daniel, A.G.S.; Schwartz, T.H. Optogenetic tools for modulating and probing the epileptic network. Epilepsy Res. 2015, 116, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Choy, M.K.; Duffy, B.A.; Lee, J.H. Optogenetic study of networks in epilepsy. J. Neurosci. Res. 2017, 95, 2325–2335. [Google Scholar] [CrossRef] [Green Version]
- Tung, J.K.; Berglund, K.; Gross, R.E. Optogenetic Approaches for Controlling Seizure Activity. Brain Stimul. 2016, 9, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, J.; Kokaia, M. Epilepsy and optogenetics: Can seizures be controlled by light? Clin. Sci. 2017, 131, 1605–1616. [Google Scholar] [CrossRef]
- Assaf, F.; Schiller, Y. The antiepileptic and ictogenic effects of optogenetic neurostimulation of PV-expressing interneurons. J. Neurophysiol. 2016, 116, 1694–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soper, C.; Wicker, E.; Kulick, C.V.; N’Gouemo, P.; Forcelli, P.A. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis. 2016, 102–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krook-Magnuson, E.; Szabo, G.G.; Armstrong, C.; Oijala, M.; Soltesz, I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Fiáth, R.; Hofer, K.T.; Csikós, V.; Horváth, D.; Nánási, T.; Tóth, K.; Pothof, F.; Böhler, C.; Asplund, M.; Ruther, P.; et al. Long-term recording performance and biocompatibility of chronically implanted cylindrically-shaped, polymer-based neural interfaces. Biomedizinische Technik 2018, 63, 301–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Understanding the Inflammatory Tissue Reaction to Brain Implants To Improve Neurochemical Sensing Performance. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P.; Island, R.; Island, R. Scanning electron microscopy of chronically implanted Intracortical Microelectrode Arrays in Non-Human Primates. J. Neural. Eng. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhong, C.; Ke, D.; Ye, F.; Tu, J.; Wang, L.; Lu, Y. Ultrasoft and Highly Stretchable Hydrogel Optical Fibers for In Vivo Optogenetic Modulations. Adv. Opt. Mater. 2018, 6, 2–7. [Google Scholar] [CrossRef]
- Sheng, H.; Wang, X.; Kong, N.; Xi, W.; Yang, H.; Wu, X.; Wu, K.; Li, C.; Hu, J.; Tang, J.; et al. Neural interfaces by hydrogels. Extrem. Mech. Lett. 2019, 30, 100510. [Google Scholar] [CrossRef]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, K.C.; Sy, J.C.; Ramadi, K.B.; Graybiel, A.M.; Langer, R.; Cima, M.J. Characterization of Mechanically Matched Hydrogel Coatings to Improve the Biocompatibility of Neural Implants. Sci. Rep. 2017, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical flexibility reduces the foreign body response to long-term implanted microelectrodes in rabbit cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Wolff, A.; Ejserholm, F.; Wallman, L.; Schouenborg, J.; Linsmeier, C.E. Influence of probe flexibility and gelatin embedding on neuronal density and glial responses to brain implants. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Kato, Y.X.; Furukawa, S.; Samejima, K.; Hironaka, N.; Kashino, M. Photosensitive-polyimide based method for fabricating various neural electrode architectures. Front. Neuroeng. 2012, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical properties of gray and white matter brain tissue by indentation. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Gilletti, A.; Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 2006, 3, 189–195. [Google Scholar] [CrossRef]
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J. Neural Eng. 2005, 2, 103–113. [Google Scholar] [CrossRef]
- Stice, P.; Gilletti, A.; Panitch, A.; Muthuswamy, J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J. Neural Eng. 2007, 4, 42–53. [Google Scholar] [CrossRef]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H. Tungsten Microelectrode for Recording from Single Units. Science 1957, 125, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Welle, E.J.; Patel, P.R.; Woods, J.E.; Petrossians, A.; Della Valle, E.; Vega-Medina, A.; Richie, J.M.; Cai, D.; Weiland, J.D.; Chestek, C.A. Ultra-small carbon fiber electrode recording site optimization and improved in vivo chronic recording yield. J. Neural Eng. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, K.; Zhong, C.; Wang, L.; Lu, Y. Fabrication and modification of implantable optrode arrays for in vivo optogenetic applications. Biophys. Rep. 2018, 4, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-T.; Wolfe, J.C.; Dani, J.A.; Shih, W.-C. Flexible optitrode for localized light delivery and electrical recording. Bone 2008, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Barna, J.S.; Arezzo, J.C.; Vaughan, H.G. A new multielectrode array for the simultaneous recording of field potentials and unit activity. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 494–496. [Google Scholar] [CrossRef]
