A Transit Time-Resolved Microflow Cytometry-Based Agglutination Immunoassay for On-Site C-Reactive Protein Detection
Abstract
:1. Introduction
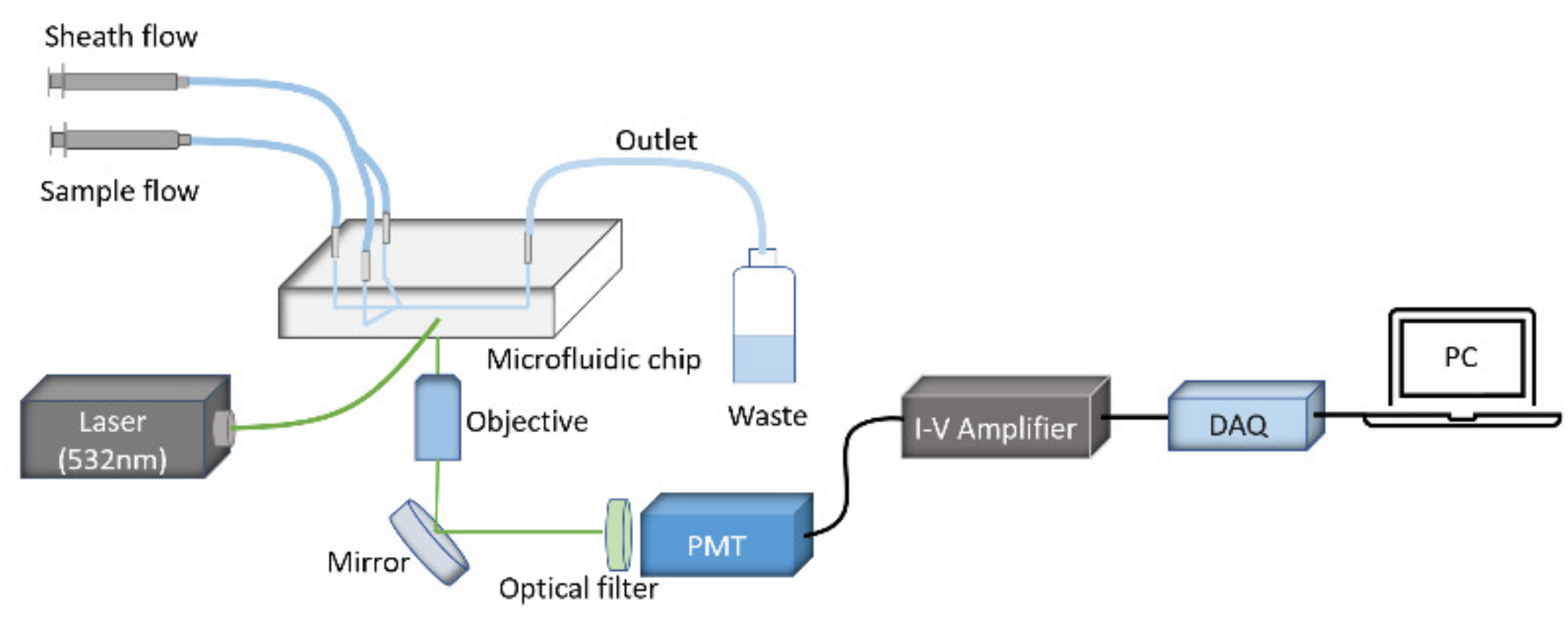
2. Materials and Methods
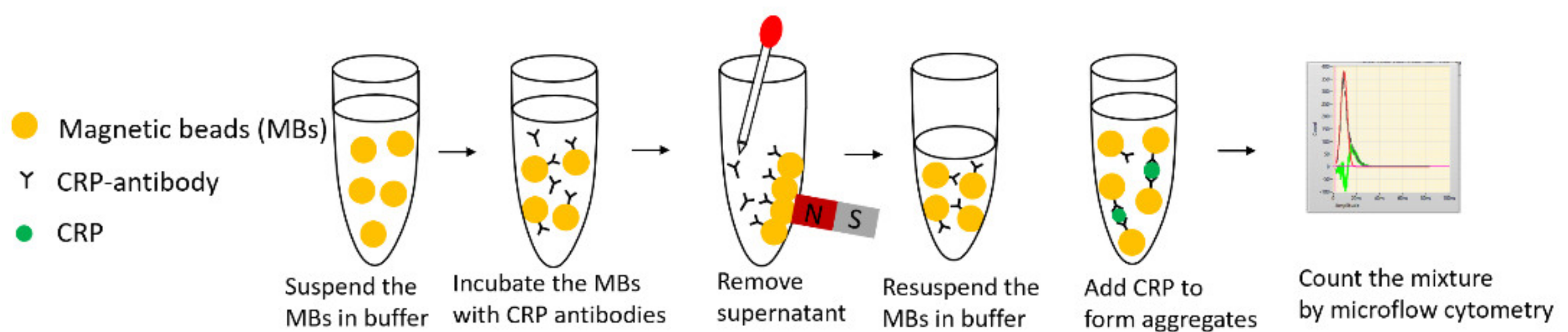
3. Procedure for Reaction
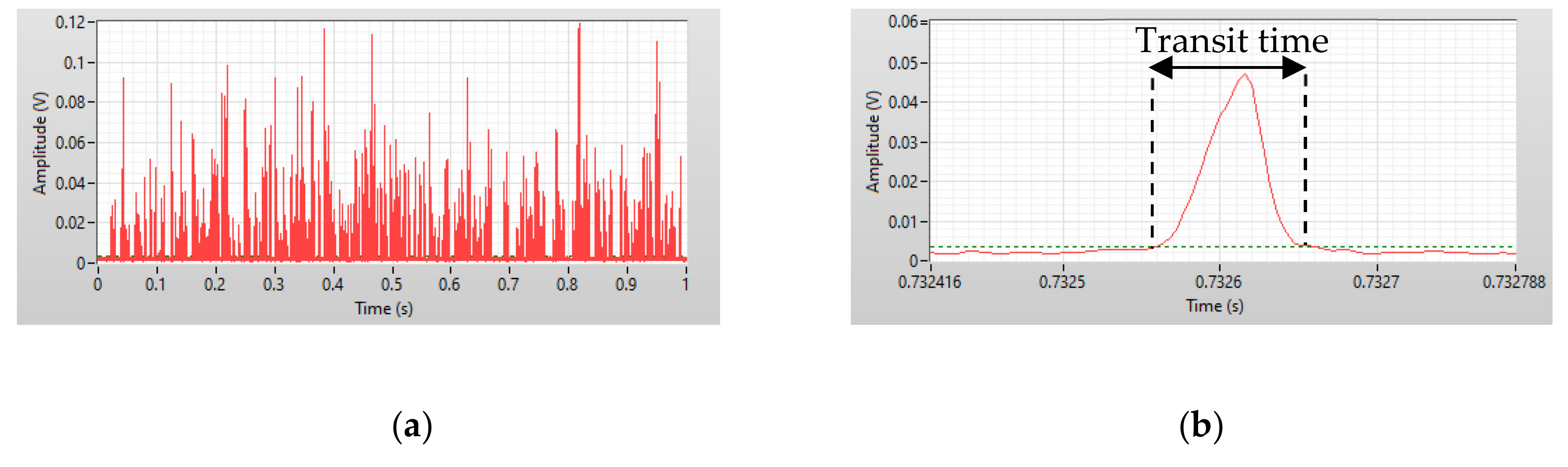
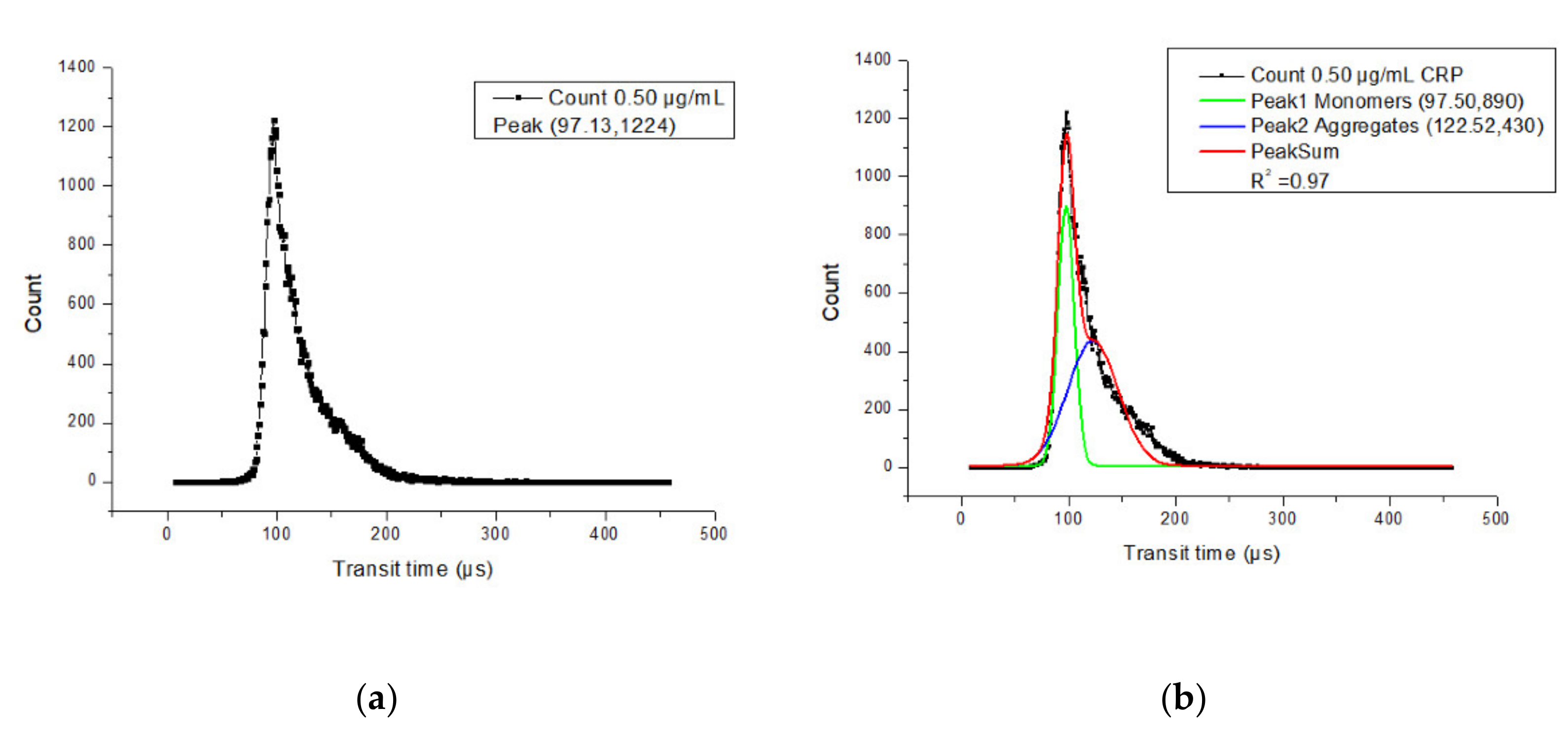
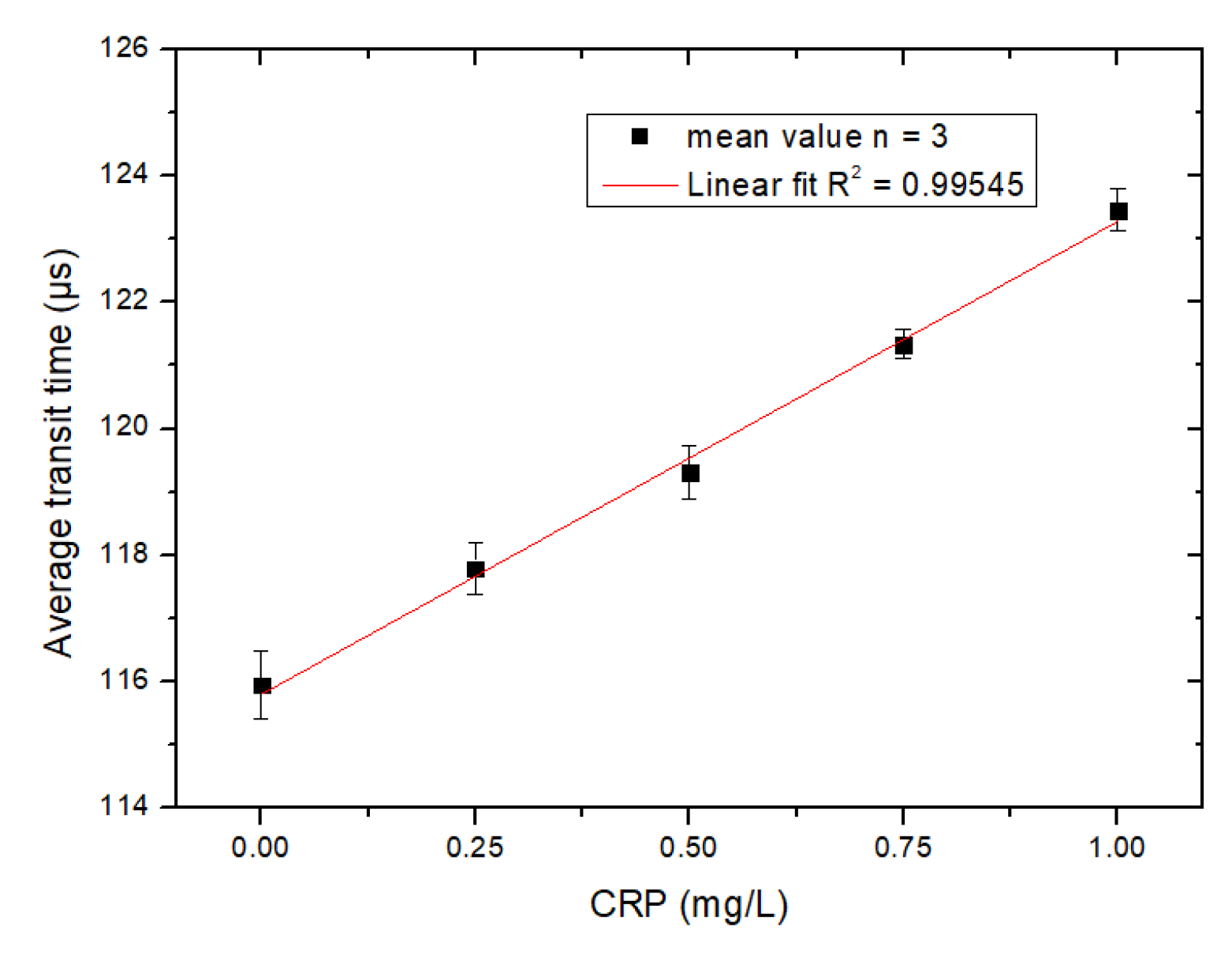
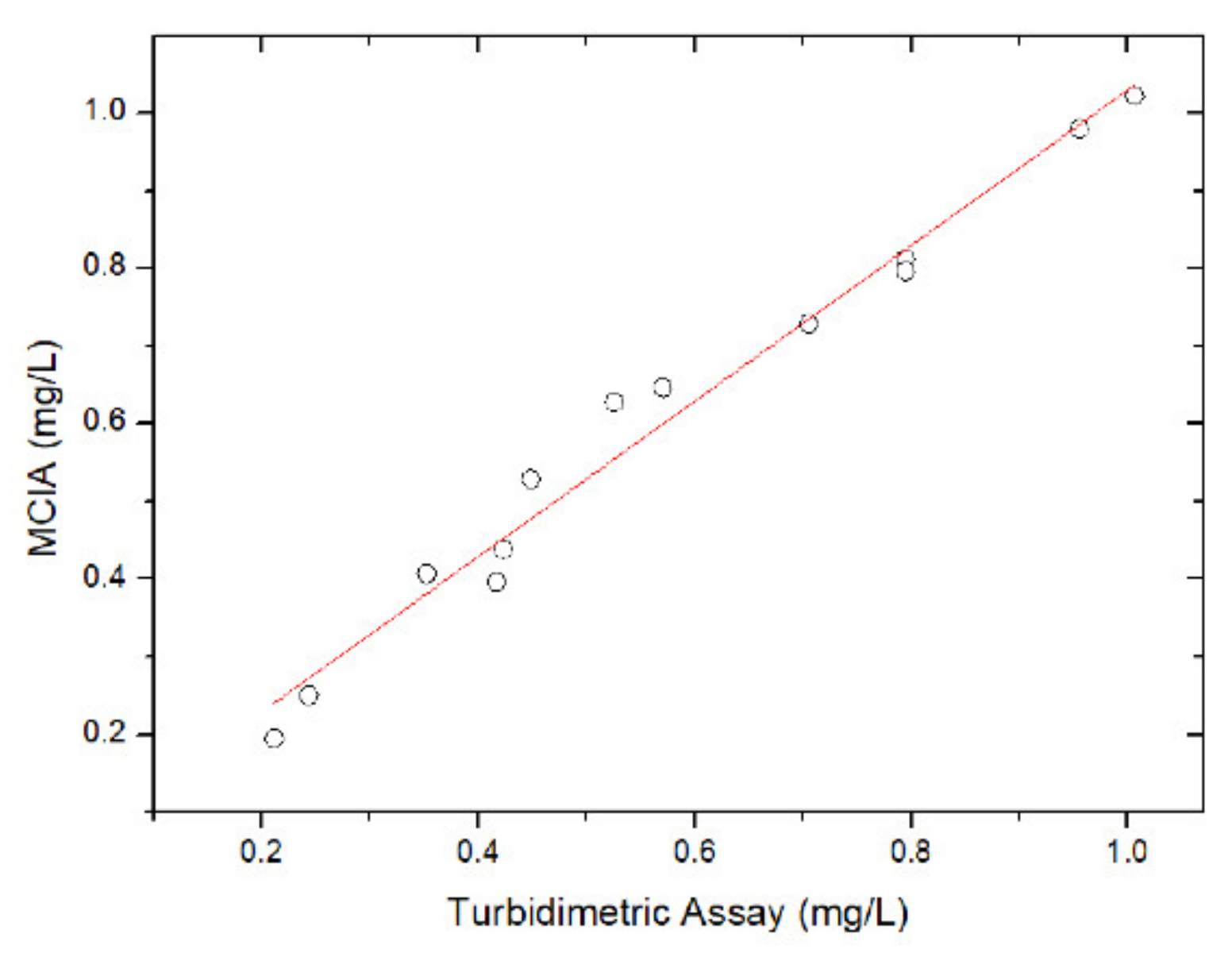
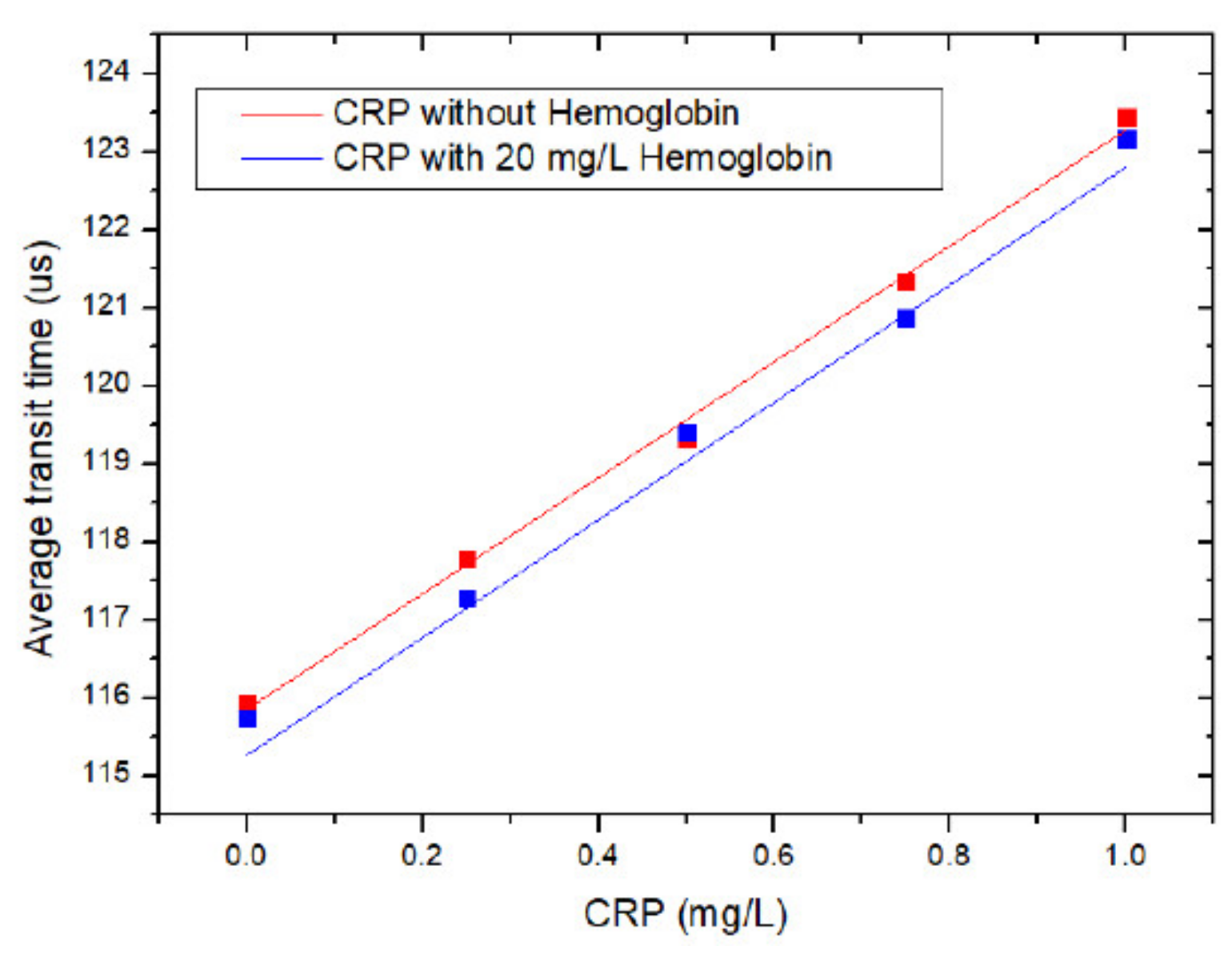
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cambiaso, C.L.; Leek, A.E.; De Steenwinkel, F.; Billen, J.; Masson, P.L. Particle Counting Immunoassay (Pacia). I. A General Method for the Determination of