Clinical Applications of Laser Technology: Laser Balloon Ablation in the Management of Atrial Fibrillation
Abstract
:1. Introduction
2. Biophysics and Biomechanics of Laser Balloon Ablation
3. Procedural Access
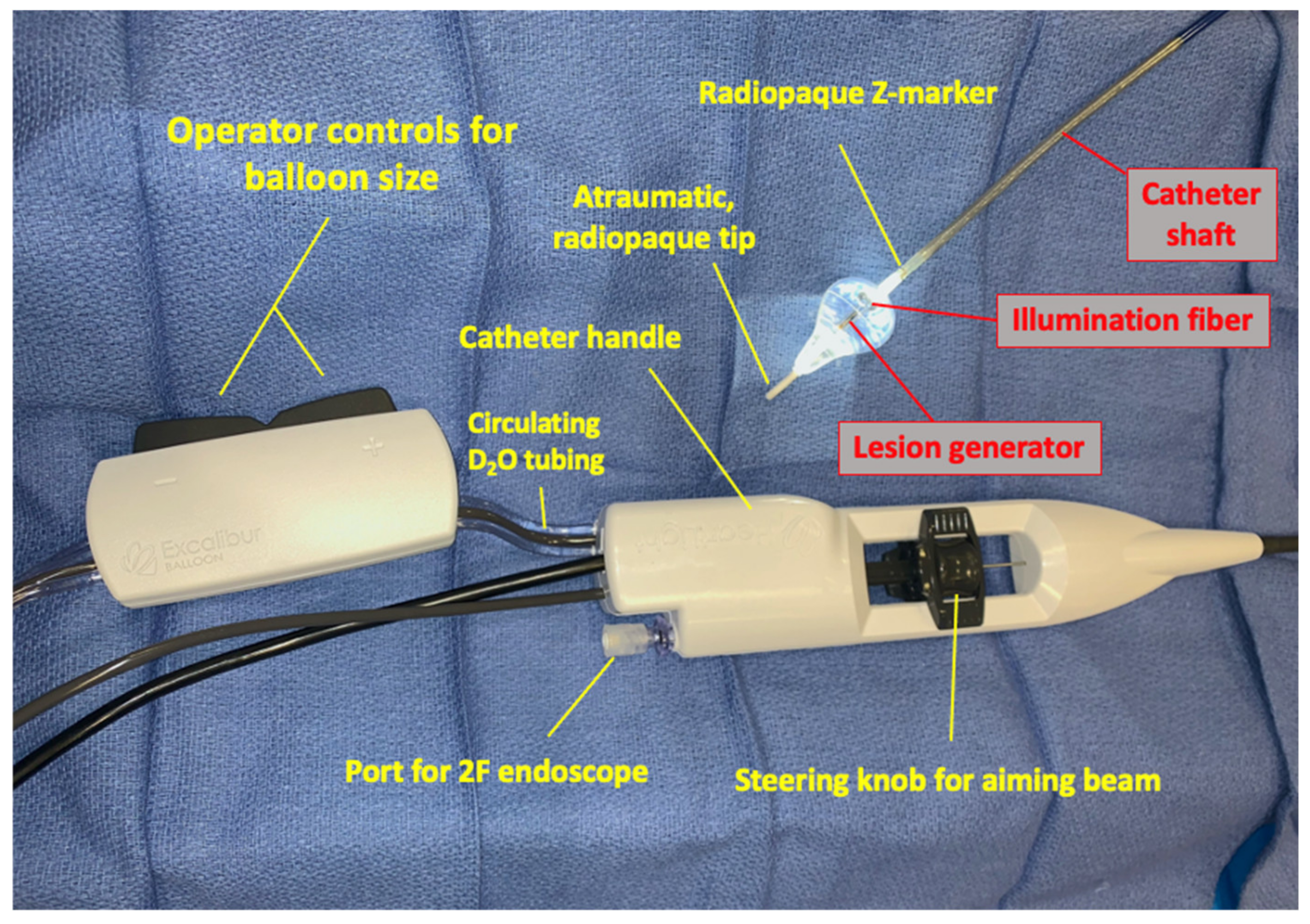
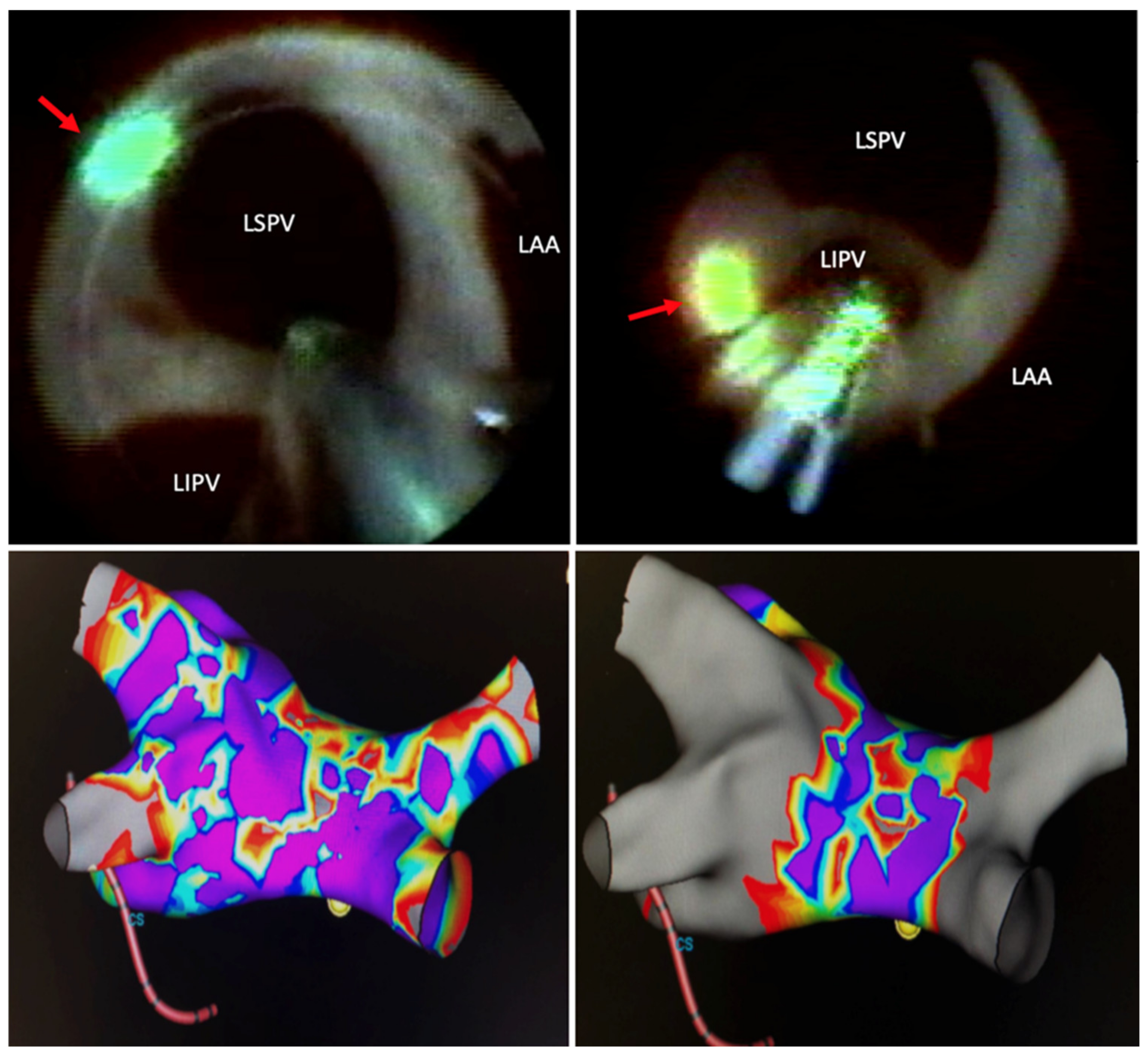
4. Lesion Generation
5. Technology Development and Technique Modification
6. Factors Influencing Success in Pulmonary Vein Isolation
7. Comparisons to Other Techniques
8. Technique Advances and Emerging Technologies
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoshitake, T.; Goya, M.; Sasaki, T.; Shiohira, S.; Sekigawa, M.; Shirai, Y.; Lee, K.; Yagishita, A.; Maeda, S.; Takahashi, Y.; et al. Safety and Efficacy of Transvenous Lead Extraction with a High-Frequency Excimer Laser—A Single Center Experience. Circ. J. 2018, 82, 2992–2997. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kuck, K.-H.; Neuzil, P.; Woollett, I.; Kautzner, J.; McElderry, H.T.; Schmidt, B.; Gerstenfeld, E.P.; Doshi, S.K.; Horton, R.; et al. Pulmonary Vein Isolation Using a Visually Guided Laser Balloon Catheter. Circ. Arrhythm. Electrophysiol. 2013, 6, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary. J. Am. Coll. Cardiol. 2018, 138, e210–e271. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.-Y.; Betts, T.R.; Chen-Yang, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Neuzil, P.; Themistoclakis, S.; Danik, S.B.; Bonso, A.; Rossillo, A.; Raviele, A.; Schweikert, R.; Ernst, S.; Kuck, K.-H.; et al. Visually-Guided Balloon Catheter Ablation of Atrial Fibrillation. Circulation 2009, 120, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation with Pulsed Electric Fields. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef]
