A Portable Device for LAMP Based Detection of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of SARS-CoV-2 and Viral RNA Extraction
2.2. Design and Experimental Conditions for SARS-CoV-2 Specific LAMP Assay
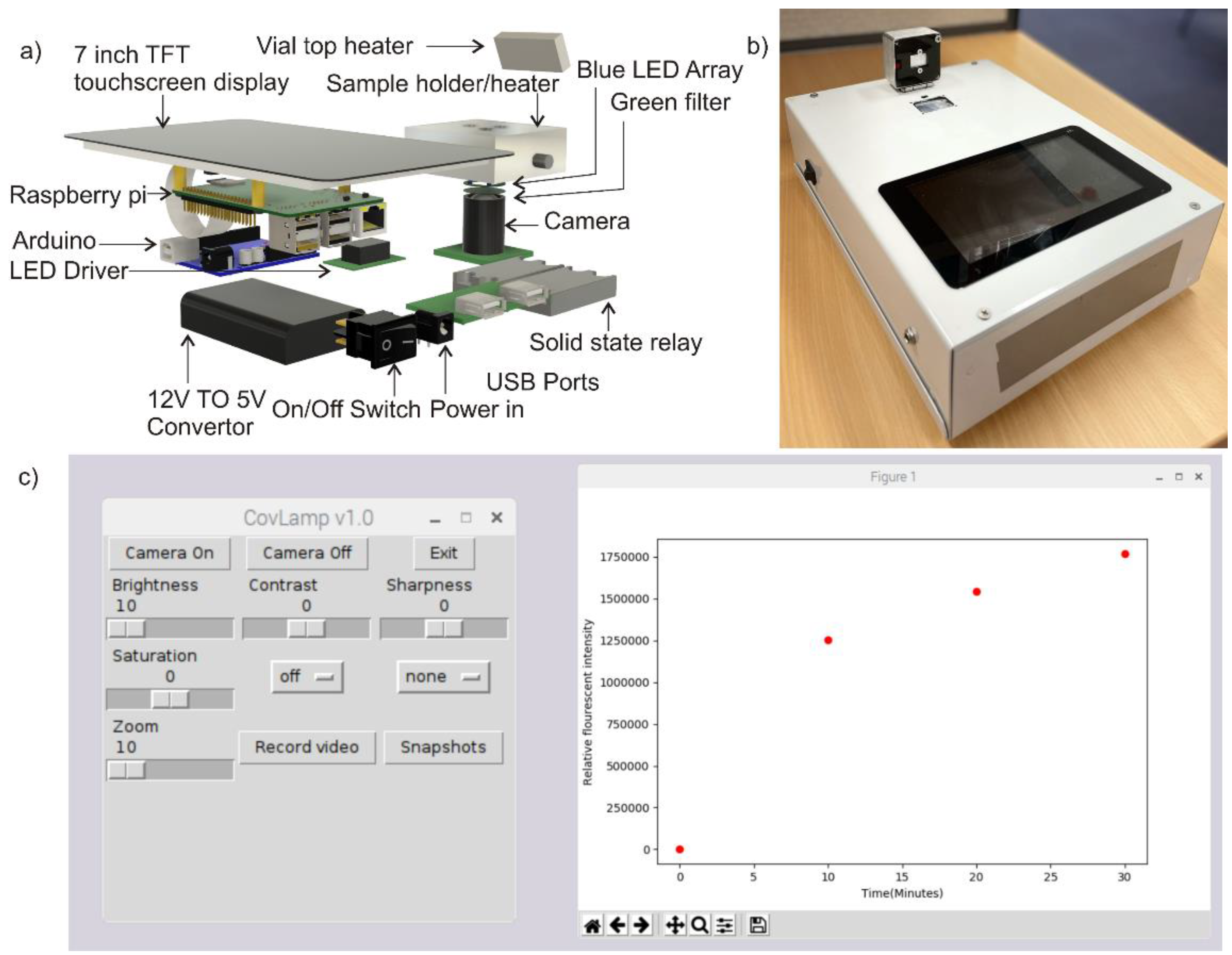
2.3. Device Design
2.4. Experimental
3. Results and Discussion
3.1. SARS-CoV-2 Specific LAMP Assay Using Conventional Thermocycler
3.2. Performance of Portable Device for LAMP Based Detection of SARS-CoV-2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organisation, W.H. Weekly Epidemiological Update on COVID-19—20 July 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---20-july-2021 (accessed on 22 July 2021).
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, C. COVID-19 spurs wave of innovative diagnostics. Nat. Biotechnol. 2020, 38, 769–772. [Google Scholar] [CrossRef]
- Organisation, W.H. Public Health Emergency of International Concern (PHEIC). Available online: https://www.who.int/blueprint/priority-diseases/key-action/Global_Research_Forum_FINAL_VERSION_for_web_14_feb_2020.pdf?ua=1 (accessed on 23 April 2020).
