Recent Progress on Stability and Thermo-Physical Properties of Mono and Hybrid towards Green Nanofluids
Abstract
:1. Development of Nanofluids using Green Technology
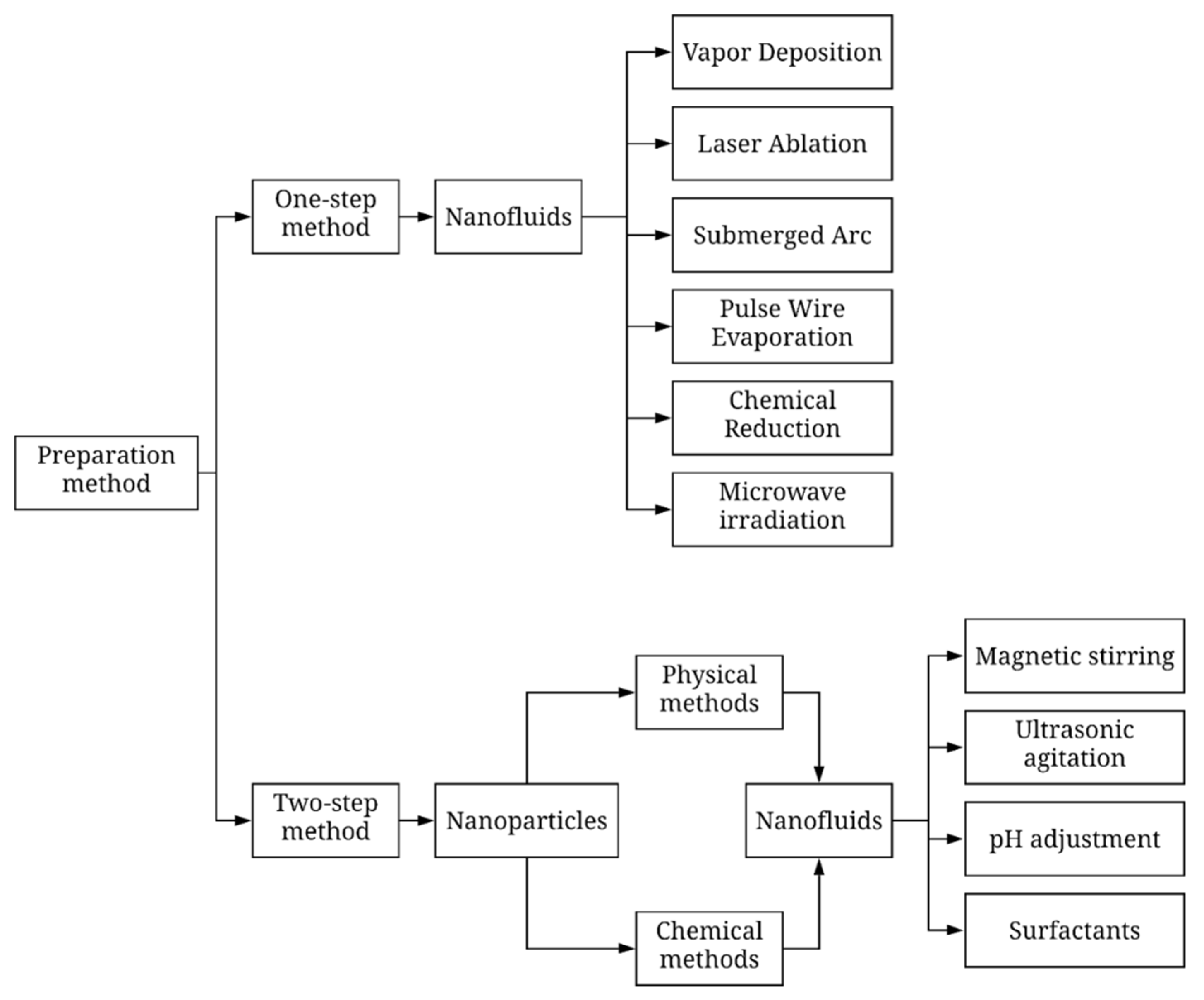
2. Preparation of Nanofluids
2.1. One-Step Method
2.2. Two-Step Method
3. Stability of Nanofluids
3.1. Stability Improvement Methods
3.2. Stability Evaluation Methods
3.2.1. Visual Observation
3.2.2. Micrograph and Imaging Observation
3.2.3. Zeta Potential Analysis
3.2.4. Ultraviolet-Visible Spectroscopy
4. Thermo-Physical Properties of Nanofluids
4.1. Thermal Conductivity
4.1.1. Effect of Particle Concentrations
4.1.2. Effect of Temperature
4.1.3. Effect of Size and Shape
4.2. Dynamic Viscosity
4.2.1. Effect of Particle Concentration and Temperature
4.2.2. Effect of Size and Shape
4.3. Density
4.4. Specific Heat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colangelo, G.; Favale, E.; Milanese, M.; de Risi, A.; Laforgia, D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017, 127, 421–435. [Google Scholar] [CrossRef]
- Visconti, P.; Primiceri, P.; Costantini, P.; Colangelo, G.; Cavalera, G. Measurement and control system for thermosolar plant and performance comparison between traditional and nanofluid solar thermal collectors. Int. J. Smart Sens. Intell. Syst. 2016, 9, 1220–1242. [Google Scholar] [CrossRef] [Green Version]
- Abdolbaqi, M.; Azmi, W.; Mamat, R.; Mohamed, N.; Najafi, G. Experimental investigation of turbulent heat transfer by counter and co-swirling flow in a flat tube fitted with twin twisted tapes. Int. Commun. Heat Mass Transf. 2016, 75, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Singh, V.; Kumar, S.; Kumar, S.; Dilbaghi, N.; Said, Z. Up to date review on the synthesis and thermophysical properties of hybrid nanofluids. J. Clean. Prod. 2018, 190, 169–192. [Google Scholar] [CrossRef]
- Colangelo, G.; Milanese, M.; De, R.A. Numerical simulation of thermal efficiency of an innovative Al2O3 nanofluid solar thermal collector: Influence of nanoparticles concentration. Therm. Sci. 2017, 21, 2769–2779. [Google Scholar] [CrossRef]
- Potenza, M.; Milanese, M.; Colangelo, G.; de Risi, A. Experimental investigation of transparent parabolic trough collector based on gas-phase nanofluid. Appl. Energy 2017, 203, 560–570. [Google Scholar] [CrossRef]
- Maksimović, M.; Omanović-Mikličanin, E. Towards green nanotechnology: Maximizing benefits and minimizing harm. In CMBEBIH 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 164–170. [Google Scholar]
- Ke, T.T.H.A. The National Green Technology Policy; Ministry of Energy, Technology, and Water Ministry of Energy: Putrajaya, Malaysia, 2009; Volume 1.
