Exploring the Effect of a MnO2 Coating on the Electrochemical Performance of a Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bare LTMO
2.2. Preparation of MnO2-Coated LTMO
2.3. Material Characterization
2.4. Electrochemical Evaluations
3. Results and Discussion
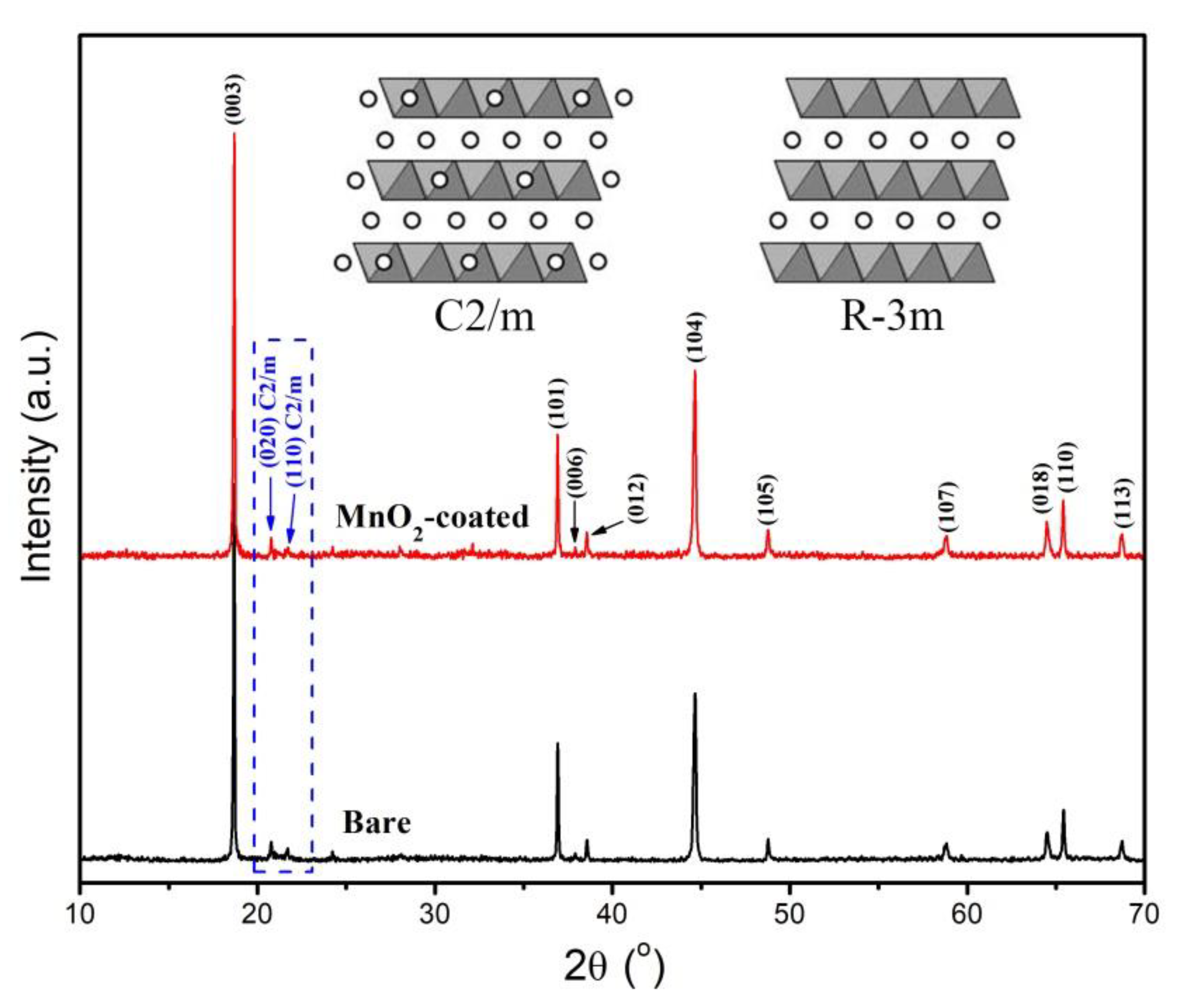
3.1. Structures of Bare and MnO2-Coated LTMO
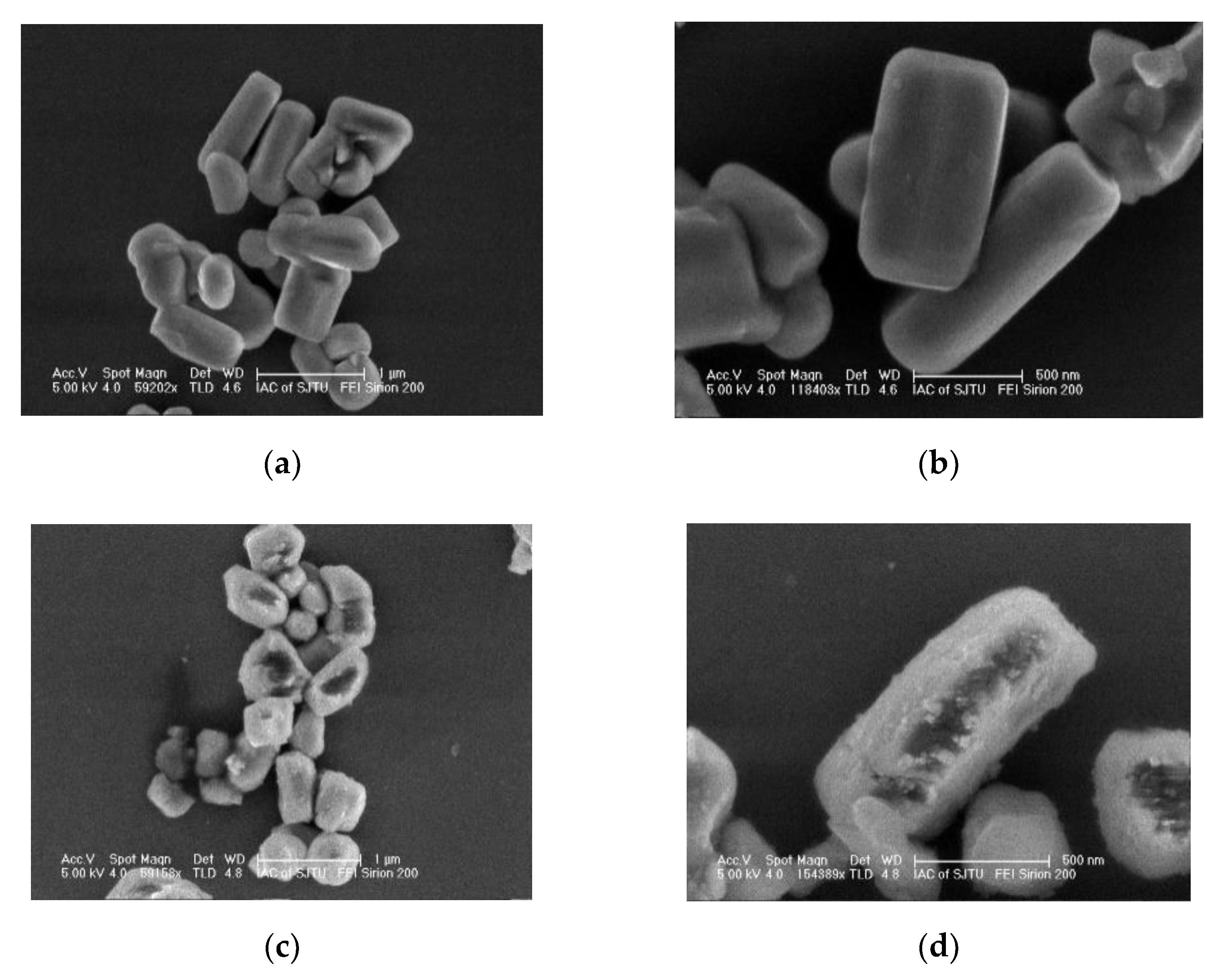
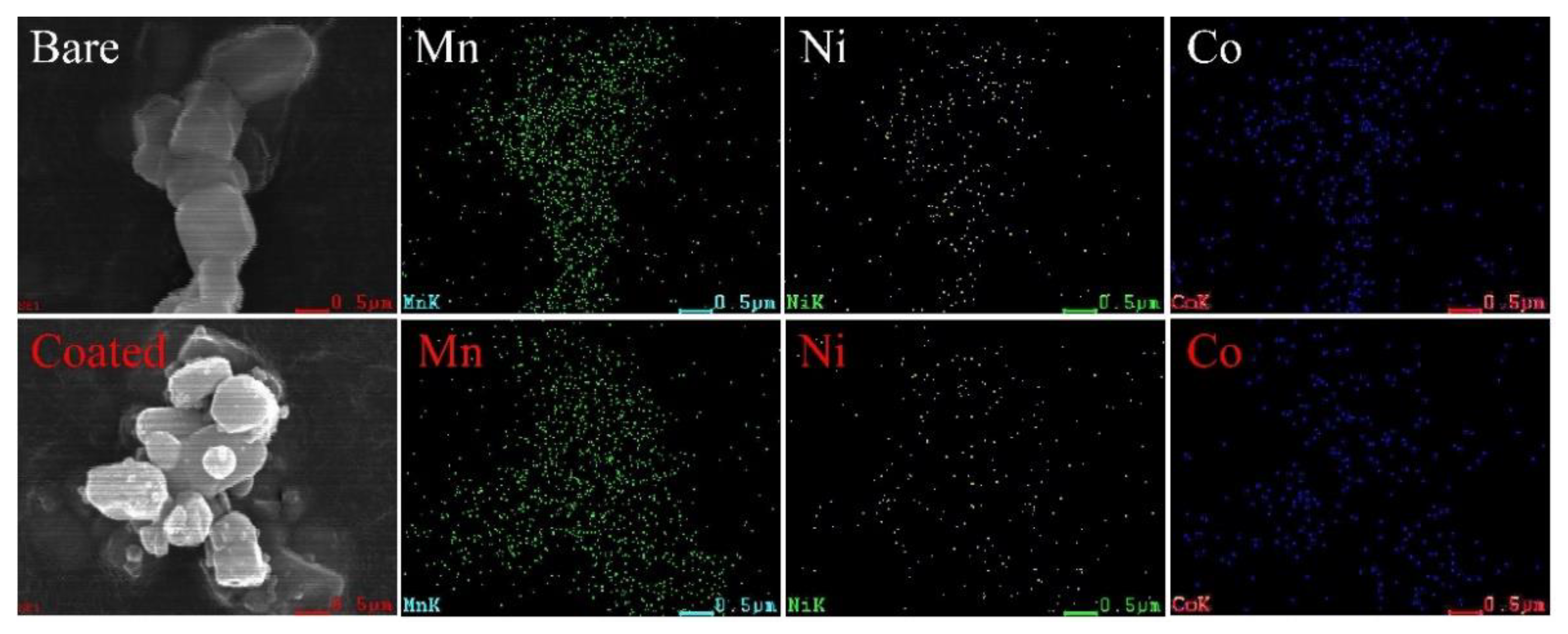
3.2. Microscopic Morphologies of Bare and MnO2-Coated LTMO
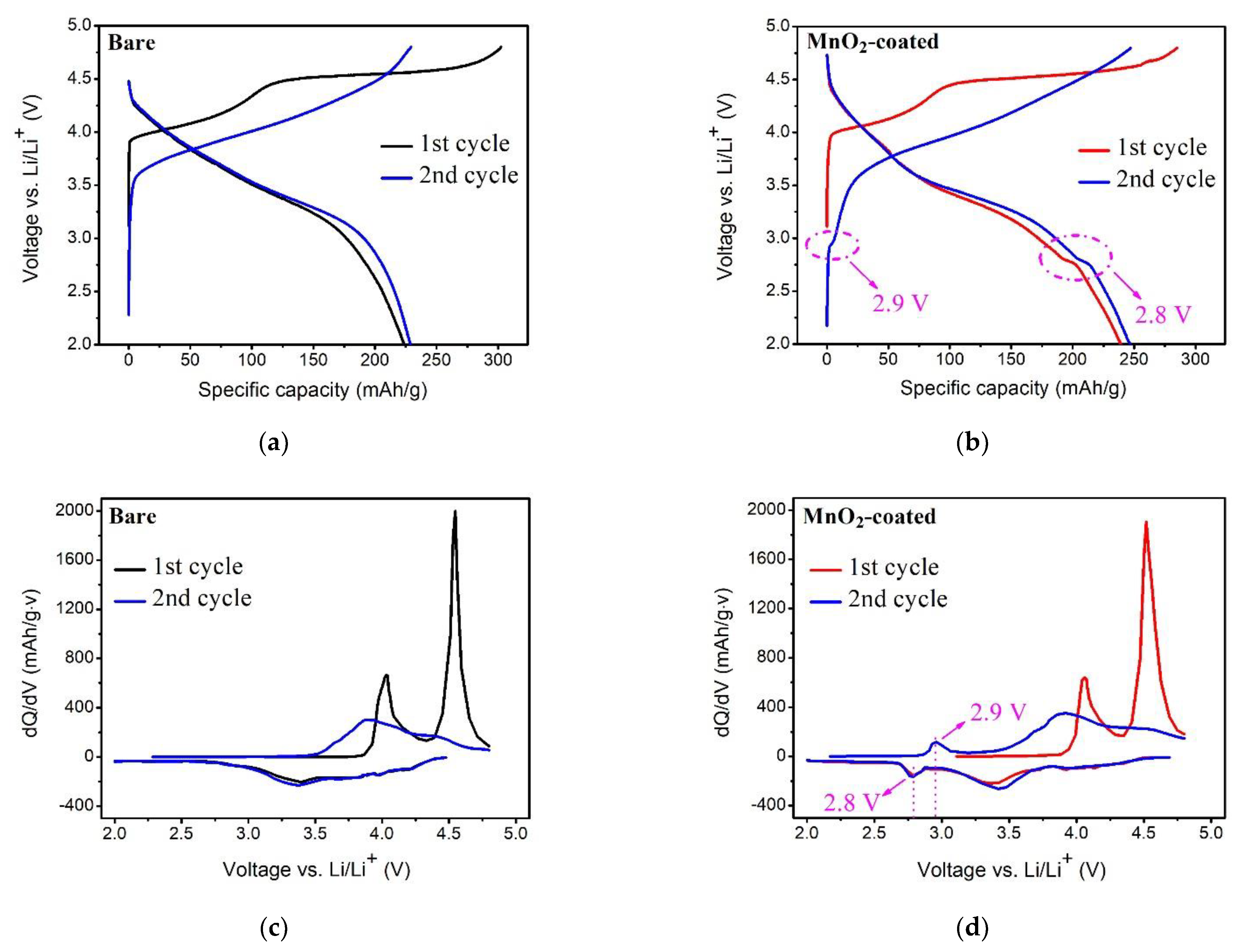
3.3. The Electrochemical Characteristics of Bare and MnO2-Coated LTMO at 0.05 C
3.4. The Cyclic Performance of Bare and MnO2-Coated LTMO
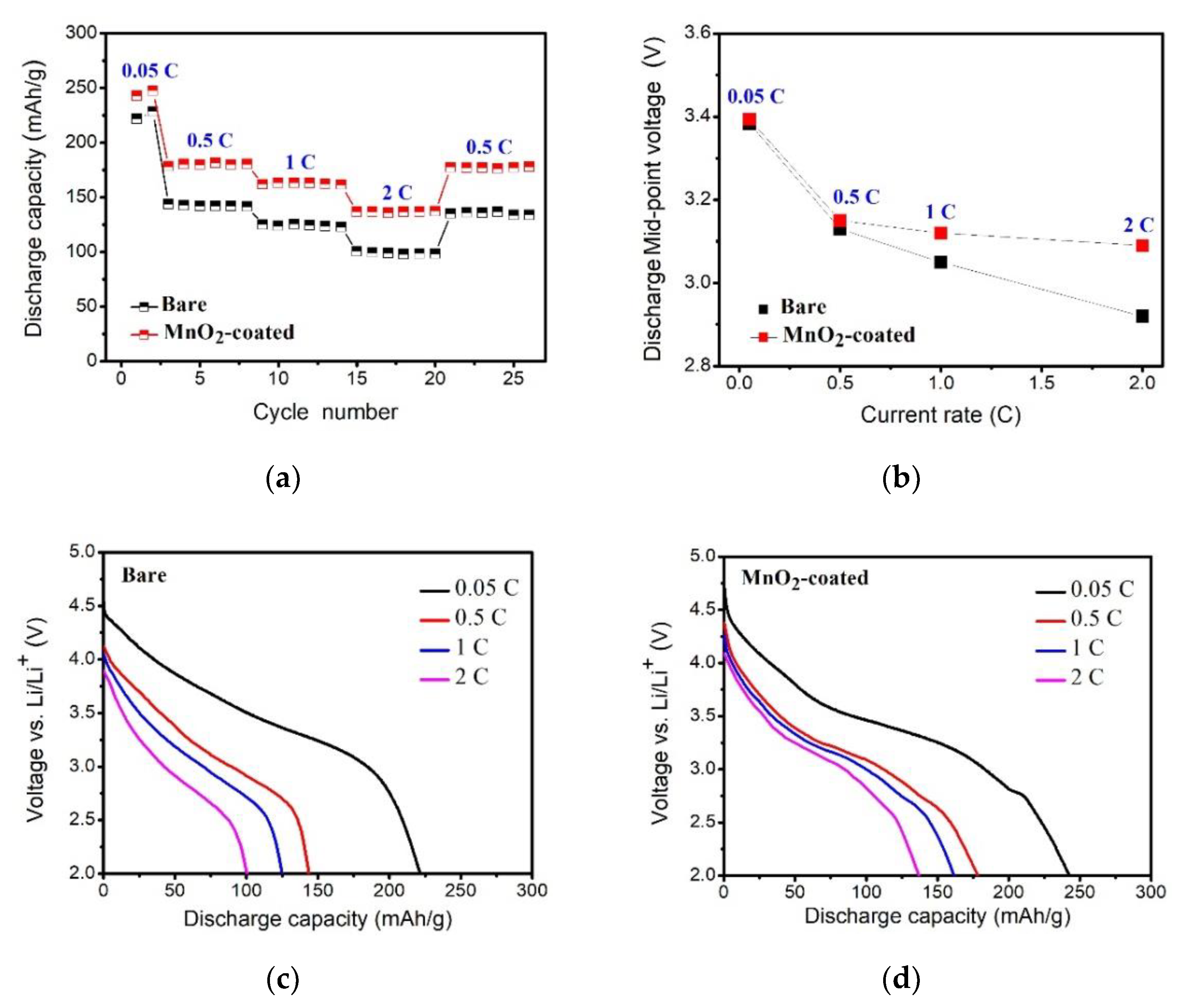
3.5. The Rate Capabilities of Bare and MnO2-Coated LTMO
3.6. The EIS Measurements of Bare and MnO2-Coated LTMO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, C.; Liu, D.; Lochala, J.A.; Anderson, C.S.; Cao, X.; Gross, M.E.; Xu, W.; Zhang, J.-G.; Whittingham, M.S.; Xiao, J.; et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 2021, 6, 723–732. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Sun, H.H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ. Sci. 2021, 14, 844–852. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface Modification of Li-Rich Mn-Based Layered Oxide Cathodes: Challenges, Materials, Methods, and Characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, C.; Yang, K.; Cao, B.; Xu, S.; Yang, N.; Zhao, W.; Chen, H.; Zhang, M.; et al. Recent progress in Li and Mn rich layered oxide cathodes for Li-ion batteries. J. Energy Chem. 2021, 61, 368–385. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.; Noh, J.-K.; Yu, S.; Kim, S.-J.; Chang, W.; Choi, W.C.; Chung, K.Y.; Cho, B.-W. Synthesis of layered–layered xLi2MnO3·(1−x)LiMO2 (M = Mn, Ni, Co) nanocomposite electrodes materials by mechanochemical process. J. Power Sources 2012, 220, 422–429. [Google Scholar] [CrossRef]
