A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue
Abstract
:1. Introduction
2. Materials and Methods
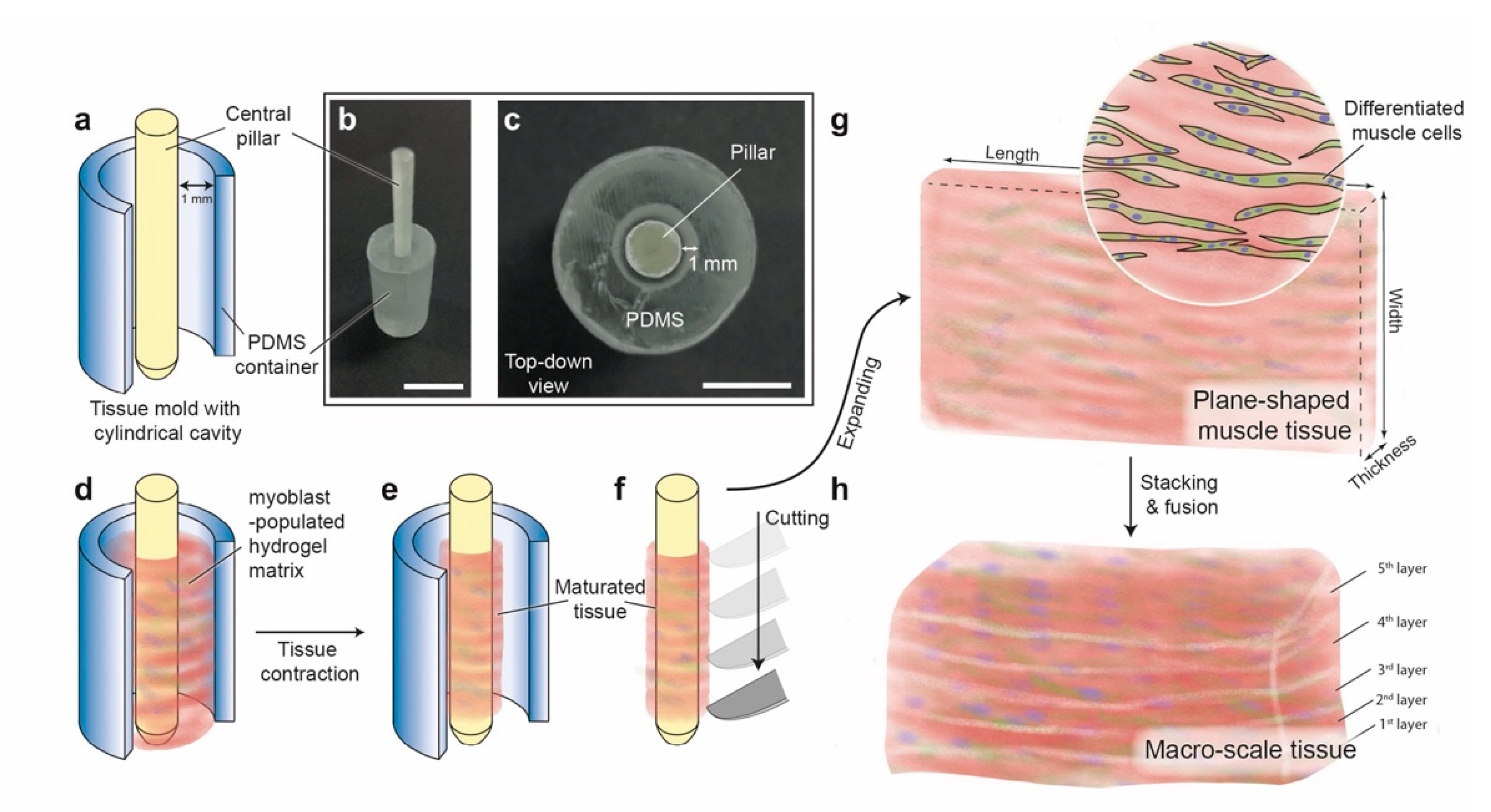
2.1. Design and Fabrication of the Tissue Mold
2.2. Cell Culture and Tissue Construction
2.3. Cell Distribution Analysis
2.4. Fluorescent Staining of Plane-Shaped Skeletal Muscle Tissue
2.5. Width and Thickness Measurement of the Tissue
2.6. Tissue Block Assembly by Stacking the Plane-Shaped Skeletal Muscle Tissues
2.7. Statistical Analysis
3. Results and Discussions
3.1. Shape and Homogeneity of the Biofabricated Plane-Shaped Skeletal Muscle Tissues
3.2. Optimization on the Hydrogel Composition to Facilitate Myogenic Differentiation
3.3. Assembly of Subcentimeter-Order Tissue Block by Stacking the Plane-Shaped Skeletal Muscle Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 2013, 10, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feric, N.T.; Pallotta, I.; Singh, R.; Bogdanowicz, D.R.; Gustilo, M.; Chaudhary, K.; Willette, R.N.; Chendrimada, T.; Xu, X.; Graziano, M.P.; et al. Engineered Cardiac Tissues Generated in the BiowireTM II: A Platform for Human-Based Drug Discovery. Toxicol. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Mori, S.; Sakai, F.; Takeuchi, S. Human induced pluripotent stem cell-derived fiber-shaped cardiac tissue on a chip. Lab Chip 2016, 16, 2295–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakar, M.S.; Neal, D.; Boudou, T.; Borochin, M.A.; Li, Y.; Weiss, R.; Kamm, R.D.; Chen, C.S.; Asada, H.H. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 2012, 12, 4976–4985. [Google Scholar] [CrossRef] [Green Version]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.A.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef] [Green Version]
- Raman, R.; Cvetkovic, C.; Uzel, S.G.M.; Platt, R.J.; Sengupta, P.; Kamm, R.D.; Bashir, R. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 2016, 113, 3497–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Onoe, H.; Takeuchi, S. Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci. Robot. 2018, 3, eaat4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Onoe, H.; Takeuchi, S. Biohybrid robot with skeletal muscle tissue covered with a collagen structure for moving in air. APL Bioeng. 2020, 4, 026101. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Fornetti, E.; Fuoco, C.; Sanchez Riera, C.; Nie, M.; Bernardini, S.; Rainer, A.; Baldi, J.; Zoccali, C.; et al. Biofabricating murine and human myo-substitutes for rapid volumetric muscle loss restoration. EMBO Mol. Med. 2021, 13, e12778. [Google Scholar] [CrossRef]