Antibodies, Antigens, and Haptens. J. Immunol. Methods 1977, 18, 33–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Watts, B.R.; Guo, T.; Zhang, Z.; Xu, C.; Fang, Q. Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review. Micromachines 2016, 7, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamme, N.; Koyama, R.; Manz, A. Counting and Sizing of Particles and Particle Agglomerates in a Microfluidic Device Using Laser Light Scattering: Application to a Particle-Enhanced Immunoassay. Lab Chip 2003, 3, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, P.; Cheng, Y.; Xie, S.; Zhang, S.; Ye, X. Homogeneous Agglutination Assay Based on Micro-Chip Sheathless Flow Cytometry. Biomicrofluidics 2015, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashist, S.K.; Venkatesh, A.G.; Marion Schneider, E.; Beaudoin, C.; Luppa, P.B.; Luong, J.H.T. Bioanalytical Advances in Assays for C-Reactive Protein. Biotechnol. Adv. 2016, 34, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A Label-Free Fiber Optic SPR Biosensor for Specific Detection of C-Reactive Protein. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The C-Reactive Protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Matas, A.J.; Gillingham, K.J.; Payne, W.D.; Najarian, J.S.; Pepys, M.B.; Hirschfield, G.M.; Nickeleit, V.; Mihatsch, M.J.; Schwedler, S.B.; Meier-Kriesche, H.