- He, L.; Xu, J.; Han, T.; Han, H.; Wang, Y.; Yang, J.; Wang, J.; Zhu, W.; Zhang, C.; Zhang, Y. SmPO4-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a cathode material with enhanced cycling stability for lithium ion batteries. Ceram. Int. 2017, 43, 5267–5273. [Google Scholar] [CrossRef]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 2005937. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Xue, Q.; Dai, Y.; Zhou, H.; Wang, X.; Kang, F. Elevated electrochemical performance of (NH4)3AlF6-coated 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material via a novel wet coating method. Electrochim. Acta 2014, 117, 41–47. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2016, 7, 1601284. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, E.; Guo, L.; Shi, C.; He, C.; Li, J.; Zhao, N. Cycle performance improvement of Li-rich layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by ZrO2 coating. Surf. Coat. Technol. 2013, 235, 570–576. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Y.; Huang, J.; Fang, X.; Wang, T.; Liu, W.; Wang, Y.; Jin, Y.; Tang, X. Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with Li+-conductive Li2SiO3 for lithium ion batteries. J. Alloys Compd. 2017, 724, 991–999. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshii, K.; Myung, S.T.; Nakai, I.; Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 2011, 133, 4404–4419. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, J.; Li, Q.; Wang, X.; Huang, M.; Liu, Z.; Han, C.; Mai, L. Novel MOF shell-derived surface modification of Li-rich layered oxide cathode for enhanced lithium storage. Sci. Bull. 2018, 63, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Li, J.; Deng, F.; Xu, G.; Liu, Y.; Yang, K.; Kang, F. Surface Heterostructure Induced by PrPO4 Modification in Li1.2[Mn0.54Ni0.13Co0.13]O2 Cathode Material for High-Performance Lithium-Ion Batteries with Mitigating Voltage Decay. ACS Appl. Mater. Interfaces 2017, 9, 27936–27945. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Zhang, P.; Cooper, A.J.; Abdelkader, A.M.; Ren, X.; Deng, L. Mesoporous Li1.2Mn0.54Ni0.13Co0.13O2 nanotubes for high-performance cathodes in Li-ion batteries. J. Power Sources 2016, 311, 35–41. [Google Scholar] [CrossRef]
- Lou, M.; Zhong, H.; Yu, H.-T.; Fan, S.-S.; Xie, Y.; Yi, T.-F. Li1.2Mn0.54Ni0.13Co0.13O2 hollow hierarchical microspheres with enhanced electrochemical performances as cathode material for lithium-ion battery application. Electrochim. Acta 2017, 237, 217–226. [Google Scholar] [CrossRef]
- Deng, B.; Lin, Z.; Chen, Y.; He, W.; Wang, J.; Xie, Q.; Wang, L.; Peng, D.-L. Preparation of porous Li1.2Mn0.54Ni0.13Co0.13O2 micro-cubes for high-capacity lithium-ion batteries. J. Alloys Compd. 2020, 834, 155152. [Google Scholar] [CrossRef]
- Lou, M.; Fan, S.-S.; Yu, H.-T.; Xie, Y.; Zhang, Q.; Zhu, Y.-R.; Yi, T.-F.; Tian, G.-H. Mg-doped Li1.2Mn0.54Ni0.13Co0.13O2 nano flakes with improved electrochemical performance for lithium-ion battery application. J. Alloys Compd. 2018, 739, 607–615. [Google Scholar] [CrossRef]
- Li, H.; Jian, Z.; Yang, P.; Li, J.; Xing, Y.; Zhang, S. Niobium doping of Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials with enhanced structural stability and electrochemical performance. Ceram. Int. 2020, 46, 23773–23779. [Google Scholar] [CrossRef]
- Xu, L.; Meng, J.; Yang, P.; Xu, H.; Zhang, S. Cesium-doped layered Li1.2Mn0.54Ni0.13Co0.13O2 cathodes with enhanced electrochemical performance. Solid State Ion. 2021, 361, 115551. [Google Scholar] [CrossRef]