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Chapter 5—Green Nanotechnology. In Interface Science and Technology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 145–198. [Google Scholar]
- Sadri, R.; Kamali, K.Z.; Hosseini, M.; Zubir, N.; Kazi, S.N.; Ahmadi, G.; Dahari, M.; Huang, N.M.; Golsheikh, A.M. Experimental study on thermo-physical and rheological properties of stable and green reduced graphene oxide nanofluids: Hydrothermal assisted technique. J. Dispers. Sci. Technol. 2017, 38, 1302–1310. [Google Scholar] [CrossRef]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.K.; Kulkarni, R.R.; Thilakavathy, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [Green Version]
- Das, D.K.; Chakraborty, A.; Bhattacharjee, S.; Dey, S. Biosynthesis of stabilised gold nanoparticle using an aglycone flavonoid, quercetin. J. Exp. Nanosci. 2012, 8, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Narchin, F.; Larijani, K.; Rustaiyan, A.; Ebrahimi, S.N.; Tafvizi, F. Phytochemical Synthesis of Silver Nanoparticles by Two Techniques Using Saturaja rechengri Jamzad Extract: Identifying and Comparing in Vitro Anti-Proliferative Activities. Adv. Pharm. Bull. 2018, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, E.Y.; Lee, J. Non-toxic nanoparticles from phytochemicals: Preparation and biomedical application. Bioprocess. Biosyst. Eng. 2013, 37, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Bahiraei, M.; Naghibzadeh, S.M.; Jamshidmofid, M. Efficacy of an eco-friendly nanofluid in a miniature heat exchanger regarding to arrangement of silver nanoparticles. Energy Convers. Manag. 2017, 144, 224–234. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Stephen, A.; Seethalakshmi, S. Phytochemical Synthesis and Preliminary Characterization of Silver Nanoparticles Using Hesperidin. J. Nanosci. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Kazerouni, A.M.; Bakhtiari, R.; Asadi, A.; Afrand, M. An experimental study on stability and thermal conductivity of water/silica nanofluid: Eco-friendly production of nanoparticles. J. Clean. Prod. 2019, 206, 1089–1100. [Google Scholar] [CrossRef]
- Khdher, A.M.; Azwadi, C.S.N.; Hamzah, W.A.W.; Mamat, R. An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al 2 O 3 nanofluids and development of new correlation. Int. Commun. Heat Mass Transf. 2016, 73, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Abdolbaqi, M.; Azmi, W.; Mamat, R.; Sharma, K.; Najafi, G. Experimental investigation of thermal conductivity and electrical conductivity of BioGlycol–water mixture based Al2O3 nanofluid. Appl. Therm. Eng. 2016, 102, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Abdolbaqi, M.; Sidik, N.A.C.; Aziz, A.; Mamat, R.; Azmi, W.H.; Yazid, M.N.A.W.M.; Najafi, G. An experimental determination of thermal conductivity and viscosity of BioGlycol/water based TiO2 nanofluids. Int. Commun. Heat Mass Transf. 2016, 77, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Abdolbaqi, M.; Sidik, N.A.C.; Rahim, M.F.A.; Mamat, R.; Azmi, W.; Yazid, M.N.A.W.M.; Najafi, G. Experimental investigation and development of new correlation for thermal conductivity and viscosity of BioGlycol/water based SiO2 nanofluids. Int. Commun. Heat Mass Transf. 2016, 77, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Rani, K.; Sridevi, V. An overview on role of nanotechnology in green and clean technology. Austin Environ. Sci. 2017, 2, 1026. [Google Scholar]
- Mohammadpoor, M.; Sabbaghi, S.; Zerafat, M.; Manafi, Z. Investigating heat transfer properties of copper nanofluid in ethylene glycol synthesized through single and two-step routes. Int. J. Refrig. 2019, 99, 243–250. [Google Scholar] [CrossRef]
- De Oliveira, L.R.; Ribeiro, S.R.F.L.; Reis, M.H.M.; Cardoso, V.L.; Filho, E.P.B. Experimental study on the thermal conductivity and viscosity of ethylene glycol-based nanofluid containing diamond-silver hybrid material. Diam. Relat. Mater. 2019, 96, 216–230. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Sharma, K. Experimental measurements of thermal conductivity and viscosity of ethylene glycol-based hybrid nanofluid with TiO2-CuO/C inclusions. J. Mol. Liq. 2017, 246, 396–405. [Google Scholar] [CrossRef]
- Nikkam, N.; Ghanbarpour, M.; Khodabandeh, R.; Toprak, M.S. The effect of particle size and base liquid on thermo-physical properties of ethylene and diethylene glycol based copper micro- and nanofluids. Int. Commun. Heat Mass Transf. 2017, 86, 143–149. [Google Scholar] [CrossRef]
- Dalkilic, A.S.; Açıkgöz, Ö.; Küçükyıldırım, B.O.; Eker, A.A.; Lüleci, B.; Jumpholkul, C.; Wongwises, S. Experimental investigation on the viscosity characteristics of water based SiO2-graphite hybrid nanofluids. Int. Commun. Heat Mass Transf. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Moldoveanu, G.M.; Huminic, G.; Minea, A.A.; Huminic, A. Experimental study on thermal conductivity of stabilized Al2O3 and SiO2 nanofluids and their hybrid. Int. J. Heat Mass Transf. 2018, 127, 450–457. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Bikić, S.; Wanic, M. Ethylene glycol-based silicon nitride nanofluids: An experimental study on their thermophysical, electrical and optical properties. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 104, 82–90. [Google Scholar] [CrossRef]
- Maddah, H.; Aghayari, R.; Mirzaee, M.; Ahmadi, M.H.; Sadeghzadeh, M.; Chamkha, A.J. Factorial experimental design for the thermal performance of a double pipe heat exchanger using Al2O3-TiO2 hybrid nanofluid. Int. Commun. Heat Mass Transf. 2018, 97, 92–102. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ozkaymak, M.; Sözen, A.; Menlik, T.; Fahed, A. Improving car radiator performance by using TiO2-water nanofluid. Eng. Sci. Technol. Int. J. 2018, 21, 996–1005. [Google Scholar] [CrossRef]
- Esfe, M.H.; Esfandeh, S.; Amiri, M.K.; Afrand, M. A novel applicable experimental study on the thermal behavior of SWCNTs(60%)-MgO(40%)/EG hybrid nanofluid by focusing on the thermal conductivity. Powder Technol. 2019, 342, 998–1007. [Google Scholar] [CrossRef]
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Inst. Chem. Eng. 2019, 97, 458–465. [Google Scholar] [CrossRef]