-U.; et al. Dissociation of Pentameric to Monomeric C-Reactive Protein on Activated Platelets Localizes Inflammation to Atherosclerotic Plaques. Circulation 2014, 366, 857–859. [Google Scholar]
- Jia, R.F.; Li, L.; Li, H.; Cao, X.J.; Ruan, Y.; Meng, S.; Wang, J.Y.; Jin, Z.N. Meta-Analysis of C-Reactive Protein and Risk of Angina Pectoris. Am. J. Cardiol. 2020, 125, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-Reactive Protein, Inflammation and Coronary Heart Disease. Egypt. Hear. J. 2015, 67, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, D.; Badawi, A. C-Reactive Protein as a Biomarker of Severe H1N1 Influenza. Inflamm. Res. 2019, 68, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Xiao, Y.; Wu, J.; Gan, L.; Huang, Y.; Wang, J. C-Reactive Protein and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, M.; Khalaj, M.R.; Zareizadeh, M.; Najafipour, F. Relationship between High-Sensitivity C-Reactive Protein and Components of Metabolic Syndrome. J. Res. Med. Dent. Sci. 2018, 6, 7–11. [Google Scholar]
- Ablij, H.C.; Meinders, A.E. C-Reactive Protein: History and Revival. Eur. J. Intern. Med. 2002, 13, 412–422. [Google Scholar] [CrossRef]
- Watts, B.R.; Kowpak, T.; Zhang, Z.; Xu, C.Q.; Zhu, S.; Cao, X.; Lin, M. Fabrication and Performance of a Photonic-Microfluidic Integrated Device. Micromachines 2012, 3, 62–77. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Wei, Y.; Xu, C.; Watts, B.R.; Zhang, Z.; Fang, Q.; Zhang, H.; Selvaganapathy, P.R.; Deen, M.J. Counting of Escherichia Coli by a Microflow Cytometer Based on a Photonic-Microfluidic Integrated Device. Electrophoresis 2015, 36, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri Fernando, S.; Wilson, G.S. Studies of the “hook” Effect in the One-Step Sandwich Immunoassay. J. Immunol. Methods 1992, 151, 47–66. [Google Scholar] [CrossRef]
- Azman, W.N.W.; Omar, J.; Koon, T.S.; Ismail, T.S.T. Hemolyzed Specimens: Major Challenge for Identifying and Rejecting Specimens in Clinical Laboratories. Oman Med. J. 2019, 34, 94–98. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Zhang, Y.; Chenier, M.; Xu, C.-q.; Chen, L.; Wan, Y. A Transit Time-Resolved Microflow Cytometry-Based Agglutination Immunoassay for On-Site C-Reactive Protein Detection. Micromachines 2021, 12, 109. https://doi.org/10.3390/mi12020109
Qu J, Zhang Y, Chenier M, Xu C-q, Chen L, Wan Y. A Transit Time-Resolved Microflow Cytometry-Based Agglutination Immunoassay for On-Site C-Reactive Protein Detection. Micromachines. 2021; 12(2):109. https://doi.org/10.3390/mi12020109
Chicago/Turabian StyleQu, Jianxi, Yushan Zhang, Mathieu Chenier, Chang-qing Xu, Lan Chen, and Yonghong Wan. 2021. "A Transit Time-Resolved Microflow Cytometry-Based Agglutination Immunoassay for On-Site C-Reactive Protein Detection" Micromachines 12, no. 2: 109. https://doi.org/10.3390/mi12020109