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab A Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Schubert, S.; Richter, S.; Dötzel, W. Hybrid-assembled micro dosing system using silicon-based micropump/valve and mass flow sensor. Sens. Actuators A Phys. 1998, 69, 85–91. [Google Scholar] [CrossRef]
- Shaffaf, T.; Ghafar-Zadeh, E. COVID-19 diagnostic strategies. Part I: Nucleic acid-based technologies. Bioengineering 2021, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Umer, M.; Singha, P.; Nguyen, N.-K.; Kasetsirikul, S.; Ooi, C.H.; Shiddiky, M.J.A.; Nguyen, N.-T. Loop-mediated isothermal amplification in a core-shell bead assay for the detection of tyrosine kinase AXL overexpression. Micromachines 2021, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2018, 60, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, faster, and sensitive zika virus assay using smartphone detection of loop-mediated isothermal amplification on paper microfluidic chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab A Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Liu, Y.; Kong, J.; Jiang, X. Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal. Chem. 2010, 82, 3002–3006. [Google Scholar] [CrossRef]
- Coelho, B.J.; Veigas, B.; Águas, H.; Fortunato, E.; Martins, R.; Baptista, P.V.; Igreja, R. A digital microfluidics platform for loop-mediated isothermal amplification detection. Sensors 2017, 17, 2616. [Google Scholar] [CrossRef] [Green Version]
- Day, C.J.; Bailly, B.; Guillon, P.; Dirr, L.; Jen, F.E.-C.; Spillings, B.L.; Mak, J.; Itzstein, M.v.; Haselhorst, T.; Jennings, M.P.; et al. Multidisciplinary approaches identify compounds that bind to human ACE2 or SARS-CoV-2 spike protein as candidates to block SARS-CoV-2–ACE2 receptor interactions. mBio 2021, 12, e03681-20. [Google Scholar] [CrossRef]
- Haque, M.F.U.; Bukhari, S.S.; Ejaz, R.; Zaman, F.U.; Sreejith, K.R.; Rashid, N.; Umer, M.; Shahzad, N. A novel RdRp-based colorimetric RT-LAMP assay for rapid and sensitive detection of SARS-CoV-2 in clinical and sewage samples from Pakistan. Virus Res. 2021, 302, 198484. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Stratton, H.; Dao, D.V.; Nguyen, N.-T. Liquid marbles as biochemical reactors for the polymerase chain reaction. Lab A Chip 2019, 19, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Takei, T.; Hayase, G.; Stratton, H.; Lamb, K.; Shiddiky, M.; Dao, D.V.; et al. Core-shell beads made by composite liquid marble technology as a versatile microreactor for polymerase chain reaction. Micromachines 2020, 11, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorgannezhad, L.; Sreejith, K.R.; Christie, M.; Jin, J.; Ooi, C.H.; Katouli, M.; Stratton, H.; Nguyen, N.-T. Core-Shell beads as microreactors for phylogrouping of E. coli strains. Micromachines 2020, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Bandara, T.; Nguyen, N.T.; Rosengarten, G. Slug flow heat transfer without phase change in microchannels: A review. Chem. Eng. Sci. 2015, 126, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Yap, Y.-F.; Tan, S.-H.; Nguyen, N.-T.; Murshed, S.M.S.; Wong, T.-N.; Yobas, L. Thermally mediated control of liquid microdroplets at a bifurcation. J. Phys. D Appl. Phys. 2009, 42, 065503. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.; Qamar, A.; Woodfield, P.; Nguyen, N.; Dao, D.V. Thermoresistive effect for advanced thermal sensors: Fundamentals, design considerations, and applications. J. Microelectromech. Syst. 2017, 26, 966–986. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.-P.; Dao, D.V.; Woodfield, P.; Qamar, A.; Nguyen, N.-T. Graphite on paper as material for sensitive thermoresistive sensors. J. Mater. Chem. C 2015, 3, 8776–8779. [Google Scholar] [CrossRef] [Green Version]


| Sequence | |
|---|---|
| Cor-RdRp-F3 | GCTCGCAAACATACAACGT |
| Cor-RdRp-B3 | GTTACCATCAGTAGATAAAAGTGCA |
| Cor-RdRp-FIP | CGCCACACATGACCATTTCACTCAATTTTGTTGTAGCTTGTCACACCGT |
| Cor-RdRp-BIP | AGGTGGAACCTCATCAGGAGATGTTTTAACATTGGCCGTGACAGC |
| Cor-RdRp-LF | CTTGAGCACACTCATTAGCTAATC |
| Cor-RdRp-LB | CCACAACTGCTTATGCTAATAGTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreejith, K.R.; Umer, M.; Dirr, L.; Bailly, B.; Guillon, P.; von Itzstein, M.; Soda, N.; Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.-T. A Portable Device for LAMP Based Detection of SARS-CoV-2. Micromachines 2021, 12, 1151. https://doi.org/10.3390/mi12101151
Sreejith KR, Umer M, Dirr L, Bailly B, Guillon P, von Itzstein M, Soda N, Kasetsirikul S, Shiddiky MJA, Nguyen N-T. A Portable Device for LAMP Based Detection of SARS-CoV-2. Micromachines. 2021; 12(10):1151. https://doi.org/10.3390/mi12101151
Chicago/Turabian StyleSreejith, Kamalalayam Rajan, Muhammad Umer, Larissa Dirr, Benjamin Bailly, Patrice Guillon, Mark von Itzstein, Narshone Soda, Surasak Kasetsirikul, Muhammad J. A. Shiddiky, and Nam-Trung Nguyen. 2021. "A Portable Device for LAMP Based Detection of SARS-CoV-2" Micromachines 12, no. 10: 1151. https://doi.org/10.3390/mi12101151