- Guitchounts, G.; Markowitz, J.E.; Liberti, W.A.; Gardner, T.J. A carbon-fiber electrode array for long-term neural recording. Res. Commun. Chem. Pathol. Pharmacol. 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Branner, A.; Gulati, T.; Giszter, S.F. Braided multi-electrode probes: Mechanical compliance characteristics and recordings from spinal cords. J. Neural Eng. 2013, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Nicolelis, M.A.L.; Ghazanfar, A.A.; Faggin, B.M.; Votaw, S.; Oliveira, L.M.O. Reconstructing the engram: Simultaneous, multisite, many single neuron recordings. Neuron 1997, 18, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Nicolelis, M.A.L.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [Green Version]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Egert, D.; Pettibone, J.R.; Lemke, S.; Patel, P.R.; Caldwell, C.M.; Cai, D.; Ganguly, K.; Chestek, C.A.; Berke, J.D. Cellular-scale silicon probes for high-density, precisely localized neurophysiology. J. Neurophysiol. 2020, 124, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Kampasi, K.; English, D.F.; Seymour, J.; Stark, E.; McKenzie, S.; Vöröslakos, M.; Buzsáki, G.; Wise, K.D.; Yoon, E. Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes. Microsyst. Nanoeng. 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Scholvin, J.; Kinney, J.P.; Bernstein, J.G.; Moore-Kochlacs, C.; Kopell, N.; Fonstad, C.G.; Boyden, E.S. Close-packed silicon microelectrodes for scalable spatially oversampled neural recording. IEEE Trans. Biomed. Eng. 2016, 63, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Raducanu, B.C.; Yazicioglu, R.F.; Lopez, C.M.; Ballini, M.; Putzeys, J.; Wang, S.; Andrei, A.; Rochus, V.; Welkenhuysen, M.; van Helleputte, N.; et al. Time multiplexed active neural probe with 1356 parallel recording sites. Sensors (Switzerland) 2017, 17, 2388. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B.; Trautmann, E.M.; Welkenhuysen, M.; Shenoy, K.V.; Andrei, A.; Harris, T.D.; Lopez, C.M.; O’Callahan, J.; Putzeys, J.; Raducanu, B.C.; et al. The Neuropixels probe: A CMOS based integrated microsystems platform for neuroscience and brain-computer interfaces. Tech. Dig.-Int. Electron Devices Meet. IEDM 2019, 202–205. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydin, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, N.A.; Aydin, C.; Lebedeva, A.; Okun, M.; Pachitariu, M.; Bauza, M.; Beau, M.; Bhagat, J.; Böhm, C.; Broux, M.; et al. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. bioRxiv 2020. [Google Scholar] [CrossRef]
- Juavinett, A.L.; Bekheet, G.; Churchland, A.K. Chronically implanted neuropixels probes enable high-yield recordings in freely moving mice. Elife 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Steinmetz, N.A.; Koch, C.; Harris, K.D.; Carandini, M. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Curr. Opin. Neurobiol. 2018, 50, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Fort, M.; Bracey, E.F.; Keshavarzi, S.; Rousseau, C.V.; Cossell, L.; Lenzi, S.C.; Strom, M.; Margrie, T.W. A Circuit for Integration of Head- and Visual-Motion Signals in Layer 6 of Mouse Primary Visual Cortex. Neuron 2018, 98, 179–191.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schevon, C.A.; Tobochnik, S.; Eissa, T.; Merricks, E.; Gill, B.; Parrish, R.R.; Bateman, L.M.; McKhann, G.; Emerson, R.G.; Trevelyan, A.J. Multiscale recordings reveal the dynamic spatial structure of human seizures. Neurobiol. Dis. 2019, 127, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.G.R.; Tu, H.; Luo, H.; Liu, B.; Bao, S.; Zhang, J.; Xu, Y. 3D silicon neural probe with integrated optical fibers for optogenetic modulation. Lab. Chip. 2015, 15, 2939–2949. [Google Scholar] [CrossRef]
- Chung, J.E.; Joo, H.R.; Fan, J.L.; Liu, D.F.; Barnett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-Density, Long-Lasting, and Multi-region Electrophysiological Recordings Using Polymer Electrode Arrays. Neuron 2019, 101, 21–31.e5. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 1642–1667. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J.; Shain, W.; Kipke, D.R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410–423. [Google Scholar] [CrossRef]
- Wu, F.; Im, M.; Yoon, E. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 966–969. [Google Scholar]
- Massey, T.L.; Kuo, L.S.; Fan, J.L.; Maharbiz, M.M. An Actuated Neural Probe Architecture for Reducing Gliosis-Induced Recording Degradation. IEEE Trans. Nanobioscience 2019, 18, 220–225. [Google Scholar] [CrossRef]
- Egert, D.; Najafi, K. New Class of Chronic Recording Multichannel Neural Probes with Post-Implant Self-Deployed Satellite Recording Sites. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 958–961. [Google Scholar]
- Agorelius, J.; Tsanakalis, F.; Friberg, A.; Thorbergsson, P.T.; Pettersson, L.M.E.; Schouenborg, J. An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation-initial evaluation in cortex cerebri of awake rats. Front. Neurosci. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Khilwani, R.; Gilgunn, P.J.; Kozai, T.D.Y.; Ong, X.C.; Korkmaz, E.; Gunalan, P.K.; Cui, X.T.; Fedder, G.K.; Ozdoganlar, O.B. Ultra-miniature ultra-compliant neural probes with dissolvable delivery needles: Design, fabrication and characterization. Biomed. Microdevices 2016, 18. [Google Scholar] [CrossRef]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Liu, J.; Fu, T.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, T.; Hong, G.; Viveros, R.D.; Zhou, T.; Lieber, C.M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, H.S.; Moon, K.S.; Cannon, A.; Hesketh, P.J.; Wong, C.P. Wafer bonding using microwave heating of parylene intermediate layers. J. Micromech. Microeng. 2004, 14, 625–631. [Google Scholar] [CrossRef]