- Lemery, R.; Veinot, J.P.; Tang, A.S.; Green, M.; Farr, N.; Baxter, L.; McIntyre, J.; Sinofsky, E. Fiberoptic balloon catheter ablation of pulmonary vein ostia in pigs using photonic energy delivery with Diode laser. Pacing Clin. Electrophysiol. 2002, 25, 32–36. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Houghtaling, C.; Fallon, J.; Fischer, G.; Farr, N.; Clarke, J.; McIntyre, J.; Sinofsky, E.; Ruskin, J.N.; Keane, D. Use of a diode laser balloon ablation catheter to generate circumferential pulmonary venous lesions in an open-thoracotomy caprine model. Pacing Clin. Electrophysiol. 2004, 27, 52–57. [Google Scholar] [CrossRef]
- Welch, A.J.; Torres, J.H.; Cheong, W.-F. Laser Physics and Laser-Tissue Interaction. Tex. Hear. Inst. J. 1989, 16, 141–149. [Google Scholar]
- Dukkipati, S.R.; Neužil, P.; Skoda, J.; Petru, J.; D’Avila, A.; Doshi, S.K.; Reddy, V.Y. Visual Balloon-Guided Point-by-Point Ablation. Circ. Arrhythm. Electrophysiol. 2010, 3, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, D.L.; Boor, P.; Yang, C.; Gowda, A.; Grady, J.J.; Motamedi, M. Slow Intramural Heating with Diffused Laser Light. Circulation 1999, 99, 1630–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Metzner, A.; Chun, K.R.J.; Leftheriotis, D.; Yoshiga, Y.; Fuernkranz, A.; Neven, K.; Tilz, R.R.; Wissner, E.; Ouyang, F.; et al. Feasibility of Circumferential Pulmonary Vein Isolation Using a Novel Endoscopic Ablation System. Circ. Arrhythm. Electrophysiol. 2010, 3, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, M.R.; Zheng, Q.; Doros, G. Laser balloon ablation for AF: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2018, 29, 1363–1370. [Google Scholar] [CrossRef]