- Graves, J.; Latvytė, E.; Greenwood, A.; Emekwuru, N. Ultrasonic preparation, stability and thermal conductivity of a capped copper-methanol nanofluid. Ultrason. Sonochem. 2019, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Alarifi, I.M.; Foong, L.K. An experimental study on characterization, stability and dynamic viscosity of CuO-TiO2/water hybrid nanofluid. J. Mol. Liq. 2020, 307, 112987. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Sidik, N.A.C.; Ken, T.L.; Mamat, R.; Najafi, G. Factors affecting the performance of hybrid nanofluids: A comprehensive review. Int. J. Heat Mass Transf. 2017, 115, 630–646. [Google Scholar] [CrossRef]
- Babu, J.R.; Kumar, K.K.; Rao, S.S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced Thermal Conductivity through the Development of Nanofluids. MRS Proc. 1996, 457, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Munkhbayar, B.; Tanshen, R.; Jeoun, J.; Chung, H.; Jeong, H.-M. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram. Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Angayarkanni, S.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Mao, M.; Huang, J.-N. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean. Prod. 2020, 257, 120408. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Manna, I. Synthesis, characterization and application of nanofluid—An overview. J. Indian Inst. Sci. 2012, 89, 21–33. [Google Scholar]
- Sezer, N.; Atieh, M.A.; Koç, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Nabil, M.; Azmi, W.H.; Hamid, K.A.; Mamat, R. Experimental investigation of heat transfer and friction factor of TiO2-SiO2 nanofluids in water:ethylene glycol mixture. Int. J. Heat Mass Transf. 2018, 124, 1361–1369. [Google Scholar] [CrossRef]
- Zhao, M.-W.; Lv, W.; Li, Y.; Dai, C.; Zhou, H.; Song, X.; Wu, Y. A Study on Preparation and Stabilizing Mechanism of Hydrophobic Silica Nanofluids. Materials 2018, 11, 1385. [Google Scholar] [CrossRef] [Green Version]
- Hamid, K.A.; Azmi, W.; Mamat, R.; Sharma, K. Heat transfer performance of TiO2–SiO2 nanofluids in a tube with wire coil inserts. Appl. Therm. Eng. 2019, 152, 275–286. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.; Mamat, R. Experimental investigation of nanoparticle mixture ratios on TiO2–SiO2 nanofluids heat transfer performance under turbulent flow. Int. J. Heat Mass Transf. 2018, 118, 617–627. [Google Scholar] [CrossRef]
- Azmi, W.H.; Hamid, K.A.; Usri, N.A.; Mamat, R.; Mohamad, M. Heat transfer and friction factor of water and ethylene glycol mixture based TiO 2 and Al 2 O 3 nanofluids under turbulent flow. Int. Commun. Heat Mass Transf. 2016, 76, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Azmi, W.; Hamid, K.A.; Usri, N.; Mamat, R.; Sharma, K. Heat transfer augmentation of ethylene glycol: Water nanofluids and applications—A review. Int. Commun. Heat Mass Transf. 2016, 75, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Zawawi, N.; Azmi, W.; Redhwan, A.; Sharif, M.; Sharma, K. Thermo-physical properties of Al2O3-SiO2/PAG composite nanolubricant for refrigeration system. Int. J. Refrig. 2017, 80, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Redhwan, A.; Azmi, W.; Sharif, M.; Mamat, R. Development of nanorefrigerants for various types of refrigerant based: A comprehensive review on performance. Int. Commun. Heat Mass Transf. 2016, 76, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; de Risi, A. A critical analysis of clustering phenomenon in Al2O3 nanofluids. J. Therm. Anal. Calorim. 2019, 135, 371–377. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Jamil, M.M.; Japar, W.M.A.A.; Adamu, I.M. A review on preparation methods, stability and applications of hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 80, 1112–1122. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al 2 O 3 -diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Nithila, S.R.; Anandkumar, B.; Vanithakumari, S.; George, R.; Mudali, U.K.; Dayal, R. Studies to control biofilm formation by coupling ultrasonication of natural waters and anodization of titanium. Ultrason. Sonochem. 2014, 21, 189–199. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An overview on the effect of ultrasonication duration on different properties of nanofluids. J. Therm. Anal. Calorim. 2018, 135, 393–418. [Google Scholar] [CrossRef]
- Kaur, I.; Ellis, L.-J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; ’Hermite, M.M.-L.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Protocol Optimization. J. Vis. Exp. 2017, 130, e56074. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Preparation, sedimentation, and agglomeration of nanofluids. Chem. Eng. Technol. 2014, 37, 2011–2021. [Google Scholar] [CrossRef]
- Nabil, M.; Azmi, W.; Hamid, K.A.; Mamat, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO 2 -SiO 2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Chen, Z.; Shahsavar, A.; Al-Rashed, A.A.; Afrand, M. The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: An experimental assessment. Powder Technol. 2020, 360, 1134–1142. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Effect of prolonged ultrasonication on the thermal conductivity of ZnO–ethylene glycol nanofluids. Thermochim. Acta 2012, 535, 58–65. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Saidur, R.; Amalina, M.; Elcioglu, E.; Okutucu-Ozyurt, T. Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrason. Sonochem. 2015, 26, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Mahbubul, I.; Elcioglu, E.B.; Saidur, R.; Amalina, M. Optimization of ultrasonication period for better dispersion and stability of TiO2–water nanofluid. Ultrason. Sonochem. 2017, 37, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Kamalgharibi, M.; Zamzamian, S.A.; Hormozi, F. Experimental study of the stability of deionized water based copper oxide nanofluid and achievement to the optimal stability conditions. Amirkabir J. Mech. Eng. 2015, 48, 17–30. [Google Scholar]
- Witharana, S.; Palabiyik, I.; Musina, Z.; Ding, Y. Stability of glycol nanofluids—The theory and experiment. Powder Technol. 2013, 239, 72–77. [Google Scholar] [CrossRef]