- Kim, E.G.R.; John, J.K.; Tu, H.; Zheng, Q.; Loeb, J.; Zhang, J.; Xu, Y. A hybrid silicon – parylene neural probe with locally flexible regions. Sens. Actuators B Chem. 2014, 195, 416–422. [Google Scholar] [CrossRef]
- Seymour, J.P.; Langhals, N.B.; Anderson, D.J.; Kipke, D.R. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed. Microdevices 2011, 13, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, 1–14. [Google Scholar] [CrossRef]
- Sohal, H.; Vassilevski, K.V.; Jackson, A.; Baker, S. A Novel Flexible Sinusoidal Probe for Chronic Extracellular Brain Recording A Novel Flexible. Quant. Biol. 2015, 23, 188–194. [Google Scholar]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Zhou, T.; Hong, G.; Fu, T.M.; Yang, X.; Schuhmann, T.G.; Viveros, R.D.; Lieber, C.M. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. USA 2017, 114, 5894–5899. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Viveros, R.D.; Zwang, T.J.; Yang, X.; Charles, M. Tissue-like neural probes for understanding and modulating the brain. Biochemistry 2018, 57, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; He, J.; Singh, A.; Massia, S.; Ehteshami, G.; Kim, B.; Raupp, G. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng. 2004, 14, 32–37. [Google Scholar] [CrossRef]
- Lee, C.D.; Hara, S.A.; Yu, L.; Kuo, J.T.W.; Kim, B.J.; Hoang, T.; Pikov, V.; Meng, E. Matrigel coatings for Parylene sheath neural probes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Lee, H.J.; Kim, J.; Shin, H.; Choi, N.; Lee, C.J.; Yoon, E.; Yoon, E.; Wise, K.D.; Kim, T.G.; et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Nat. Publ. Gr. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alla, K.; Yuri, C.; Anatoliy, L.; Volodymyr, L.; Yuriy, S. Interface Nerve Tissue-Silicon Nanowire for Regeneration of Injured Nerve and Creation of Bio- Electronic Device; IntechOpen: London, UK, 2019. [Google Scholar]
- Du, J.; Riedel-Kruse, I.H.; Nawroth, J.C.; Roukes, M.L.; Laurent, G.; Masmanidis, S.C. High-resolution three-dimensional extracellular recording of neuronal activity with microfabricated electrode arrays. J. Neurophysiol. 2009, 101, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, T.; Simon, D.; Liu, C.; Musa, T.; Vasudevan, S.; Sloan, A.; Keefer, E.W.; Ii, R.L.R.; Voit, W. Thiol-ene/acrylate substrates for softening intracortical electrodes. J. Biomed. Mater Res. B. Appl. Biomater. 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Stieglitz, T.; Asplund, M. Biomaterials Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef]
- Xiang, Z.; Yen, S.C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.R.; Morgan, S.; Chen, J.; Kapsa, R.M.; Wallace, G.G. Conducting polymer coated neural recording electrodes. J. Neural Eng. 2013, 10, 1–16. [Google Scholar] [CrossRef]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.S.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M. PEDOT-CNT composite microelectrodes for recording and electrostimulation applications: Fabrication, morphology, and electrical properties. Front. Neuroeng. 2012, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, A.; Castagnola, V.; Descamps, E.; Dahan, L.; Blatché, M.C.; Dinis, T.M.; Leclerc, E.; Egles, C.; Bergaud, C. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: Toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 2015, 25. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pas, J.; Rutz, A.L.; Quilichini, P.P.; Slézia, A.; Ghestem, A.; Kaszas, A.; Donahue, M.J.; Curto, V.F.; O’Connor, R.P.; Bernard, C.; et al. A bilayered PVA/PLGA-bioresorbable shuttle to improve the implantation of flexible neural probes. J. Neural Eng. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Schander, A.; Tesmann, T.; Strokov, S.; Stemmann, H.; Kreiter, A.K.; Lang, W. In-vitro evaluation of the long-term stability of PEDOT:PSS coated microelectrodes for chronic recording and electrical stimulation of neurons. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2016, 6174–6177. [Google Scholar] [CrossRef]
- Shain, W.; Spataro, L.; Dilgen, J.; Haverstick, K.; Retterer, S.; Isaacson, M.; Saltzman, M.; Turner, J.N. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 186–188. [Google Scholar] [CrossRef]
- Zhong, Y.; McConnell, G.C.; Ross, J.D.; Deweerth, S.P.; Bellamkonda, R.V. A novel dexamethasone-releasing, anti-inflammatory coating for neural implants. In Proceedings of the Conference 2nd International IEEE EMBS Conference on Neural Engineering, Arlington, VA, USA, 16–19 March 2005; pp. 522–525. [Google Scholar] [CrossRef]
- Jhaveri, S.J.; Hynd, M.R.; Dowell-Mesfin, N.; Turner, J.N.; Shain, W.; Ober, C.K. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules 2009, 10, 174–183. [Google Scholar] [CrossRef]
- Mattis, J.; Tye, K.M.; Ferenczi, E.A.; Ramakrishnan, C.; Daniel, J. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 2012, 9, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Kale, R.P.; Kouzani, A.Z.; Walder, K.; Berk, M.; Tye, S.J. Evolution of optogenetic microdevices. Neurophotonics 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y.; et al. An Ultra-Sensitive Step-Function Opsin for Minimally Invasive Optogenetic Stimulation in Mice and Macaques. Neuron 2020, 107, 38–51.e8. [Google Scholar] [CrossRef]
- ChR Viewer | TeamPrigge. Available online: http://teamprigge.de/chr-viewer/ (accessed on 22 June 2020).