- Van Eelen, W.F.; Van Kooten, W.J.; Westerhof, W. Industrial Scale Production of Meat from In Vitro Cell Cultures. World Patent WO1999031222A1, 24 June 1999. [Google Scholar]
- Furuhashi, M.; Morimoto, Y.; Shima, A.; Nakamura, F.; Ishikawa, H.; Takeuchi, S. Formation of contractile 3D bovine muscle tissue for construction of millimetre-thick cultured steak. NPJ Sci. Food 2021, 5, 6. [Google Scholar] [CrossRef]
- Jo, B.; Nie, M.; Takeuchi, S. Manufacturing of animal products by the assembly of microfabricated tissues. Essays Biochem. 2021. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. “Printability” of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.G.; Kosnik, P.E., 2nd. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 327–335. [Google Scholar] [CrossRef]
- Khodabukus, A.; Baar, K. Regulating fibrinolysis to engineer skeletal muscle from the C2C12 cell line. Tissue Eng. Part C Methods 2009, 15, 501–511. [Google Scholar] [CrossRef]
- Lam, M.T.; Huang, Y.-C.; Birla, R.K.; Takayama, S. Microfeature guided skeletal muscle tissue engineering for highly organized 3-dimensional free-standing constructs. Biomaterials 2009, 30, 1150–1155. [Google Scholar] [CrossRef]
- Langen, R.C.J.; Schols, A.M.W.J.; Kelders, M.C.J.M.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W. Enhanced myogenic differentiation by extracellular matrix is regulated at the early stages of myogenesis. In Vitro Cell. Dev. Biol. Anim. 2003, 39, 163–169. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, M.; Shima, A.; Fukushima, K.; Morimoto, Y.; Takeuchi, S. A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue. Micromachines 2021, 12, 1411. https://doi.org/10.3390/mi12111411
Nie M, Shima A, Fukushima K, Morimoto Y, Takeuchi S. A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue. Micromachines. 2021; 12(11):1411. https://doi.org/10.3390/mi12111411
Chicago/Turabian StyleNie, Minghao, Ai Shima, Kenta Fukushima, Yuya Morimoto, and Shoji Takeuchi. 2021. "A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue" Micromachines 12, no. 11: 1411. https://doi.org/10.3390/mi12111411
APA StyleNie, M., Shima, A., Fukushima, K., Morimoto, Y., & Takeuchi, S. (2021). A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue. Micromachines, 12(11), 1411. https://doi.org/10.3390/mi12111411