- Choudhary, R.; Khurana, D.; Kumar, A.; Subudhi, S. Stability analysis of Al2O3/water nanofluids. J. Exp. Nanosci. 2017, 12, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, I.; Sefid, M.; Afrand, M. A novel comparative experimental study on rheological behavior of mono & hybrid nanofluids concerned graphene and silica nano-powders: Characterization, stability and viscosity measurements. Powder Technol. 2020, 366, 216–229. [Google Scholar] [CrossRef]
- Leong, K.Y.; Razali, I.; Ahmad, K.K.; Ong, H.C.; Ghazali, M.; Rahman, M.R.A. Thermal conductivity of an ethylene glycol/water-based nanofluid with copper-titanium dioxide nanoparticles: An experimental approach. Int. Commun. Heat Mass Transf. 2018, 90, 23–28. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Mamat, R.; Sharma, K. Experimental investigation on heat transfer performance of TiO 2 nanofluids in water–ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2016, 73, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Azmi, W.H.; Hamid, K.A.; Mamat, R.; Sharma, K.; Mohamad, M. Effects of working temperature on thermo-physical properties and forced convection heat transfer of TiO 2 nanofluids in water—Ethylene glycol mixture. Appl. Therm. Eng. 2016, 106, 1190–1199. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.; Shabani, B.; Andrews, J.; Rosengarten, G. Experimental investigation of using ZnO nanofluids as coolants in a PEM fuel cell. Int. J. Hydrog. Energy 2017, 42, 19272–19286. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.; Mamat, R.; Sharma, K. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152. [Google Scholar] [CrossRef]
- Baer, D.R. Surface Characterization of Nanoparticles: Critical needs and significant challenges. J. Surf. Anal. 2011, 17, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Kumar, D.D.; Arasu, A.V. A comprehensive review of preparation, characterization, properties and stability of hybrid nanofluids. Renew. Sustain. Energy Rev. 2018, 81, 1669–1689. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Behera, D.K.; Pal, S.K.; Chakraborty, S. Experimental investigation on the effect of dispersant addition on thermal and rheological characteristics of TiO2 nanofluid. Powder Technol. 2017, 307, 10–24. [Google Scholar] [CrossRef]
- Chiam, H.W.; Azmi, W.H.; Usri, N.A.; Mamat, R.; Adam, N. Thermal conductivity and viscosity of Al 2 O 3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.M. Experimental studies on thermal and rheological properties of Al 2 O 3 –ethylene glycol nanofluid. Int. J. Refrig. 2018, 89, 122–130. [Google Scholar] [CrossRef]
- Mukherjee, S.; Paria, S. Preparation and stability of nanofluids—A review. IOSR J. Mech. Civil Eng. 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Chapter 3—Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 59–94. [Google Scholar] [CrossRef]
- Raja, P.M.V.; Barron, A.R. Physical Methods in Chemistry and Nano Science; Rice University: Houston, TX, USA, 2019. [Google Scholar]
- Safaei-Naeini, Y.; Aminzare, M.; Golestani-Fard, F.; Khorasanizadeh, F.; Salahi, E. Suspension stability of titania nanoparticles studied by UV-Vis spectroscopy method. Iran. J. Mater. Sci. Eng. 2012, 9, 62–68. [Google Scholar]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid. Nanofluidics 2014, 18, 1023–1030. [Google Scholar] [CrossRef]
- Yu, H.; Hermann, S.; Schulz, S.E.; Gessner, T.; Dong, Z.; Li, W.J. Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chem. Phys. 2012, 408, 11–16. [Google Scholar] [CrossRef]
- Gangadevi, R.; Vinayagam, B.; Senthilraja, S. Effects of sonication time and temperature on thermal conductivity of CuO/water and Al 2 O 3 /water nanofluids with and without surfactant. Mater. Today Proc. 2018, 5, 9004–9011. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C. Heat transfer and friction factor of nanodiamond-nickel hybrid nanofluids flow in a tube with longitudinal strip inserts. Int. J. Heat Mass Transf. 2018, 121, 390–401. [Google Scholar] [CrossRef]
- Akhgar, A.; Toghraie, D. An experimental study on the stability and thermal conductivity of water-ethylene glycol/TiO2-MWCNTs hybrid nanofluid: Developing a new correlation. Powder Technol. 2018, 338, 806–818. [Google Scholar] [CrossRef]
- Dalkilic, A.S.; Yalçın, G.; Küçükyıldırım, B.O.; Öztuna, S.; Eker, A.A.; Jumpholkul, C.; Nakkaew, S.; Wongwises, S. Experimental study on the thermal conductivity of water-based CNT-SiO2 hybrid nanofluids. Int. Commun. Heat Mass Transf. 2018, 99, 18–25. [Google Scholar] [CrossRef]
- Khairul, M.A.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of surfactant on stability and thermo-physical properties of metal oxide nanofluids. Int. J. Heat Mass Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Kumar, P.M.; Palanisamy, K.; Vijayan, V. Stability analysis of heat transfer hybrid/water nanofluids. Mater. Today Proc. 2020, 21, 708–712. [Google Scholar] [CrossRef]
- Sahooli, M.; Sabbaghi, S. Investigation of thermal properties of CuO nanoparticles on the ethylene glycol–water mixture. Mater. Lett. 2013, 93, 254–257. [Google Scholar] [CrossRef]
- Yasinskiy, A.; Navas, J.; Aguilar, T.; Alcántara, R.; Gallardo, J.J.; Sánchez-Coronilla, A.; Martín, E.I.; Santos, D.D.L.; Fernández-Lorenzo, C. Dramatically enhanced thermal properties for TiO2-based nanofluids for being used as heat transfer fluids in concentrating solar power plants. Renew. Energy 2018, 119, 809–819. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Takabi, B.; Salehi, S. Augmentation of the Heat Transfer Performance of a Sinusoidal Corrugated Enclosure by Employing Hybrid Nanofluid. Adv. Mech. Eng. 2014, 6, 147059. [Google Scholar] [CrossRef]
- Lim, S.; Azmi, W.; Yusoff, A. Investigation of thermal conductivity and viscosity of Al2O3/water–ethylene glycol mixture nanocoolant for cooling channel of hot-press forming die application. Int. Commun. Heat Mass Transf. 2016, 78, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Teng, T.-P.; Hung, Y.-H. Estimation and experimental study of the density and specific heat for alumina nanofluid. J. Exp. Nanosci. 2012, 9, 707–718. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharma, K.; Mamat, R.; Najafi, G.; Mohamad, M. The enhancement of effective thermal conductivity and effective dynamic viscosity of nanofluids—A review. Renew. Sustain. Energy Rev. 2016, 53, 1046–1058. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Ghajar, A.J. Heat and Mass Transfer: Fundamentals & Applications/Heat and Mass Transfer: Fundamentals & Applications, 4th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Das, P.K. A review based on the effect and mechanism of thermal conductivity of normal nanofluids and hybrid nanofluids. J. Mol. Liq. 2017, 240, 420–446. [Google Scholar] [CrossRef]