- Gysbrechts, B.; Wang, L.; Nguyen, N.; Trong, D.; Cabral, H.; Navratilova, Z.; Battaglia, F.; Saeys, W.; Bartic, C. Light distribution and thermal effects in the rat brain under optogenetic stimulation. J. Biophotonics. 2016, 585, 576–585. [Google Scholar] [CrossRef]
- Liu, Y.; Jacques, S.L.; Azimipour, M.; Rogers, J.D.; Pashaie, R.; Eliceiri, K.W. OptogenSIM: A 3D Monte Carlo simulation platform for light delivery design in optogenetics. Biomed. Opt. Express. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hin, Y.O.S.; Oo, M.I.Y.; Im, H.Y.U.N.K.; Am, S.U.N.G.I.N.; Im, H.Y.H.L.K.; Ee, S.U.N.Y.U.L.; Im, S.O.K.; Ang, H.Y.U.K. Characterization of fiber-optic light delivery and light-induced temperature changes in a rodent brain for precise optogenetic neuromodulation. Biomed. Opt. Express. 2016, 7, 525–534. [Google Scholar]
- Andersen, P.; Moser, E.I. Brain temperature and hippocampal function. Hippocampus 1995, 5, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S. Pathologic analysis of photothermal and photomechnical effects of laser-tissue interactions. Photochem. Photobiol. 1991, 53, 825–835. [Google Scholar] [CrossRef]
- Scharf, R.; Tsunematsu, T.; McAlinden, N.; Dawson, M.D.; Sakata, S.; Mathieson, K. Depth-specific optogenetic control in vivo with a scalable, high-density μlED neural probe. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- McAlinden, N.; Massoubre, D.; Richardson, E.; Gu, E.; Sakata, S.; Dawson, M.D.; Mathieson, K. Thermal and optical characterization of micro-LED probes for in vivo optogenetic neural stimulation. Opt. Lett. 2013, 38, 992. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Soltan, A.; Maaskant, P.; Dong, N.; Sun, X.; Degenaar, P. A Scalable Optoelectronic Neural Probe Architecture with Self-Diagnostic Capability. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 2431–2442. [Google Scholar] [CrossRef]
- Schwaerzle, M.; Nehlich, J.; Ayub, S.; Paul, O.; Ruther, P. Led-based optical cochlear implant on highly flexible triple layer polyimide substrates. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 395–398. [Google Scholar] [CrossRef]
- Reddy, J.W.; Kimukin, I.; Stewart, L.T.; Ahmed, Z.; Barth, A.L.; Towe, E.; Chamanzar, M. High density, double-sided, flexible optoelectronic neural probes with embedded µLEDs. Front. Genet. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Kwon, K.Y.; Weber, A.J.; Li, W. An implantable, miniaturized SU-8 optical probe for optogenetics-based deep brain stimulation. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBC, Chicago, IL, USA, 26–30 August 2014; pp. 450–453. [Google Scholar] [CrossRef]
- Cao, H.; Gu, L.; Mohanty, S.K.; Chiao, J.C. An integrated µLED optrode for optogenetic stimulation and electrical recording. IEEE Trans. Biomed. Eng. 2013, 60, 225–229. [Google Scholar] [CrossRef]
- Schwaerzle, M.; Pothof, F.; Paul, O.; Ruther, P. High-resolution neural depth probe with integrated 460 NM light emitting diode for optogenetic applications. In Proceedings of the 2015 Transducers-2015 18th International Conference Solid-State Sensors, Actuators Microsystems, Transducers, Anchorage, AK, USA, 21–25 June 2015; pp. 1774–1777. [Google Scholar] [CrossRef]
- Sun, J.; Lee, S.J.; Wu, L.; Sarntinoranont, M.; Xie, H. Refractive index measurement of acute rat brain tissue slices using optical coherence tomography. Opt. Express. 2012, 20, 1084–1095. [Google Scholar] [CrossRef]
- Wu, F.; Stark, E.; Im, M.; Cho, I.J.; Yoon, E.S.; Buzsáki, G.; Wise, K.D.; Yoon, E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J. Neural Eng. 2013, 10. [Google Scholar] [CrossRef]
- Schwaerzle, M.; Paul, O.; Ruther, P. Compact silicon-based optrode with integrated laser diode chips, SU-8 waveguides and platinum electrodes for optogenetic applications. J. Micromech. Microeng. 2017, 27. [Google Scholar] [CrossRef]
- Kampasi, K.; Stark, E.; Seymour, J.; Na, K.; Winful, H.G.; Buzsáki, G.; Wise, K.D.; Yoon, E. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Son, Y.; Chae, U.; Kim, J.; Choi, N.; Woo, J.; Cho, Y.; Yang, S.H.; Lee, C.J.; Cho, I.; et al. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nat. Commun. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sileo, L.; Bitzenhofer, S.H.; Spagnolo, B.; Pöpplau, J.A.; Holzhammer, T.; Pisanello, M.; Pisano, F.; Bellistri, E.; Maglie, E.; De Vittorio, M.; et al. Tapered Fibers Combined With a Multi-Electrode Array for Optogenetics in Mouse Medial Prefrontal Cortex. Front. Neurosci. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Iseri, E.; Shi, Y.; Kuzum, D. A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes. Front. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Reddy, J.W.; Lassiter, M.; Chamanzar, M. Parylene photonics: A flexible, broadband optical waveguide platform with integrated micromirrors for biointerfaces. Microsystems Nanoeng. 2020, 6. [Google Scholar] [CrossRef]