- Jafta, C.J.; Ozoemena, K.I.; Mathe, M.K.; Roos, W.D. Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery. Electrochim. Acta 2012, 85, 411–422. [Google Scholar] [CrossRef]
- Ghorbanzadeh, M.; Allahyari, E.; Riahifar, R.; Hadavi, S.M.M. Effect of Al and Zr co-doping on electrochemical performance of cathode Li[Li0.2Ni0.13Co0.13Mn0.54]O2 for Li-ion battery. J. Solid State Electrochem. 2017, 22, 1155–1163. [Google Scholar] [CrossRef]
- Wu, Y.; Manthiram, A. High Capacity, Surface-Modified Layered Li[Li(1−x)/3Mn(2−x)/3Nix/3Cox/3]O2 Cathodes with Low Irreversible Capacity Loss. Electrochem. Solid-State Lett. 2006, 9, A221–A224. [Google Scholar] [CrossRef]
- Şahan, H.; Göktepe, H.; Patat, Ş.; Yıldız, S.; Özdemir, B.; Ülgen, A.; Mukerjee, S.; Abraham, K.M. Effect of silver coating on electrochemical performance of 0.5Li2MnO3.0.5 LiMn1/3Ni1/3Co1/3O2 cathode material for lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 1593–1604. [Google Scholar] [CrossRef]
- Zheng, J.M.; Li, J.; Zhang, Z.R.; Guo, X.J.; Yang, Y. The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery. Solid State Ion. 2008, 179, 1794–1799. [Google Scholar] [CrossRef]
- Wu, Y.; Ming, J.; Zhuo, L.; Yu, Y.; Zhao, F. Simultaneous surface coating and chemical activation of the Li-rich solid solution lithium rechargeable cathode and its improved performance. Electrochim. Acta 2013, 113, 54–62. [Google Scholar] [CrossRef]
- Shi, S.J.; Tu, J.P.; Tang, Y.Y.; Liu, X.Y.; Zhang, Y.Q.; Wang, X.L.; Gu, C.D. Enhanced cycling stability of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification of MgO with melting impregnation method. Electrochim. Acta 2013, 88, 671–679. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Zhai, H.-F.; Qian, X.; Wang, M.; Wang, Q.-Z.; Li, A.-D.; Li, H.; Zhou, F. Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with ultrathin ZnO. J. Alloys Compd. 