- Osca, J.; Andrés, A.; Cano, O.; Alonso, P.; Tello, M.J.S.; Olagüe, J.; Martinez-Dolz, L.; Salvador, A. Electrical Isolation of Pulmonary Veins Using Laser Catheter in the Treatment of Paroxysmal and Persistent Atrial Fibrillation. One-year Results. Revista Española de Cardiología 2016, 69, 488–493. [Google Scholar] [CrossRef]
- Reissmann, B.; Budelmann, T.; Wissner, E.; Schlüter, M.; Heeger, C.-H.; Mathew, S.; Maurer, T.; Lemes, C.; Fink, T.; Rillig, A.; et al. Five-year clinical outcomes of visually guided laser balloon pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Clin. Res. Cardiol. 2018, 107, 405–412. [Google Scholar] [CrossRef]
- Metzner, A.; Wissner, E.; Schoonderwoerd, B.; Burchard, A.; Tilz, R.R.; Furnkranz, A.; Rillig, A.; Mathew, S.; Ouyang, F.; Kuck, K.-H. The influence of varying energy settings on efficacy and safety of endoscopic pulmonary vein isolation. Hear. Rhythm. 2012, 9, 1380–1385. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Cuoco, F.; Kutinsky, I.; Aryana, A.; Bahnson, T.D.; Lakkireddy, D.; Woollett, I.; Issa, Z.F.; Natale, A.; Reddy, V.Y. Pulmonary Vein Isolation Using the Visually Guided Laser Balloon. J. Am. Coll. Cardiol. 2015, 66, 1350–1360. [Google Scholar] [CrossRef] [Green Version]
- Ücer, E.; Janeczko, Y.; Seegers, J.; Fredersdorf, S.; Friemel, S.; Poschenrieder, F.; Maier, L.S.; Jungbauer, C.G. A RAndomized Trial to compare the acute reconnection after pulmonary vein ISolation with Laser-BalloON versus radiofrequency Ablation: RATISBONA trial. J. Cardiovasc. Electrophysiol. 