- Libbrecht, S.; Hoffman, L.; Welkenhuysen, M.; Van den Haute, C.; Baekelandt, V.; Braeken, D.; Haesler, S. Proximal and distal modulation of neural activity by spatially confined optogenetic activation with an integrated high-density optoelectrode. J. Neurophysiol. 2018, 120, 149–161. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, H.; Jun, S.B.; Kim, Y.K.; Ji, C.H. Disposable MEMS optrode array integrated with single LED for neurostimulation. Sens. Actuators A Phys. 2018, 273, 276–284. [Google Scholar] [CrossRef]
- Stark, E.; Koos, T.; Buzsáki, G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J. Neurophysiol. 2012, 108, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanello, F.; Mandelbaum, G.; Pisanello, M.; Oldenburg, I.A.; Sileo, L.; Markowitz, J.E.; Peterson, R.E.; Della Patria, A.; Haynes, T.M.; Emara, M.S.; et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 2017, 20, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reupert, A.; Heck, M.; Nolte, S.; Wondraczek, L. Side-emission properties of femtosecond laser induced scattering centers in optical fibers. Opt. Mater. Express 2019, 9, 2497. [Google Scholar] [CrossRef]
- Lanzarini-Lopes, M.; Cruz, B.; Garcia-Segura, S.; Alum, A.; Abbaszadegan, M.; Westerhoff, P. Nanoparticle and Transparent Polymer Coatings Enable UV-C Side-Emission Optical Fibers for Inactivation of Escherichia coli in Water. Environ. Sci. Technol. 2019, 53, 10880–10887. [Google Scholar] [CrossRef]
- Fischer, U.H.P.; Haupt, M.; Joncic, M. Optical Transmission Systems Using Polymeric Fibers; Intech open: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Szczurowski, M.K.; Martynkien, T.; Statkiewicz-Barabach, G.; Khan, L.; Webb, D.J.; Ye, C.; Dulieu-Barton, J.; Urbanczyk, W. Measurements of stress-optic coefficient and Young’s modulus in PMMA fibers drawn under different conditions. Photonic Cryst. Fibers IV 2010, 7714, 77140G. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Park, S.; Richner, T.J.; Derry, A.; Brown, I.; Hou, C.; Rao, S.; Kang, J.; Moritz, C.T.; Fink, Y.; et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Guo, Y.; Jia, X.; Choe, H.K.; Grena, B.; Kang, J.; Park, J.; Lu, C.; Canales, A.; Chen, R.; et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 2017, 20, 612–619. [Google Scholar] [CrossRef]
- Aden, M.; Roesner, A.; Olowinsky, A. Optical Characterization of Polycarbonate: Influence of Additives on Optical Properties. Ploymer Phys. 2004, 48, 451–455. [Google Scholar] [CrossRef]
- Niles, W.D.; Coassin, P.J. Cyclic olefin polymers: Innovative materials for high-density multiwell plates. Assay Drug Dev. Technol. 2008, 6, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Khanarian, G. Optical properties of cyclic olefin copolymers. Opt. Eng. 2001, 40, 1024. [Google Scholar] [CrossRef]
- Shabahang, S.; Forward, S.; Yun, S.-H. Polyethersulfone optical fibers with thermally induced microbubbles for custom side-scattering profiles. Opt. Express 2019, 27, 7560. [Google Scholar] [CrossRef] [PubMed]
- Pazokian, H.; Barzin, J.; Mollabashi, M.; Jelvani, S.; Abolhosseini, S. Exploring the influence of a XeCl laser treatment on biocompatibility of polyethersulfone film. Laser Phys. 2012, 22, 922–929. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Gather, M.C.; Humar, M.; Choi, M.; Kim, S.; Kim, K.S.; Hahn, S.K.; Scarcelli, G.; Randolph, M.; Redmond, R.W.; et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gierej, A.; Vagenende, M.; Filipkowski, A.; Siwicki, B.; Buczynski, R.; Thienpont, H.; Van Vlierberghe, S.; Geernaert, T.; Dubruel, P.; Berghmans, F. Poly(D,L-Lactic Acid) (PDLLA) Biodegradable and Biocompatible Polymer Optical Fiber. J. Light. Technol. 2019, 37, 1916–1923. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, Q.; Rogin, P.; de Oliveira, P.W.; del Campo, A. Printed soft optical waveguides of PLA copolymers for guiding light into tissue. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Fu, R.; Luo, W.; Nazempour, R.; Tan, D.; Ding, H.; Zhang, K.; Yin, L.; Guan, J.; Sheng, X. Implantable and Biodegradable Poly(l-lactic acid) Fibers for Optical Neural Interfaces. Adv. Opt. Mater. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Ohannsmeier, S.O.J.; Apa, M.A.L.T.O.; Aniele, D.; Ipresa, D.; Ipken, T.A.R.; Einemann, D.A.G.H.; Eisterkamp, A.L.H. Hydrogels for targeted waveguiding and light diffusion. Opt. Matercial Express 2019, 9, 3925. [Google Scholar] [CrossRef]
- Choi, M.; Choi, J.W.; Kim, S.; Nizamoglu, S.; Hahn, S.K.; Yun, S.H. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics 2013, 7, 987–994. [Google Scholar] [CrossRef]
