A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform
Abstract
1. Introduction
2. Experimental Results
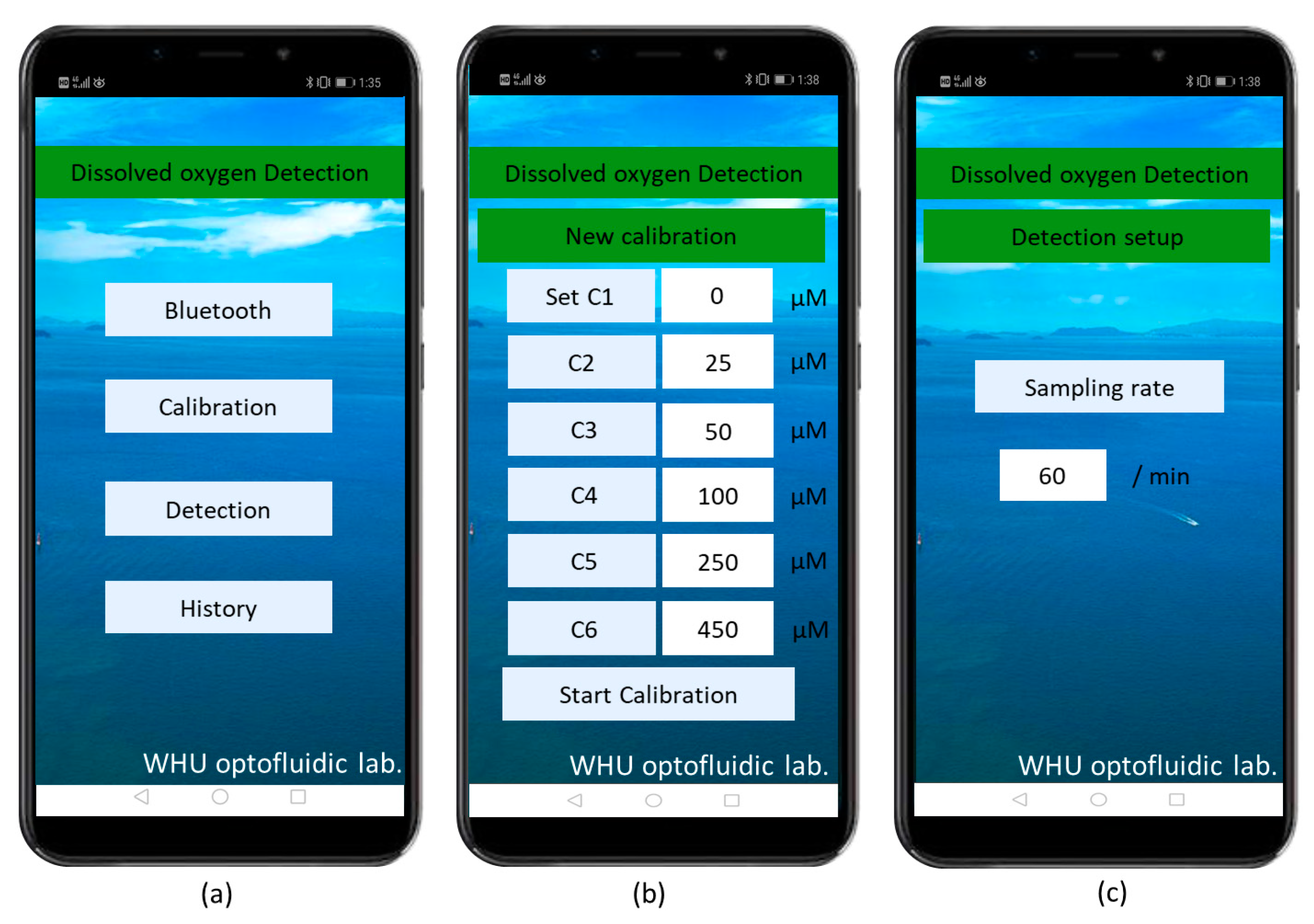
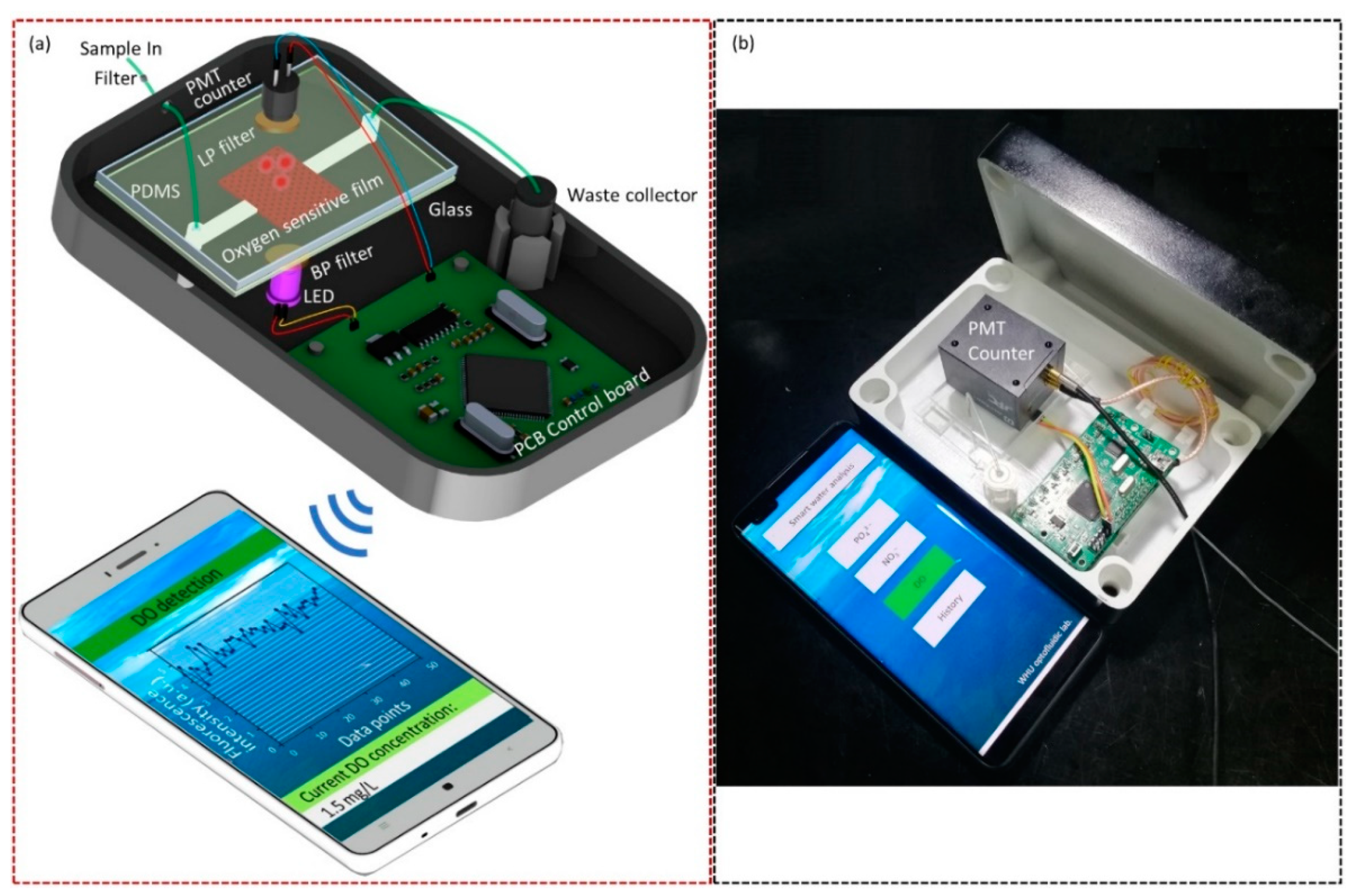
2.1. Sensor Setup
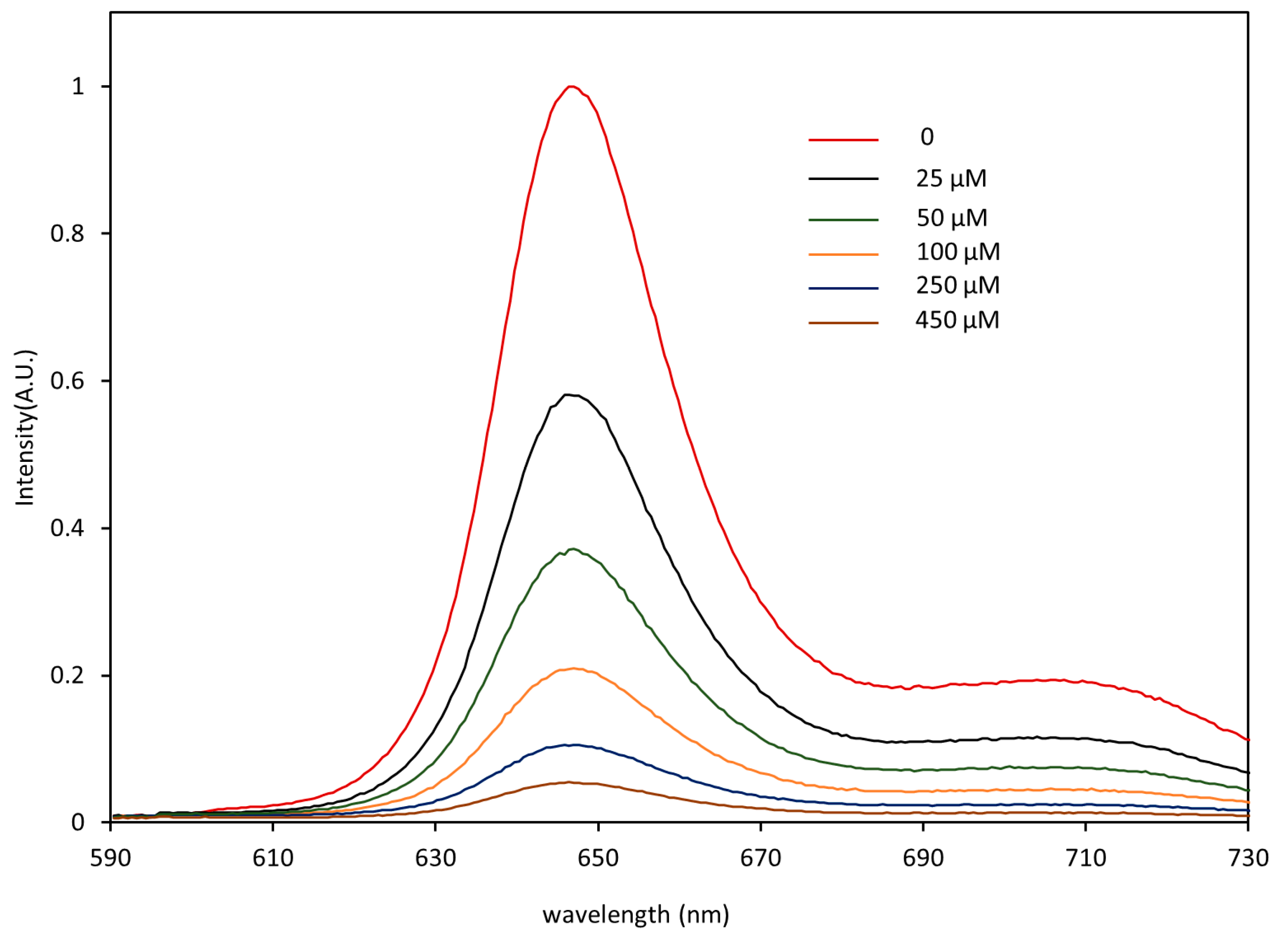
2.2. Principle of Phosphorescence Detection
2.3. Sensor Calibration
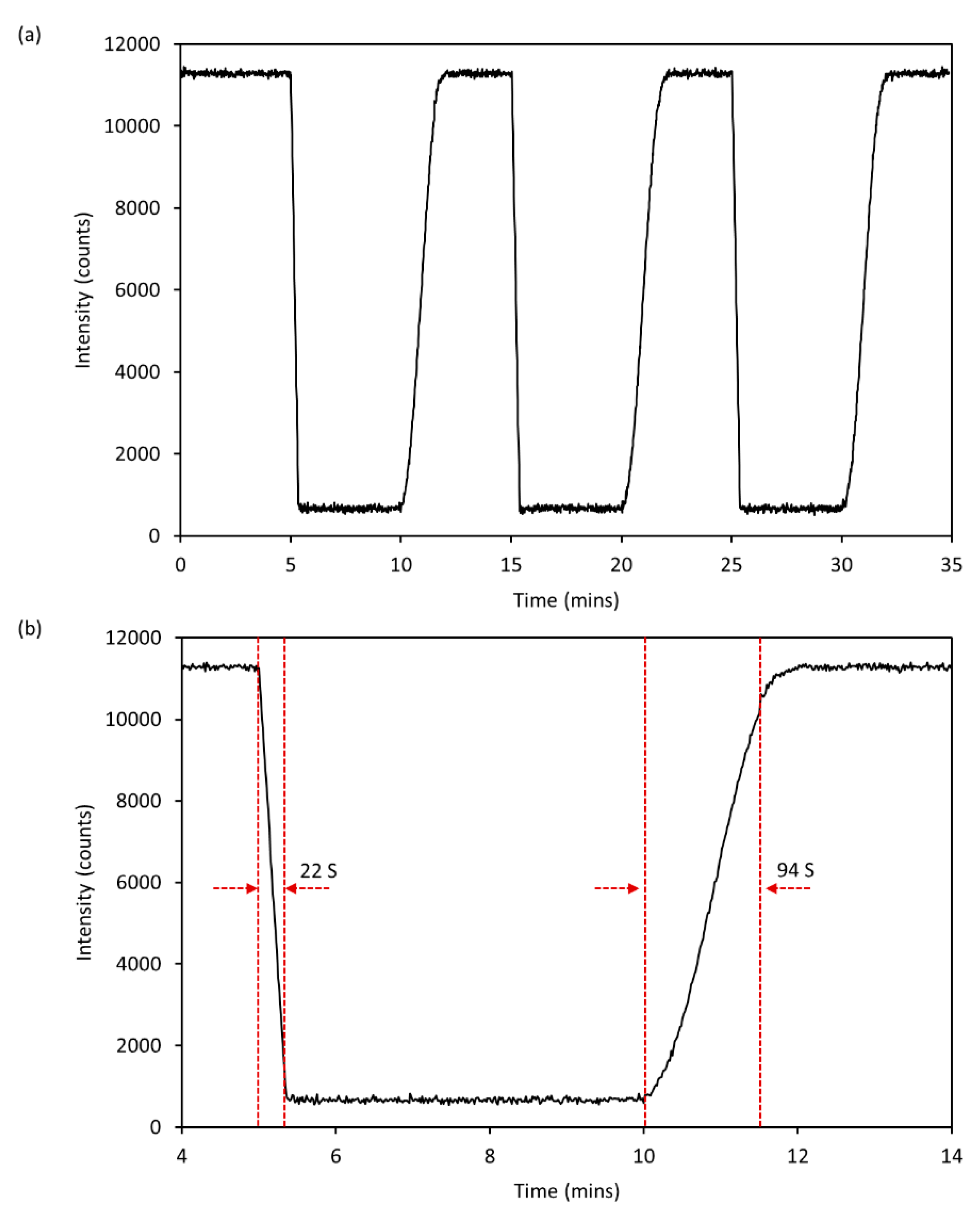
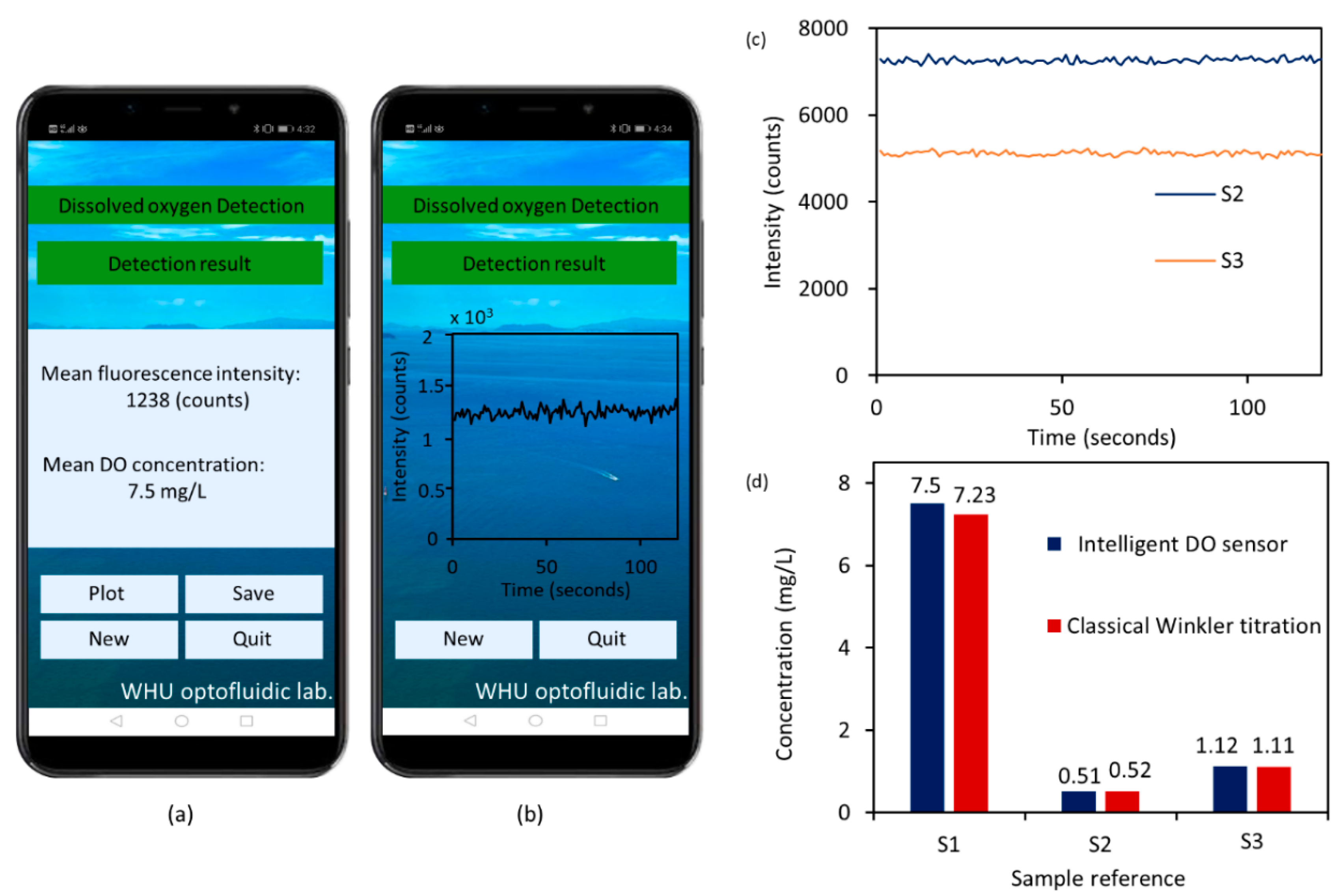
2.4. Sensor Response Time and Performance
3. Materials and Methods
3.1. Chemicals
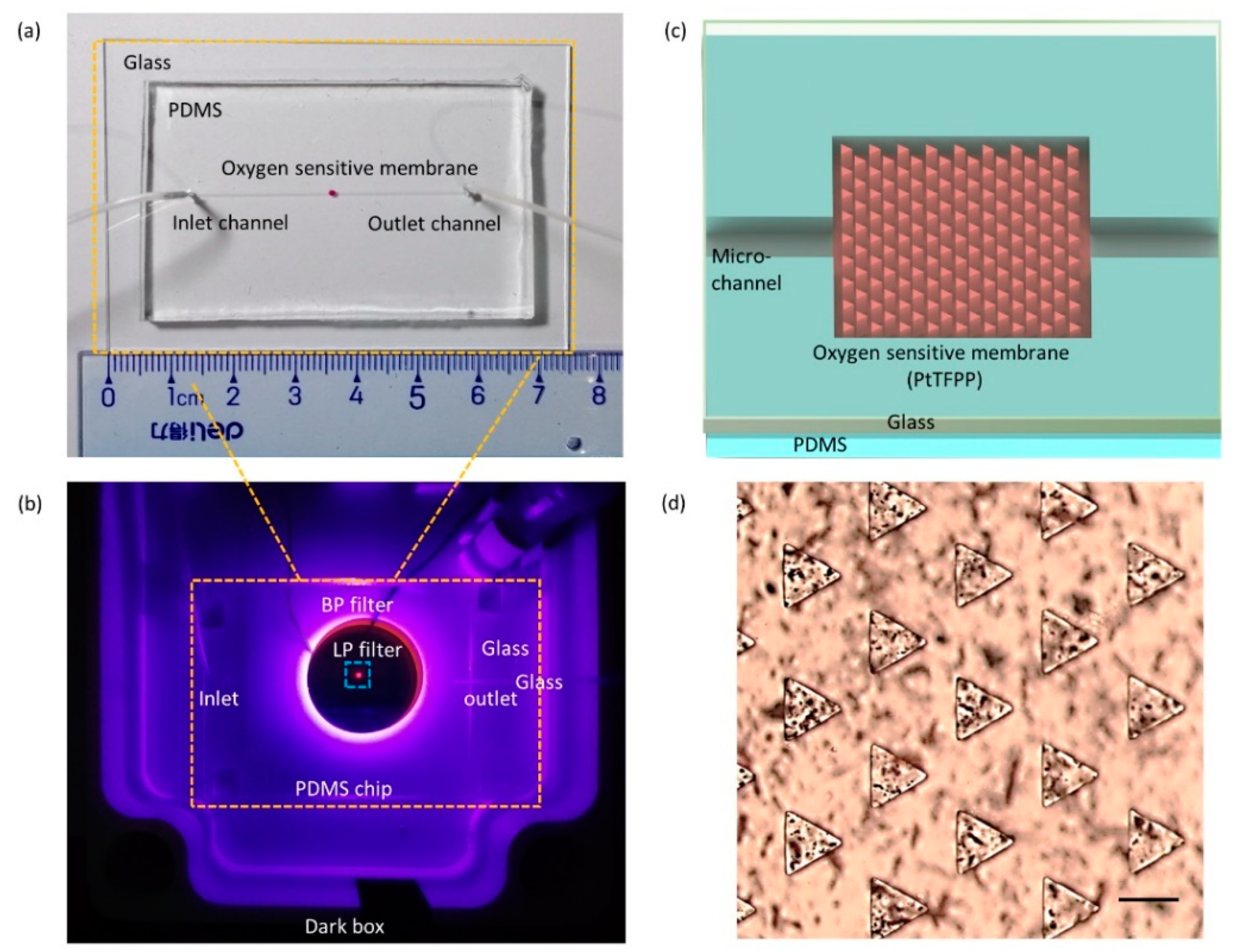
3.2. Oxygen-Sensitive Membrane and Microfluidic Chip Fabrications
3.3. Instruments
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Schmidtko, S.; Stramma, L.; Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Life with oxygen. Science 2007, 318, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Papkovsky, D.B.; Dmitriev, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef] [PubMed]