2018, 29, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Neužil, P.; Luik, A.; Asensi, J.O.; Schrickel, J.W.; Deneke, T.; Bordignon, S.; Petru, J.; Merkel, M.; Sediva, L.; et al. Laser Balloon or Wide-Area Circumferential Irrigated Radiofrequency Ablation for Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2017, 10, e005767. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, N.; Jin, Q.; Pan, W.; Xie, Y.; Chen, K.; Ling, T.; Lin, C.; Bao, Y.; Luo, Q.; et al. Comparison of efficacy and safety of laser balloon and cryoballoon ablation for atrial fibrillation—A meta-analysis. J. Interv. Card. Electrophysiol. 2019, 54, 237–245. [Google Scholar] [CrossRef]
- Luik, A.; Kunzmann, K.; Hörmann, P.; Schmidt, K.; Radzewitz, A.; Bramlage, P.; Schenk, T.; Schymik, G.; Merkel, M.; Kieser, M.; et al. Cryoballoon vs. open irrigated radiofrequency ablation for paroxysmal atrial fibrillation: Long-term FreezeAF outcomes. BMC Cardiovasc. Disord. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Galizia Brito, V.; Vecchio, N.; Tomas, L.; Jarma, J.J.; Mondragon, L.; Burgo, L.; Ordonez, S.; Rivera, S.; Albia, G.; Giniger, A.; et al. Second generation cryoballoon, vs. radiofrequency ablation in paroxysmal atrial fibrillation: Outcomes beyond one-year follow-up. J. Atr. Fibrill. 2019, 11, 1–6. [Google Scholar]
- Bordignon, S.; Chun, K.R.J.; Gunawardene, M.; Fuernkranz, A.; Urban, V.; Schulte-Hahn, B.; Nowak, B.; Schmidt, B. Comparison of Balloon Catheter Ablation Technologies for Pulmonary Vein Isolation: The Laser Versus Cryo Study. J. Cardiovasc. Electrophysiol. 2013, 24, 987–994. [Google Scholar] [CrossRef]
- Clinical Study of the CardioFocus Endoscopic Ablation System for the Treatment of Symptomatic AF ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT00958165 (accessed on 31 January 2021).