- Torres-Mapa, M.L.; Singh, M.; Simon, O.; Mapa, J.L.; Machida, M.; Günther, A.; Roth, B.; Heinemann, D.; Terakawa, M.; Heisterkamp, A. Fabrication of a monolithic lab-on-a-chip platform with integrated hydrogel waveguides for chemical sensing. Sensors (Switzerland) 2019, 19, 4333. [Google Scholar] [CrossRef] [Green Version]
- Reid, B.; Gibson, M.; Singh, A.; Taube, J.; Furlong, C.; MUrcia, M.; Elisseeff, J. PEG hydrogel degradation and the role of the surronding tissue environment. J. Tissue Eng. Regen MED 2015, 9, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, M.B.; Cosgriff-Hernandez, E. Development of a biostable replacement for PEGDA hydrogels. Biomacromolecules 2012, 13, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J. Biomed. Mater. Res. Q. 2014, 102, 4244–4251. [Google Scholar] [CrossRef]
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 2014, 35, 4848–4861. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Jiang, N.; Fallahi, A.; Montelongo, Y.; Ruiz-Esparza, G.U.; Tamayol, A.; Zhang, Y.S.; Mahmood, I.; Yang, S.A.; Kim, K.S.; et al. Glucose-Sensitive Hydrogel Optical Fibers Functionalized with Phenylboronic Acid. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1. [Google Scholar] [CrossRef]
- Choi, M.; Humar, M.; Kim, S.; Yun, S.H. Step-Index Optical Fiber Made of Biocompatible Hydrogels. Adv. Mater. 2015, 27, 4081–4086. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Shan, D.; Zhang, C.; Kalaba, S.; Mehta, N.; Kim, G.B.; Liu, Z.; Yang, J. Flexible biodegradable citrate-based polymeric step-index optical fiber. Biomaterials 2017, 143, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; O’Brien, K.; Li, S.; Shepherd, R.F. Optoelectronically innervated soft prosthetic hand via stretchable optical waveguides. Sci. Robot. 2016, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, S.J.J.; Forward, S.; Wertheimer, C.M.; Liapis, A.C.; Lin, H.H.; Kim, M.; Seiler, T.G.; Birngruber, R.; Kochevar, I.E.; Seiler, T.; et al. Selective equatorial sclera crosslinking in the orbit using a metal-coated polymer waveguide. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2563–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermette, P.; Griesser, H.J.; Laroche, G.; Guidoin, R. Tissue Engineering Intelligence Unit 6; Landes Bioscience: Eurekah, TX, USA, 2001; ISBN 158706023X. [Google Scholar]
- Kwok, S.J.J.; Kim, M.; Lin, H.H.; Seiler, T.G.; Beck, E.; Shao, P.; Kochevar, I.E.; Seiler, T.; Yun, S.H. Flexible optical waveguides for uniform periscleral cross-linking. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sheng, B.; Peng, L.; Huang, Y.; Ni, Z. Flexible and optical fiber sensors composited by graphene and PDMS for motion detection. Polymers (Basel) 2019, 11, 1433. [Google Scholar] [CrossRef] [Green Version]
- Ersen, A.; Sahin, M. Polydimethylsiloxane-based optical waveguides for tetherless powering of floating microstimulators. J. Biomed. Opt. 2017, 22. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24. [Google Scholar] [CrossRef]
- Ochsner, M.; Dusseiller, M.R.; Grandin, H.M.; Luna-Morris, S.; Textor, M.; Vogel, V.; Smith, M.L. Micro-well arrays for 3D shape control and high resolution analysis of single cells. Lab Chip 2007, 633, 2–3. [Google Scholar] [CrossRef]
- Missinne, J.; Kalathimekkad, S.; Van Hoe, B.; Bosman, E.; Vanfleteren, J.; Steenberge, G. Van Stretchable optical waveguides. Opt. Soc. Amerika 2014, 22, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Martincek, I.; Pudis, D.; Chalupova, M. Technology for the preparation of PDMS optical fibers and some fiber structures. IEEE Photonics Technol. Lett. 2014, 26, 1446–1449. [Google Scholar] [CrossRef]
- Zhao, H. Recent progress of development of optogenetic implantable neural probes. Int. J. Mol. Sci. 2017, 18, 1751. [Google Scholar] [CrossRef] [PubMed]
- Francone, A.; Kehoe, T.; Obieta, I.; Saez-Martinez, V.; Bilbao, L.; Khokhar, A.Z.; Gadegaard, N.; Simao, C.D.; Kehagias, N.; Torres, C.M.S. Integrated 3D Hydrogel Waveguide Out-Coupler by Step-and-Repeat Thermal Nanoimprint Lithography: A Promising Sensor Device for Water and pH. Sensors 2018, 18, 3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bélanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Park, J.Y.; Hwang, C.M.; Lee, S.H. Effective methods to improve the biocompatibility of poly (dimethylsiloxane). Biochip J. 2008, 2, 39–43. [Google Scholar]
- Ionescu, M.; Winton, B.; Wexler, D.; Siegele, R.; Deslantes, A.; Stelcer, E.; Atanacio, A.; Cohen, D.D. Enhanced biocompatibility of PDMS (polydimethylsiloxane) polymer films by ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 273, 161–163. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.H.; Kim, J.H.; Jeong, S.M.; Lee, S.H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Eng. Lett. 2011, 1, 199–203. [Google Scholar] [CrossRef]