- Nnanna, A.G.A. Experimental Model of Temperature-Driven Nanofluid. J. Heat Transf. 2007, 129, 697–704. [Google Scholar] [CrossRef]
- Sundar, L.S.; Farooky, H.; Sarada, S.N.; Singh, M.K. Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int. Commun. Heat Mass Transf. 2013, 41, 41–46. [Google Scholar] [CrossRef]
- Esfahani, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO–Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Harandi, S.S.; Karimipour, A.; Afrand, M.; Akbari, M.; D’Orazio, A. An experimental study on thermal conductivity of F-MWCNTs–Fe 3 O 4 /EG hybrid nanofluid: Effects of temperature and concentration. Int. Commun. Heat Mass Transf. 2016, 76, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Madhesh, D.; Parameshwaran, R.; Kalaiselvam, S. Experimental investigation on convective heat transfer and rheological characteristics of Cu–TiO2 hybrid nanofluids. Exp. Therm. Fluid Sci. 2014, 52, 104–115. [Google Scholar] [CrossRef]
- Zadkhast, M.; Toghraie, D.; Karimipour, A. Developing a new correlation to estimate the thermal conductivity of MWCNT-CuO/water hybrid nanofluid via an experimental investigation. J. Therm. Anal. Calorim. 2017, 129, 859–867. [Google Scholar] [CrossRef]
- Esfe, M.H.; Behbahani, P.M.; Arani, A.A.A.; Sarlak, M.R. Thermal conductivity enhancement of SiO2–MWCNT (85:15%)–EG hybrid nanofluids. J. Therm. Anal. Calorim. 2017, 128, 249–258. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Montazer, E.; Arzani, H.K.; Sadri, R.; Dahari, M.; Kazi, S. Nanofluid based on activated hybrid of biomass carbon/graphene oxide: Synthesis, thermo-physical and electrical properties. Int. Commun. Heat Mass Transf. 2016, 72, 10–15. [Google Scholar] [CrossRef]
- Sharma, K.; Azmi, W.; Kamal, S.; Sarma, P.K.; Vijayalakshmi, B. Theoretical analysis of heat transfer and friction factor for turbulent flow of nanofluids through pipes. Can. J. Chem. Eng. 2016, 94, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Usri, N.; Azmi, W.; Mamat, R.; Hamid, K.A.; Najafi, G. Thermal Conductivity Enhancement of Al2O3 Nanofluid in Ethylene Glycol and Water Mixture. Energy Proc. 2015, 79, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Mostafizur, R.; Saidur, R.; Aziz, A.A.; Bhuiyan, M. Thermophysical properties of methanol based Al2O3 nanofluids. Int. J. Heat Mass Transf. 2015, 85, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Aparna, Z.; Michael, M.; Pabi, S.; Ghosh, S. Thermal conductivity of aqueous Al2O3/Ag hybrid nanofluid at different temperatures and volume concentrations: An experimental investigation and development of new correlation function. Powder Technol. 2019, 343, 714–722. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Parashar, S.K.S.; Chaudhuri, P. Role of temperature on thermal conductivity of nanofluids: A brief literature review. Heat Mass Transf. 2016, 52, 2575–2585. [Google Scholar] [CrossRef]
- Riahi, A.; Khamlich, S.; Balghouthi, M.; Khamliche, T.; Doyle, T.B.; Dimassi, W.; Guizani, A.; Maaza, M. Study of thermal conductivity of synthesized Al2O3-water nanofluid by pulsed laser ablation in liquid. J. Mol. Liq. 2020, 304, 112694. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; de Risi, A. An explanation of the Al2O3 nanofluid thermal conductivity based on the phonon theory of liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Shima, P.D.; Philip, J.; Raj, B. Synthesis of Aqueous and Nonaqueous Iron Oxide Nanofluids and Study of Temperature Dependence on Thermal Conductivity and Viscosity. J. Phys. Chem. C 2010, 114, 18825–18833. [Google Scholar] [CrossRef]
- Megatif, L.; Ghozatloo, A.; Arimi, A.; Shariati-Niasar, M. Investigation of Laminar Convective Heat Transfer of a Novel Tio2–Carbon Nanotube Hybrid Water-Based Nanofluid. Exp. Heat Transf. 2015, 29, 124–138. [Google Scholar] [CrossRef]
- Azmi, W.; Usri, N.; Mamat, R.; Sharma, K.; Noor, M. Force convection heat transfer of Al 2 O 3 nanofluids for different based ratio of water: Ethylene glycol mixture. Appl. Therm. Eng. 2017, 112, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xu, J.; Du, K.; Zhang, X. Recent developments on viscosity and thermal conductivity of nanofluids. Powder Technol. 2017, 317, 348–369. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ho, C.; Chang, C.; Yan, W.-M.; Amani, P. A combined numerical and experimental study on the forced convection of Al2O3-water nanofluid in a circular tube. Int. J. Heat Mass Transf. 2018, 120, 66–75. [Google Scholar] [CrossRef]
- Afrand, M.; Toghraie, D.; Ruhani, B. Effects of temperature and nanoparticles concentration on rheological behavior of Fe 3 O 4 –Ag/EG hybrid nanofluid: An experimental study. Exp. Therm. Fluid Sci. 2016, 77, 38–44. [Google Scholar] [CrossRef]
- Saeedi, A.H.; Akbari, M.; Toghraie, D. An experimental study on rheological behavior of a nanofluid containing oxide nanoparticle and proposing a new correlation. Phys. E 2018, 99, 285–293. [Google Scholar] [CrossRef]
- Asirvatham, L.G.; Vishal, N.; Gangatharan, S.K.; Lal, D.M. Experimental Study on Forced Convective Heat Transfer with Low Volume Fraction of CuO/Water Nanofluid. Energies 2009, 2, 97–119. [Google Scholar] [CrossRef]
- Gu, B.; Hou, B.; Lu, Z.; Wang, Z.; Chen, S. Thermal conductivity of nanofluids containing high aspect ratio fillers. Int. J. Heat Mass Transf. 2013, 64, 108–114. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; de Risi, A. An investigation of layering phenomenon at the liquid–solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Milanese, M.; Starace, G.; de Risi, A. Experimental Measurements of Al2O3 and CuO Nanofluids Interaction with Microwaves. J. Energy Eng. 2017, 143, 04016045. [Google Scholar] [CrossRef]