2017, 694, 848–856. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Yu, T.; Ding, F.; Xu, G.; Li, Z.; Zhao, Y.; Kang, F. Stabilizing the structure and suppressing the voltage decay of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 cathode materials for Li-ion batteries via multifunctional Pr oxide surface modification. Ceram. Int. 2016, 42, 18620–18630. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, H.; Tang, T.; Du, W.; Gao, M.; Liu, Y.; Jian, D.; Pan, H. In Situ Encapsulation of the Nanoscale Er2O3 Phase To Drastically Suppress Voltage Fading and Capacity Degradation of a Li- and Mn-Rich Layered Oxide Cathode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 33863–33875. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-D.; Yao, Z.-L.; Xu, J.; Tang, P.; Xiong, X. Surface-modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2 nanoparticles with LaF3 as cathode for Li-ion battery. Ionics 2017, 23, 549–558. [Google Scholar] [CrossRef]
- Geng, X.; Guo, H.; Wang, C.; Cheng, M.; Li, Y.; Zhang, H.; Huo, H. Surface modification of Li1.20Mn0.54Ni0.13Co0.13O2 cathode materials with SmF3 and the improved electrochemical properties. J. Mater. Sci.-Mater. Electron. 2018, 29, 19207–19218. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, F.; Li, L.; Chen, M.; Zhong, X.; Li, W.; Lu, L. Effect of Li3PO4 coating of layered lithium-rich oxide on electrochemical performance. J. Power Sources 2017, 341, 147–155. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, B.; Liu, J.; Kaliyappan, K.; Sun, Q.; Liu, Y.; Dadheech, G.; Balogh, M.P.; Yang, L.; Sham, T.-K.; et al. Highly stable Li1.2Mn0.54Co0.13Ni0.13O2 enabled by novel atomic layer deposited AlPO4 coating. Nano Energy 2017, 34, 120–130. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, S.; Shi, Z.; Xu, H.; Xu, H.; Deng, Y.; Zhang, Z.; Chen, G. The effects of persulfate treatment on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. J. Power Sources 2013, 221, 108–113. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Xue, Q.; Ren, X.; Yan, G.; Wang, X.; Kang, F. Enhanced oxygen reducibility of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material with mild acid treatment. J. Power Sources 2014, 248, 894–899. [Google Scholar] [CrossRef]
- Liu, X.; Huang, T.; Yu, A. A new, high energy rechargeable lithium ion battery with a surface-treated Li1.2Mn0.54Ni0.13Co0.13O2 cathode and a nano-structured Li4Ti5O12 anode. J. Alloys Compd. 2015, 648, 7–12. [Google Scholar] [CrossRef]
- Li, S.; Ji, J.; Li, Z.; Yan, L.; Jiang, W.; Ling, M.; Lin, Z.; Liang, C. Pre-activation and Defects Introduced via Citric Acid to Mitigate Capacity and Voltage Fading in Li-rich Cathode. Z. Anorg. Allg. Chem. 2020, 646, 1285–1291. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Tynan, B.; Sha, Z.; Huang, F.; Islam, M.S.; Zhang, J.; Rider, A.N.; Dai, L.; Chu, D.; et al. High-performance hierarchical MnO2/CNT electrode for multifunctional supercapacitors. Carbon 2021, 184, 504–513. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.-S.; Wang, J.; Tan, Y.; Bai, L.; Liu, A. MnO2/multi-walled carbon nanotubes based nanocomposite with enhanced electrocatalytic activity for sensitive amperometric glucose biosensing. J. Electroanal. Chem. 2020, 878, 114602. [Google Scholar] [CrossRef]
- Huyan, Y.; Chen, J.; Yang, K.; Zhang, Q.; Zhang, B. Tailoring carboxyl tubular carbon nanofibers/MnO2 composites for high-performance lithium-ion battery anodes. J. Am. Ceram. Soc. 2020, 104, 1402–1414. [Google Scholar] [CrossRef]