- Huang, H.D.; Rodriguez, J.M.; Serafini, N.J.; Macias, C.; Winterfield, J.; Sharma, P.S.; Larsen, T.; Krishnan, K.; Trohman, R.G. Comparison between minimal fluoroscopy and conventional approaches for visually guided laser balloon pulmonary vein isolation ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- US FDA Approval Granted for HeartLight X3 Endoscopic Ablation System. Available online: https://cardiacrhythmnews.com/us-fda-approval-granted-for-heartlight-x3-endoscopic-ablation-system/ (accessed on 6 December 2020).
- CardioFocus Announces Results From HeartLight X3 Ablation System Pivotal Study|DAIC. Available online: https://www.dicardiology.com/content/cardiofocus-announces-results-heartlight-x3-ablation-system-pivotal-study (accessed on 6 December 2020).
- Heeger, C.-H.; Tiemeyer, C.M.; Phan, H.-L.; Meyer-Saraei, R.; Fink, T.; Sciacca, V.; Liosis, S.; Brüggemann, B.; Große, N.; Fahimi, B.; et al. Rapid pulmonary vein isolation utilizing the third-generation laserballoon—The PhoeniX registry. IJC Hear. Vasc. 2020, 29, 100576. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Dorwarth, U.; Straube, F.; Daccarett, M.; Rieber, J.; Wankerl, M.; Krieg, J.; Leber, A.W.; Ebersberger, U.; Huber, A.; et al. Cryoballoon in AF ablation: Impact of PV ovality on AF recurrence. Int. J. Cardiol. 2013, 167, 114–120. [Google Scholar] [CrossRef]
- Kubala, M.; Hermida, J.-S.; Nadji, G.; Quenum, S.; Traulle, S.; Jarry, G. Normal Pulmonary Veins Anatomy is Associated with Better AF-Free Survival after Cryoablation as Compared to Atypical Anatomy with Common Left Pulmonary Vein. Pacing Clin. Electrophysiol. 2011, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Gal, P.; Ooms, J.F.; Ottervanger, J.P.; Smit, J.J.; Adiyaman, A.; Ramdat Misier, A.R.; Delnoy, P.P.; Jager, P.L.; Elvan, A. Association between pulmonary vein orientation and atrial fibrillation-free survival in patients undergoing endoscopic laser balloon ablation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkle, R.A.; Mohanty, S.; Patrawala, R.A.; Mead, R.H.; Kong, M.H.; Engel, G.; Salcedo, J.; Trivedi, C.G.; Gianni, C.; Jais, P.; et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Hear. Rhythm. 2019, 16, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skeete, J.R.; Du-Fay-de-Lavallaz, J.M.; Kenigsberg, D.; Macias, C.; Winterfield, J.R.; Sharma, P.S.; Trohman, R.G.; Huang, H.D. Clinical Applications of Laser Technology: Laser Balloon Ablation in the Management of Atrial Fibrillation. Micromachines 2021, 12, 188. https://doi.org/10.3390/mi12020188
Skeete JR, Du-Fay-de-Lavallaz JM, Kenigsberg D, Macias C, Winterfield JR, Sharma PS, Trohman RG, Huang HD. Clinical Applications of Laser Technology: Laser Balloon Ablation in the Management of Atrial Fibrillation. Micromachines. 2021; 12(2):188. https://doi.org/10.3390/mi12020188
Chicago/Turabian StyleSkeete, Jamario R., Jeanne M. Du-Fay-de-Lavallaz, David Kenigsberg, Carlos Macias, Jeffrey R. Winterfield, Parikshit S. Sharma, Richard G. Trohman, and Henry D. Huang. 2021. "Clinical Applications of Laser Technology: Laser Balloon Ablation in the Management of Atrial Fibrillation" Micromachines 12, no. 2: 188. https://doi.org/10.3390/mi12020188
APA StyleSkeete, J. R., Du-Fay-de-Lavallaz, J. M., Kenigsberg, D., Macias, C., Winterfield, J. R., Sharma, P. S., Trohman, R. G., & Huang, H. D. (2021). Clinical Applications of Laser Technology: Laser Balloon Ablation in the Management of Atrial Fibrillation. Micromachines, 12(2), 188. https://doi.org/10.3390/mi12020188