- Xing, M.; Yu, J.; Wang, R. Experimental study on the thermal conductivity enhancement of water based nanofluids using different types of carbon nanotubes. Int. J. Heat Mass Transf. 2015, 88, 609–616. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Contreras, E.M.C.; Filho, E.P.B. Experimental study on the heat transfer of MWCNT/water nanofluid flowing in a car radiator. Appl. Therm. Eng. 2017, 111, 1450–1456. [Google Scholar] [CrossRef]
- Moldoveanu, G.M.; Minea, A.A.; Huminic, G.; Huminic, A. Al2O3/TiO2 hybrid nanofluids thermal conductivity. J. Therm. Anal. Calorim. 2018, 137, 583–592. [Google Scholar] [CrossRef]
- Minea, A.A. Hybrid nanofluids based on Al2O3, TiO2 and SiO2: Numerical evaluation of different approaches. Int. J. Heat Mass Transf. 2017, 104, 852–860. [Google Scholar] [CrossRef]
- Pryazhnikov, M.I.; Minakov, A.; Rudyak, V.Y.; Guzei, D.V. Thermal conductivity measurements of nanofluids. Int. J. Heat Mass Transf. 2017, 104, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Idrus, S.N.S.; Zaini, N.S.; Mohamad, I.S.; Abdullah, N.; Husin, M.H.M. Comparison of thermal conductivity for HHT-24-CNF-based nanofluid using deionized water and ethylene glycol. J. Teknol. 2015, 77, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.; Mohamad, I.; Hashim, A.Y.B.; Abdullah, N.; Wei, P.; Isa, M.M.; Abidin, S.Z. Thermal conductivity and viscosity of deionised water and ethylene glycol-based nanofluids. J. Mech. Eng. Sci. 2016, 10, 2249–2261. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Kadirgama, K.; Padmanabhan, E.; Sharma, K. Viscosity, electrical and thermal conductivities of ethylene and propylene glycol-based β-SiC nanofluids. J. Mol. Liq. 2019, 284, 780–792. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Chaichan, M.T.; Sopian, K.; Kazem, H.A. Influence of the base fluid on the thermo-physical properties of PV/T nanofluids with surfactant. Case Stud. Therm. Eng. 2019, 13, 100340. [Google Scholar] [CrossRef]
- Alirezaie, A.; Hajmohammad, M.H.; Ahangar, M.R.H.; Esfe, M.H. Price-performance evaluation of thermal conductivity enhancement of nanofluids with different particle sizes. Appl. Therm. Eng. 2018, 128, 373–380. [Google Scholar] [CrossRef]
- Sivan, S.; Venkitaraj, K.; Selvakumar, P.; Chandrasekar, M. Effect of Al2O3–Cu/water hybrid nanofluid in heat transfer. Exp. Therm. Fluid Sci. 2012, 38, 54–60. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Wongwises, S.; Toghraie, D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluids. J. Therm. Anal. Calorim. 2015, 119, 1817–1824. [Google Scholar] [CrossRef]
- Chopkar, M.; Sudarshan, S.; Das, P.; Manna, I. Effect of Particle Size on Thermal Conductivity of Nanofluid. Met. Mater. Trans. A 2008, 39, 1535–1542. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.; Liu, Y. Experimental investigation on preparation and stability of Al2O3/CuO-water nanofluids. In Proceedings of the Asia-Pacific Energy Equipment Engineering Research Conference, Zhuhai, China, 13–14 June 2015; pp. 2352–5401. [Google Scholar]
- Teng, T.-C.; Hung, Y.-H.; Mo, H.-E.; Hsu, H.-G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N. Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 148–154. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO2—water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Chen, H.; Witharana, S.; Jin, Y.; Kim, C.; Ding, Y. Predicting thermal conductivity of liquid suspensions of nanoparticles (nanofluids) based on rheology. Particuology 2009, 7, 151–157. [Google Scholar] [CrossRef]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Ghosh, M.M.; Ghosh, S.K.; Pabi, S.K. Effects of Particle Shape and Fluid Temperature on Heat-Transfer Characteristics of Nanofluids. J. Mater. Eng. Perform. 2013, 22, 1525–1529. [Google Scholar] [CrossRef]
- Farbod, M.; Asl, R.K.; Abadi, A.R.N. Morphology dependence of thermal and rheological properties of oil-based nanofluids of CuO nanostructures. Colloids Surf. A Physicochem. Eng. Asp. 2015, 474, 71–75. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.D.; Kang, S.; Bang, I.C.; Kim, J.H. Investigation of viscosity and thermal conductivity of SiC nanofluids for heat transfer applications. Int. J. Heat Mass Transf. 2011, 54, 433–438. [Google Scholar] [CrossRef]
- Nikkam, N.; Saleemi, M.; Haghighi, E.B.; Ghanbarpour, M.; Khodabandeh, R.; Muhammed, M.; Palm, B.; Toprak, M.S. Fabrication, characterization and thermophysical property evaluation of SiC nanofluids for heat transfer applications. Nano-Micro Lett. 2014, 6, 178–189. [Google Scholar] [CrossRef]
- Murshed, S.S.; Estellé, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Routbort, J.L.; Singh, D.; Timofeeva, E.V.; Yu, W.; France, D.M. Pumping power of nanofluids in a flowing system. J. Nanopart. Res. 2011, 13, 931–937. [Google Scholar] [CrossRef]
- Torii, S. Turbulent Heat Transfer Behavior of Nanofluid in a Circular Tube Heated under Constant Heat Flux. Adv. Mech. Eng. 2010, 2, 917612. [Google Scholar] [CrossRef]
- Vold, R.D.; Vold, M.J. Colloid and Interface Chemistry; Addison-Wesley Reading: Boston, MA, USA, 1983. [Google Scholar]
- Meyer, J.P.; Adio, S.A.; Sharifpur, M.; Nwosu, P.N. The Viscosity of Nanofluids: A Review of the Theoretical, Empirical, and Numerical Models. Heat Transf. Eng. 2016, 37, 387–421. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Li, Y. Investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluid. Thermochim. Acta 2009, 491, 92–96. [Google Scholar] [CrossRef]
- Sundar, L.S.; Ramana, E.V.; Graça, M.; Singh, M.K.; Sousa, A.C. Nanodiamond-Fe 3 O 4 nanofluids: Preparation and measurement of viscosity, electrical and thermal conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Adewumi, G.; Inambao, F.; Sharifpur, M.; Meyer, J. Investigation of the viscosity and stability of green nanofluids from coconut fibre carbon nanoparticles: Effect of temperature and mass fraction. Int. J. Appl. Eng. Res. 2018, 13, 8336–8342. [Google Scholar]
- Azevedo-Oliveira, G.; Bandarra-Filho, E.; Wen, D.S. Synthesis and characterization of silver/water nanofluids. High Temp. High Press. 2014, 43, 69–83. [Google Scholar]