- Li, Q.; Dhakal, R.; Kim, J. Microdroplet-based On-Demand Drawing of High Aspect-Ratio Elastomeric Micropillar and Its Contact Sensing Application. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kacik, D.; Martincek, I. Optical structure with PDMS microfibre for displacement measurement. In Proceedings of the PHOTOPTICS 2017-Proc. 5th International Conference Photonics, Optics and Laser Technology, Porto, Portugal, 21–25 February 2017; pp. 365–368. [Google Scholar] [CrossRef]
- Snell, K.; Lopez, I.; Louie, B.; Kiessling, R.; Sanii, B. Drawing and hydrophobicity-patterning long polydimethylsiloxane silicone filaments. J. Vis. Exp. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. Fabrication of strongly anchored, high aspect ratio elastomeric microwires for mechanical and optical applications. J. Micromech. Microeng. 2011, 21. [Google Scholar] [CrossRef]
- Kralik, J.D.; Dimitrov, D.F.; Krupa, D.J.; Katz, D.B.; Cohen, D.; Nicolelis, M.A.L. Techniques for long-term multisite neuronal ensemble recordings in behaving animals. Methods 2001, 25, 121–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paralikar, K.J.; Clement, R.S. Collagenase-aided intracortical microelectrode array insertion: Effects on insertion force and recording performance. IEEE Trans. Biomed. Eng. 2008, 55, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Shoffstall, A.J.; Srinivasan, S.; Willis, M.; Stiller, A.M.; Ecker, M.; Voit, W.E.; Pancrazio, J.J.; Capadona, J.R. A Mosquito Inspired Strategy to Implant Microprobes into the Brain. Sci. Rep. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafat, M.A.; Rubin, L.N.; Jefferys, J.G.R.; Irazoqui, P.P. A Method of Flexible Micro-Wire Electrode Insertion in Rodent for Chronic Neural Recording and a Device for Electrode Insertion. IEEE Trans. Neural Syst. Rehabil Eng. 2019, 27, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, B.; Huang, S.; Leo, T.; Lee, M.; Chung, P.; Chow, Y.T.; Huang, I.-W.; Monbouquette, H.G.; Maidment, N.T.; et al. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosens. Bioelectron. 2019, 131, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.R.; Fan, J.L.; Chen, S.; Pebbles, J.A.; Liang, H.; Chung, J.E.; Yorita, A.M.; Tooker, A.C.; Tolosa, V.M.; Geaghan-Breiner, C.; et al. A microfabricated, 3D-sharpened silicon shuttle for insertion of flexible electrode arrays through dura mater into brain. J. Neural Eng. 2019, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, T.L.; Diaz-Botia, C.A.; Kharazia, V.; Maharbiz, M.M.; Sabes, P.N. The “sewing machine” for minimally invasive neural recording. bioRxiv 2019, 578542. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Arreaga-Salas, D.E.; Reeder, J.; Rennaker, R.; Keefer, E.W.; Voit, W. Fabrication of responsive, softening neural interfaces. Adv. Funct. Mater. 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Rezaei, S.; Xu, Y.; Pang, S.W. Control of neural probe shank flexibility by fluidic pressure in embedded microchannel using PDMS/PI hybrid substrate. PLoS ONE 2019, 14, e0220258. [Google Scholar] [CrossRef] [Green Version]
- Felix, S.H.; Shah, K.G.; Tolosa, V.M.; Sheth, H.J.; Tooker, A.C.; Delima, T.L.; Jadhav, S.P.; Frank, L.M.; Pannu, S.S. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 2009, 2, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, X.; He, F.; Wei, X.; Lin, S. Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. J. Neural Eng. 2019, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, C.; Gao, H.; Yu, C.; Yan, Q.; Lu, Y.; Tao, Z.; Linghu, C.; Chen, Z.; Xu, K.; et al. A Removable Insertion Shuttle for Ultraflexible Neural Probe Implantation with Stable Chronic Brain Electrophysiological Recording. Adv. Mater. Interfaces 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab. Chip. 2005, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tien, L.; Chen, F.; Kaplan, D.; Berke, J.; Yoon, E. A multi-shank silk-backed parylene neural probe for reliable chronic recording. In Proceedings of the 2013 Transducers Eurosensors XXVII 17th International Conference Solid-State Sensors, Actuators Microsystems, Transducers Eurosensors, Barcelona, Spain, 16–20 June 2013; pp. 888–891. [Google Scholar] [CrossRef]
- Lind, G.; Linsmeier, C.E.; Thelin, J.; Schouenborg, J. Gelatine-embedded electrodes - A novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J. Neural Eng. 2010, 7. [Google Scholar] [CrossRef] [PubMed]

| Metal Contact | Surface Modification | Surface Area (µm2) | Original Impedance 1 [kΩ] | Modified Impedance 1 [kΩ] | Ref. |
|---|---|---|---|---|---|
| Pt/Ir (90/10) | Pt particles | - | 1520 | 11.96 | [45] |
| Pt/Ir (90/10) | IrO2 | - | 2710 | 148 | [45] |
| Au | Platinum black | 177 | - | 207 | [90] |
| Pt | Pt nanograss | 962 | 1212 ± 365 | 413 ± 309 | [27] |