- Shriwastav, A.; Sudarsan, G.; Bose, P.; Tare, V. A modified Winkler’s method for determination of dissolved oxygen concentration in water: Dependence of method accuracy on sample volume. Measurement 2017, 106, 190–195. [Google Scholar] [CrossRef]
- Horstkotte, B.; Alonso, J.C.; Miro, M.; Cerdà, V. A multisyringe flow injection Winkler-based spectrophotometric analyzer for in-line monitoring of dissolved oxygen in seawater. Talanta 2010, 80, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dziubla, T.; Eitel, R. A low temperature co-fired ceramic based microfluidic Clark-type oxygen sensor for real-time oxygen sensing. Sens. Actuators B Chem. 2017, 240, 392–397. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.A.; Borisov, S.M.; Nagl, S. In-line monitoring of pH and oxygen during enzymatic reactions in off-the shelf all-glass microreactors using integrated luminescent microsensors. Microchim. Acta 2017, 184, 621–626. [Google Scholar] [CrossRef]
- Zang, L.; Zhao, H.; Hua, J.; Qin, F.; Zheng, Y.; Zhang, Z.; Cao, W. Ratiometric dissolved oxygen sensitive indicator based on lutetium labeled hematoporphyrin monomethyl ether with balanced phosphorescence and fluorescence dual emission. Sens. Actuators B Chem. 2016, 231, 539–546. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, N.; Chen, L.; Li, B. An optical sensor for monitoring of dissolved oxygen based on phase detection. J. Opt. 2013, 15, 055502. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef] [PubMed]
- Bittig, H.C.; Arne, K.; Craig, N.; Ooijen, E.; Plant, J.N.; Hahn, J.; Johnson, K.S.; Yang, B.; Emerson, S.R. Oxygen Optode Sensors: Principle, Characterization, Calibration, and Application in the Ocean. Front. Mar. Sci. 2018, 4, 429. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Adarsh, N.; Klymchenko, A.S. Ratiometric Nanoparticle Probe Based on FRET-Amplified Phosphorescence for Oxygen Sensing with Minimal Phototoxicity. Small 2020, 16, 2002494. [Google Scholar] [CrossRef]