- Namburu, P.; Kulkarni, D.; Dandekar, A.; Das, D. Experimental investigation of viscosity and specific heat of silicon dioxide nanofluids. Micro Nano Lett. 2007, 2, 67. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based α-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; González-Fernández, L.; Grosu, Y.; Zaki, A.; Igartua, J.M.; Faik, A. Shape effect of Al2O3 nanoparticles on the thermophysical properties and viscosity of molten salt nanofluids for TES application at CSP plants. Appl. Therm. Eng. 2020, 169, 114942. [Google Scholar] [CrossRef]
- Thomas, S.; Sobhan, C.B.P. A review of experimental investigations on thermal phenomena in nanofluids. Nanoscale Res. Lett. 2011, 6, 377. [Google Scholar] [CrossRef] [Green Version]
- Ferrouillat, S.; Bontemps, A.; Poncelet, O.; Soriano, O.; Gruss, J.-A. Influence of nanoparticle shape factor on convective heat transfer and energetic performance of water-based SiO2 and ZnO nanofluids. Appl. Therm. Eng. 2013, 51, 839–851. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Akhiani, A.R.; Latibari, S.T.; Talebian, S.; Dolatshahi-Pirouz, A.; Metselaar, H.S.C.; Mehrali, M. An ecofriendly graphene-based nanofluid for heat transfer applications. J. Clean. Prod. 2016, 137, 555–566. [Google Scholar] [CrossRef]
- Hosseini, M.; Sadri, R.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Teng, C.B.; Zaharinie, T. Experimental Study on Heat Transfer and Thermo-Physical Properties of Covalently Functionalized Carbon Nanotubes Nanofluids in an Annular Heat Exchanger: A Green and Novel Synthesis. Energy Fuels 2017, 31, 5635–5644. [Google Scholar] [CrossRef]
- Sahid, N.; Rahman, M.; Kadirgama, K.; Maleque, M.A. Experimental investigation on properties of hybrid nanofluids (TiO2 and ZnO) in water–ethylene glycol mixture. J. Mech. Eng. Sci. 2017, 11, 3087–3094. [Google Scholar] [CrossRef]
- Esfe, M.H.; Raki, H.R.; Emami, M.R.S.; Afrand, M. Viscosity and rheological properties of antifreeze based nanofluid containing hybrid nano-powders of MWCNTs and TiO2 under different temperature conditions. Powder Technol. 2019, 342, 808–816. [Google Scholar] [CrossRef]
- Saidur, R.; Kazi, S.; Hossain, M.; Rahman, M.; Mohammed, H. A review on the performance of nanoparticles suspended with refrigerants and lubricating oils in refrigeration systems. Renew. Sustain. Energy Rev. 2011, 15, 310–323. [Google Scholar] [CrossRef]
- Chavan, D.; Pise, A. Experimental Investigation of Effective Viscosity and Density of Nanofluids. Mater. Today: Proc. 2019, 16, 504–515. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Kishore, P.; Sireesha, V.; Harsha, V.S.; Rao, V.D.; Solomon, A.B. Preparation, characterization and thermo-physical properties of Cu-graphene nanoplatelets hybrid nanofluids. Mater. Today Proc. 2020, 27, 610–614. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.; Bagheri, S.; Zubir, N.; Ahmadi, G.; Dahari, M.; Zaharinie, T. A novel, eco-friendly technique for covalent functionalization of graphene nanoplatelets and the potential of their nanofluids for heat transfer applications. Chem. Phys. Lett. 2017, 675, 92–97. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.; Bagheri, S.; Abdelrazek, A.H.; Ahmadi, G.; Zubir, N.; Ahmad, R.; Abidin, N. A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano-coolants toward improved thermo-physical and heat transfer properties. J. Colloid Interface Sci. 2018, 509, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Sharifpur, M.; Yousefi, S.; Meyer, J.P. A new model for density of nanofluids including nanolayer. Int. Commun. Heat Mass Transf. 2016, 78, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Boles, M.; Cengel, Y. An Engineering Approach; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–205. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transf. 2005, 128, 240–250. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Vajjha, R.S.; Das, D.K.; Oliva, D. Application of aluminum oxide nanofluids in diesel electric generator as jacket water coolant. Appl. Therm. Eng. 2008, 28, 1774–1781. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the Specific Heat Capacity of CuO Nanofluid. Adv. Mech. Eng. 2010, 2, 172085. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. An experimental study on the heat transfer performance and pressure drop of TiO2-water nanofluids flowing under a turbulent flow regime. Int. J. Heat Mass Transf. 2010, 53, 334–344. [Google Scholar] [CrossRef]
- Kumaresan, V.; Ramalingam, V. Experimental investigation of the thermo-physical properties of water–ethylene glycol mixture based CNT nanofluids. Thermochim. Acta 2012, 545, 180–186. [Google Scholar] [CrossRef]
| Author(s) | Nanoparticles/Base Fluid | Preparation Method |
|---|---|---|
| [24] | CuO/EG | One-step method (Chemical reduction method) |
| [25] | Di-Ag/EG | One-step method (Polyol method) |
| [26] | TiO2-CuO and C/EG | Two-step method |
| [27] | Cu/EG and DEG | Two-step method |
| [28] | SiO2-Graphite/Water | Two-step method |
| [29] | Al2O3-SiO2/Water | Two-step method |
| [30] | Si3N4/EG | Two-step method |
| [31] | Al2O3-TiO2/NA | Two-step method |
| [32] | TiO2/Water | Two-step method |
| [18] | Si/Water | Two-step method |
| [33] | SWCNT- MgO/EG | Two-step method |
| [34] | Ag and Au/Water | One-step method |
| [35] | Cu/Methanol | Two-step method |
| [36] | CuO-TiO2/Water | Two-step method |
| Zeta Potential (mV) | Stability Behavior |
|---|---|
| <±5 | Rapid coagulation |
| ±10 to ±30 | Incipient stability |
| ±30 to ±40 | Moderate stability |
| ±40 to ±60 | Good stability |
| >±61 | Excellent stability |
| Author(s) | Nanofluids | Enhancement Method (s) | Evaluation Method (s) | Remarks |
|---|---|---|---|---|
| [92] | CuO/W | Ultrasonication: 1–4 h | - | Thermal conductivity increases with sonication time, temperature, and amount of surfactant. |
| Al2O3/W | SDBS | |||
| [83] | TiO2/W | Ultrasonication: 1.5 h | TEM | Raw TiO2 powder: agglomerated synthesized TiO2: smaller size and spherical shape. |
| PVP Tween 20 | ||||
| [85] | Al2O3/EG | Ultrasonication: 12 h | SEM UV-Vis | There is no distinguishing difference between the absorbance of the sample that was measured on the first day and fifth day. |