| Pt | Pt nanograss | 962 | ~300 | ~20 | [91] |
| Au | Electrodeposited Au | 100 | 2100 | 200–250 | [89] |
| Au | CNT-Au nanocomposite | 707 | 1090 | 59.02 | [92] |
| Au | PEDOT | 180 | 870 ± 330 | 46 ± 26 | [82] |
| Au | PEDOT | 707 | 329 ± 33 | 20.55 ± 0.82 | [94] |
| Au | PEDOT | 1257 | 630 | ~7 | [95] |
| Au | PEDOT-PSS | 154 | - | 41.5 ± 6.4 | [97] |
| Ir | PEDOT-pTS | 413 | 192.5 ± 10.0 | 35.0 ± 6.4 | [93] |
| Au | PEDOT-CNT | 707 | 329 ± 33 | 15.55 ± 0.67 | [94] |
| Material | Refractive Index | Bulk Material Loss (λ = 473 nm) [dB/cm] | Young’s Modulus | Stability | Small-Cross-Section Fibers 1 | References |
|---|---|---|---|---|---|---|
| Thermoplastic polymers | + | +/− | − | + | + | |
| PMMA | 1.49 | 0.001 | 3 GPa | Inert | Yes | [135,136] |
| PC | 1.58–1.586 | 1.27 | 2.39 GPa | Inert | Yes | [137,138,139] |
| COC | 1.52–1.53 | 1.21–1.58 | 3 GPa | Inert | Yes | [137,138,140,141] |
| PES | 1.65 | 2.6 | 2.6 GPa | Inert | Yes | [142,143] |
| PLA (including isomers and copolymers) 2 | 1.47 | 0.1–1.8 | 3.5 GPa | Degrades (weeks, months, 1–2 years) | No | [144,145,146,147] |
| Hydrogels | + | +/− | + | − | − | |
| PEG-based (PEGDA, PEGDMA, …) | 1.33–1.46 | 0.13–1.26 3 | ≥60 kPa | Slow oxidation and hydrolysis | No | [148,149,150,151,152,153,154] |
| PAAm | 1.33–1.45 | No data | 8 kPa | Slow hydrolysis | No | [31,155,156,157,158] |
| Alginate | 1.33 | 0.25–2.96 4 | 17 kPa | Slow outdiffusion of divalent cations | / | [31,155,156,157,159,160] |
| Elastomer | + | + | + | +/− | + | |
| COCE | 1.51 | No data | 34 MPa | Inert | Yes | [137] |
| POC/POMC | 1.5 | 0.03–0.08 | 3.4–4.8 MPa | Degrades (months) | No | [161] |
| PU | 1.49 | 0.02 | 4.7–7.4 MPa | Slow oxidation and hydrolysis | No | [162,163,164] |
| PDMS | 1.40–1.55 | 0.11 (Sylgard 184) | 3 kPa–10 MPa | Inert | Yes | [165,166,167,168,169] |
| Materials (Core/Clad) | Dimensions 1 | Optical Loss [dB/cm] | λ [nm] | NA | Manufacturing Process | Ref. |
|---|---|---|---|---|---|---|
| PC/COC | 100–130 µm ⌀ 65–71 µm ⌀ | 1.90 ± 0.02 <1.5 | 473 473 | 0.43 0.42 | Thermal drawing Thermal drawing | [137,138] |
| PES | 800 µm ⌀ | 0.7–0.8 | 633 | Thermal drawing | [142] | |
| Alginate-PAAm/Alginate-PAAm | 750 µm/1100 µm ⌀ | 0.45 | 532 | 0.11 | UV-curing in tube mold + dip-coating | [156] |
| Alginate-PAAm | 300 µm ⌀ (unswollen) | 0.249 (swollen) | 472 | UV-curing in tube mold | [31] | |
| PEGDA (700Da)/Alginate | 800 µm/1000 µm ⌀ | 0.32 ± 0.02 | 492 | 0.6 | UV-curing in tube mold + dip-coating | [159] |
| P(AAm-co-PEGDA)/alginate | 200 µm/300–400 µm ⌀ | 0.30 | 532 | 0.54 | UV-curing in tube mold + dip-coating | [155] |
| COC elastomer | 125 µm × 100 µm–250 µm × 200 µm | 3.98 | 473 | Thermal drawing | [137] | |
| POMC/POC | 500 µm/800 µm–3 mm ⌀ | 0.4 | 633 | 0.1 | Thermal crosslinking in premanufactured cladding layer | [161] |
| PU/silicone | 1 mm × 1 mm/3 mm × 3 mm | 2 | 860 | 0.46 | Thermal crosslinking in premanufactured cladding layer | [162] |
| PU/PDMS | 4 mm × 1 mm/4 mm × 1.8 mm | 0.48 | Molding | [163] | ||
| PDMS | 1 mm ⌀ 5 mm × 1.4 mm | 0.63 0.45 | 441.6 445 | Thermal crosslinking in tube mold | [166,165] | |
| PDMS/PDMS | 50 µm × 50 µm/ 250 µm × 100 µm | 0.14 | 850 | 0.69 | Soft lithography + capillary filling | [170] |
| PDMS/PDMS | 800 µm/1100 µm ⌀ | 0.36 ± 0.03 | 635 | 0.64 | Tube mold + dip-coating | [167] |
| PDMS | 45 µm ⌀ | 0.5 | 632 | Fiber drawing | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandekerckhove, B.; Missinne, J.; Vonck, K.; Bauwens, P.; Verplancke, R.; Boon, P.; Raedt, R.; Vanfleteren, J. Technological Challenges in the Development of Optogenetic Closed-Loop Therapy Approaches in Epilepsy and Related Network Disorders of the Brain. Micromachines 2021, 12, 38. https://doi.org/10.3390/mi12010038
Vandekerckhove B, Missinne J, Vonck K, Bauwens P, Verplancke R, Boon P, Raedt R, Vanfleteren J. Technological Challenges in the Development of Optogenetic Closed-Loop Therapy Approaches in Epilepsy and Related Network Disorders of the Brain. Micromachines. 2021; 12(1):38. https://doi.org/10.3390/mi12010038
Chicago/Turabian StyleVandekerckhove, Bram, Jeroen Missinne, Kristl Vonck, Pieter Bauwens, Rik Verplancke, Paul Boon, Robrecht Raedt, and Jan Vanfleteren. 2021. "Technological Challenges in the Development of Optogenetic Closed-Loop Therapy Approaches in Epilepsy and Related Network Disorders of the Brain" Micromachines 12, no. 1: 38. https://doi.org/10.3390/mi12010038
APA StyleVandekerckhove, B., Missinne, J., Vonck, K., Bauwens, P., Verplancke, R., Boon, P., Raedt, R., & Vanfleteren, J. (2021). Technological Challenges in the Development of Optogenetic Closed-Loop Therapy Approaches in Epilepsy and Related Network Disorders of the Brain. Micromachines, 12(1), 38. https://doi.org/10.3390/mi12010038