- Kang, B.J.; Joo, J.-B.; Lee, J.K.; Choi, W. Surface modification of cathodes with nanosized amorphous MnO2 coating for high-power application in lithium-ion batteries. J. Electroanal. Chem. 2014, 728, 34–40. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Guo, R.; Liu, Z.; Meng, L.; Zheng, M.; Li, F.; Li, T.; Luo, Y.; Jiang, H. Amorphous MnO2-modified Li3V2(PO4)3/C as high-performance cathode for LIBs: The double effects of surface coating. J. Mater. Sci. 2017, 53, 2709–2724. [Google Scholar] [CrossRef]
- Guo, X.; Cong, L.-N.; Zhao, Q.; Tai, L.-H.; Wu, X.-L.; Zhang, J.-P.; Wang, R.-S.; Xie, H.-M.; Sun, L.-Q. Enhancement of electrochemical performance of LiNi1/3Co1/3Mn1/3O2 by surface modification with MnO2. J. Alloys Compd. 2015, 651, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, Z.; Wan, J.; Liu, S. Improved electrochemical properties of YF3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode for Li-ion batteries. Ionics 2017, 23, 1365–1374. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Wang, C.-L.; Qian, X.; Tai, G.-A.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F.; Yu, C.; Sun, Y.; et al. Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by surface modification with graphene-like lithium-active MoS2. Electrochim. Acta 2015, 174, 542–550. [Google Scholar] [CrossRef]



| Sample | a (Å) | c (Å) | c/a | I(003)/I(104) |
|---|---|---|---|---|
| Bare | 2.8501 | 14.2384 | 4.9958 | 2.24 |
| MnO2-coated | 2.8508 | 14.2364 | 4.9938 | 2.26 |
| Sample | Measured Atomic Ratio | ||
|---|---|---|---|
| Mn | Ni | Co | |
| Bare | 0.541 | 0.130 | 0.129 |
| MnO2-coated | 0.559 | 0.131 | 0.128 |
| Coating Material [References] | Initial Irreversible Capacity Loss (mAh/g) | Cyclic Retention | ||
|---|---|---|---|---|
| Bare Sample | Coated Sample | Bare Sample | Coated Sample | |
| This work MnO2 (3 wt %) | 78.2 | 46 | 85.4% (50 cycles) | 94.3% (50 cycles) |
| 71.1% (100 cycles) | 88.2% (100 cycles) | |||