| pH: 2–11 | ||||
| PVP SDBS | ||||
| pH: 2–12 | ||||
| Al2O3/W | Anionic SDBS | |||
| [93] | ND-Ni/W | Ultrasonication: 3 h | XRD DLS TEM SEM | The size of particles was also estimated using DLS: ND: 14 nm; Ni: 11 nm; ND-Ni: 28 nm
|
| Nanosperse AQ | ||||
| [94] | TiO2-MWCNT/W-EG | Ultrasonication: 45 min | Visual Observation DLS TEM SEM |
|
| pH: 3, 6, 9, 12 | ||||
| CTAB | ||||
| [95] | CNT-SiO2/W | Ultrasonication: 3 h | SEM |
|
| Gum Arabic | ||||
| [29] | Al2O3/W | Ultrasonication: 60 min |
| |
| SiO2/W | ||||
| Al2O3-SiO2/W | ||||
| [96] | Al2O3/W | pH: 5–10 | Visual Observation Zeta Potential DLS |
|
| CuO/DI-W | SDBS | |||
| [97] | Al2O3-SiO2/W | Ultrasonication: 4 h P = 100 W f = 36 ± 3 kHz | Visual Observation UV-Vis Zeta Potential |
|
| [54] | Al2O3/EG | Ultrasonication: 2 h | Visual Observation FESEM UV-Vis |
|
| pH: 5.34–5.97 | ||||
| pH: Neutralized |
| Author (s) | Nanoparticles | Base Fluids | Size (nm)/Shape | T (°C) | Vol.%/wt.% | kenhanced (%) | Green | ||
|---|---|---|---|---|---|---|---|---|---|
| [19] | Al2O3 | BG:W (60:40) | 13/spherical | 30–80 | 0.5–2.0 vol.% | T = 80 °C, ϕ = 2.0% Max60: 40: 13% Max40: 60: 24% | Yes | ||
| BW:W (40:60) | |||||||||
| [29] | Al2O3 | water | - | 20–50 | 1.0–3.0 vol.% | T = 20–50 °C ϕ = 0.5% Al2O3 + 2.5% SiO2 Max = 17.96–23.61% | No | ||
| SiO2 | Al2O3: 0.5 vol.% SiO2: 0.5–2.5 vol.% | ||||||||
| Al2O3:SiO2 | |||||||||
| [85] | Al2O3 | EG | 13 and 50/spherical | 25–50 | 0.1–1.0 vol.% | T = 50 °C, ϕ = 1.0% Max 50 nm: 38% | No | ||
| [78] | TiO2:SiO2 | 20:80 | W:EG (60:40) | TiO2: 50/Rod-like SiO2: 22/spherical | 30–80 | 1.0 vol.% | No | ||
| 40:60 | |||||||||
| 50:50 | |||||||||
| 60:40 | |||||||||
| 80:20 | |||||||||
| [18] | SiO2 | water | 40–50/spherical | 25–55 | 0–3.0% | T = 55 °C, ϕ = 3.0% Max: 38.2% | Yes | ||
| [114] | Activated hybrid carbon/graphene oxide | EG | - | 20–40 | 0.00–0.06 wt.%. | T = 40 °C, ϕ = 0.06% Max: 6.47% | Yes | ||
| [10] | h-rGO | water | Planar structure | 15–45 | 0.02–0.08 wt.%. | At T = 55 °C and 0.02 < ϕ < 0.08, k was enhanced from 8.9 to 35.7% | Yes | ||
| [160] | MWCNT: SiC (50:50) | W:EG (50:50) | 25–50/ MWCNT: Tubular surface SiC: Almost spherical | 61 | 0–0.75 vol.% | T = 50 °C, ϕ = 0.75% Max: 28.86% | No | ||
| [21] | TiO2 | W: BG | 80:20 | 50/spherical | 30–80 | 0.5–2.0 vol.% | T = 80 °C, ϕ = 2.0% Rbf: 80:20 Max: 12.6% | Yes | |
| 70:30 | |||||||||
| [84] | Al2O3 | W:EG | 40: 60 | 13/spherical | 30–70 | 0.2–1.0 vol.% | T = 70 °C, ϕ = 1.0% Rbf: 40: 60 Max: 12.8% | No | |
| 50: 50 | |||||||||
| 60: 40 | |||||||||
| [161] | SiC | water | <100/spherical | 22–23.5 | 0.001, 0.1, 1, 2, 3 vol.% | ϕ = 3.0% Max: 7.2% | No | ||
| [145] | SiC | water | - | 25–60 | 0.1–3.0 wt.% | T = 60 °C, ϕ = 3.0% Max: 2.29% | No | ||
| W:EG (65:35) | |||||||||
| W: PG (65:35) | |||||||||
| [19] | Al2O3 | BG | 13 | 30–80 | 0.1–1.0 vol.% | T = 30 °C, ϕ= 1.0% Max: 17% | Yes | ||
| Author(s) | Nanofluids | Vol.%/wt.% | T (°C) | Size (nm)/Shape | Findings | Green |
|---|---|---|---|---|---|---|
| [115] | TiO2/W:EG | 0.5–1.5 vol.% | 30–70 | - | The viscosity decreased from 2.3 to 2.4 times in temperatures between 30 and 70 °C | No |
| [177] | SiO2/W | 1.08 vol.% 2.28 vol.% | 20–70 | Spherical Banana-shaped | The viscosity of banana-shaped SiO2 nanoparticles almost similar to the spherical-shaped SiO2 nanoparticles | No |
| ZnO/W | 0.82 vol.% 0.93 vol.% | Polygonal Rod-like | The viscosity of rod-shaped ZnO nanoparticles is less than the polygonal-shaped ZnO nanoparticles | |||
| [115] | TiO2/W:EG | 0.5–1.5 vol.% | 30–80 | - | Fluctuation in the relative viscosities in the range of 4.6–33.3% at temperatures between 30 and 80 °C | No |
| [21] | SiO2/W:BG (80:20) | 0.5–2.0 vol.% | 30–80 | 22/Spherical | The viscosity increased from 16.02 to 28.9% in the temperature range of 30 to 80 °C at 2.0 vol.% | Yes |
| SiO2/W:BG (70:30) | The viscosity increased from 17.3 to 37.8% in the temperature range of 30 to 80 °C at 2.0 vol.% | |||||
| [171] | C/W:EG (40:60) | 0.04–1.0 wt.% | 15–60 | Nano sphere | The viscosity increased by up to 50% with mass fraction and no significant change with temperature. | Yes |
| [21] | TiO2/W:BG (80:20) | 0.5–2.0 vol.% | 30–80 | 50/Spherical | The viscosity increased from 20.5 to 33.8% in the temperature range between 30 and 80 °C at 2.0 vol.% | Yes |
| TiO2/W:BG (70:30) | The viscosity increased from 29.8 to 53.4% in the temperature range between 30 and 80 °C at 2.0 vol.% | |||||
| [178] | rGO/W | 1.0–4.0 vol.% | 20–70 | The rGO/water nanofluids demonstrated a Newtonian behavior. The viscosity decreased from 86.2 to 87.9% between particle concentrations | Yes | |
| [179] | C-MWCNTs/W | 0.075–0.175 wt.%. | 20–50 | - | The viscosity of nanofluids slightly increases from the base fluid. | Yes |
| [114] | Activated hybrid carbon- graphene oxide/EG | 0.00–0.06 wt.%. | 20–40 | - | The viscosity increased up to 4.16% at 0.06 wt.% | Yes |
| [180] | TiO2-ZnO (70:30)/W:EG | 0.1–1.5 vol.% | 50–70 | TiO2 (21) ZnO (10–30) | The viscosity of the hybrid nanofluids increase with the increase in the amount of TiO2 nanoparticles | No |
| TiO2-ZnO (80:20)/W:EG | ||||||
| TiO2-ZnO (90:10)/W:EG | ||||||
| [64] | TiO2-SiO2/W:EG | 0.5–3.0 vol.% | 30–80 | - | The viscosity of the hybrid nanofluids increased by up to 62.5% at 3.0 vol.% and 80 °C | No |
| [181] | MWCNT- TiO2/W:EG | 0.05–0.85 vol.% | 10–50 | - | The maximum increase in viscosity is 83%, found at 0.85 vol.% and 10 °C. | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainon, S.N.M.; Azmi, W.H. Recent Progress on Stability and Thermo-Physical Properties of Mono and Hybrid towards Green Nanofluids. Micromachines 2021, 12, 176. https://doi.org/10.3390/mi12020176
Zainon SNM, Azmi WH. Recent Progress on Stability and Thermo-Physical Properties of Mono and Hybrid towards Green Nanofluids. Micromachines. 2021; 12(2):176. https://doi.org/10.3390/mi12020176
Chicago/Turabian StyleZainon, S.N.M., and W.H. Azmi. 2021. "Recent Progress on Stability and Thermo-Physical Properties of Mono and Hybrid towards Green Nanofluids" Micromachines 12, no. 2: 176. https://doi.org/10.3390/mi12020176