| ZrO2 (1 wt %) [10] | ~70.3 | ~71.9 | 87.5% (50 cycles) | 94.9% (50 cycles) |
| Al2O3 (3 wt %) [23] | 75 | 41 | 89.5% (50 cycles) | 94% (50 cycles) |
| TiO2 (3 mol %) [25] | 75.5 | ~60 | 63% (90 cycles) | 87% (90 cycles) |
| MgO (2 wt %) [27] | 74.1 | 73.7 | 70.7% (100 cycles) | 96.4% (100 cycles) |
| ZnO (20 ALD layers) [28] | 77.5 | 50.8 | 85.3% (100 cycles) | 97.5% (100 cycles) |
| Pr6O11 (3 wt %) [29] | 73.1 | 47.8 | 79.2% (50 cycles) | 97.9% (50 cycles) |
| Er2O3 (4 wt %) [30] | 64 | 55 | 84% (300 cycles) | 89% (300 cycles) |
| Sample | Rs (Ω) | Rsf (Ω) | Rct (Ω) |
|---|---|---|---|
| Bare | 10.3 | 94.5 | 422.6 |
| MnO2-coated | 9.6 | 39.7 | 167.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yang, P.; Zheng, Z.; Pan, Q.; Liu, Y.; Li, Y.; Xuan, J. Exploring the Effect of a MnO2 Coating on the Electrochemical Performance of a Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material. Micromachines 2021, 12, 1410. https://doi.org/10.3390/mi12111410
Li Z, Yang P, Zheng Z, Pan Q, Liu Y, Li Y, Xuan J. Exploring the Effect of a MnO2 Coating on the Electrochemical Performance of a Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material. Micromachines. 2021; 12(11):1410. https://doi.org/10.3390/mi12111410
Chicago/Turabian StyleLi, Zhong, Peiyue Yang, Zhongxiang Zheng, Qiyun Pan, Yisi Liu, Yao Li, and Jinnan Xuan. 2021. "Exploring the Effect of a MnO2 Coating on the Electrochemical Performance of a Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material" Micromachines 12, no. 11: 1410. https://doi.org/10.3390/mi12111410
APA StyleLi, Z., Yang, P., Zheng, Z., Pan, Q., Liu, Y., Li, Y., & Xuan, J. (2021). Exploring the Effect of a MnO2 Coating on the Electrochemical Performance of a Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material. Micromachines, 12(11), 1410. https://doi.org/10.3390/mi12111410