- Yoshihara, T.; Hirakawa, Y.; Hosaka, M.; Nangaku, M.; Tobita, S. Oxygen imaging of living cells and tissues using luminescent molecular probes. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 71–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Luo, T.; Hong, L.; Peng, X.; Austin, R.H.; Qu, J. A platinum-porphine/poly (perfluoroether) film oxygen tension sensor for noninvasive local monitoring of cellular oxygen metabolism using phosphorescence lifetime imaging. Sens. Actuators B Chem. 2018, 269, 88–95. [Google Scholar] [CrossRef]
- Akram, M.; Mei, Z.P.; Shi, J.Y.; Wen, J.Y.; Khalid, H.; Jiang, J.P.; Tian, Y.H.; Tian, Y.Q. Electrospun nanofibers and spin coated films prepared from side-chain copolymers with chemically bounded platinum (II) porphyrin moieties for oxygen sensing and pressure sensitive paints. Talanta 2018, 188, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Gao, Y.; Wu, S.; Wu, S.; Shi, J.; Zhou, B.; Tian, Y. Highly enhanced sensitivity of optical oxygen sensors using microstructured PtTFPP/PDMS-pillar arrays sensing layer. Sens. Actuators B Chem. 2017, 251, 495–502. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, T.; Yamamoto, S.; Miyashita, T.; Mitsuishi, M. Superhydrophobic Porous Surfaces: Dissolved Oxygen Sensing. ACS Appl. Mater. Interfaces 2015, 7, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Marques, M.P.C.; Szita, N.; Mayr, T. Integration and application of optical chemical sensors in microbioreactors. Lab Chip 2017, 17, 2693–2712. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; Kelly, R.T.; Suter, J.; Anheier, N.C. Silicon-on-glass pore network micromodels with oxygen-sensing fluorophore films for chemical imaging and defined spatial structure. Lab Chip 2012, 12, 4796–4801. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Huang, Z.Y.; Chen, Q.M.; Wu, Q.; Huang, X.W.; So, P.; Shao, L.Y.; Yao, Z.P.; Jia, Y.W.; Li, Z.H.; et al. Continuous artificial synthesis of glucose precursor using enzyme-immobilized microfluidic reactors. Nat. Commun. 2019, 10, 4049. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, J.M.; Chen, L.F.; Zuo, Y.F.; Hu, X.J.; Yang, Y. Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform. Micromachines 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Zhu, T.T.; Nguyen, K.T.; Zhang, Y.; Xiong, S.; Yap, P.H.; Ser, W.; Wang, S.B.; Qiu, C.W.; Chan, C.T.; et al. Optofluidic Micro-Engine in A Dynamic Flow Environment via Self-Induced Back-Action. ACS Photonics 2020, 7, 1500–1507. [Google Scholar] [CrossRef]
- Zhu, J.M.; Zhu, X.Q.; Zuo, Y.F.; Hu, X.J.; Shi, Y.; Liang, L.; Yang, Y. Optofluidics: The interaction between light and flowing liquids in integrated devices. Opto-Electron. Adv. 2019, 2, 190007. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, L.; Zuo, Y.; Zhu, X.; Yang, Y.; Xin, H.; Li, B. Amplitude Holographic Interference-Based Microfluidic Colorimetry at the Micrometer Scale. J. Phys. Chem. Lett. 2020, 11, 4747–4754. [Google Scholar] [CrossRef]
- Wang, N.; Dai, T.; Lei, L. Optofluidic Technology for Water Quality Monitoring. Micromachines 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, H.L.; Zhu, X.Q.; Zhu, J.M.; Zuo, Y.F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H. Optofluidic differential colorimetry for rapid nitrite determination. Lab Chip 2018, 18, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wong, Y.L.; Jian, A.; Tsoi, C.C.; Wang, M.; Li, W.; Zhang, W.; Sang, S.; Zhang, X. Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles. Micromachines 2019, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Jin, Y.X.; Zhu, X.Q.; Zhou, F.L.; Yang, Y. Real-time detection and monitoring of the drug resistance of single myeloid leukemia cells by diffused total internal reflection. Lab Chip 2018, 18, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Z.; DeGrandpre, M.D.; Darlington, R.C. Autonomous Optofluidic Chemical Analysers for Marine Applications: Insights from the Submersible Autonomous Moored Instruments (SAMI) for pH and pCO2. Front. Mar. Sci. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Campos, C.D.; Silva, J.A. Applications of autonomous microfluidic systems in environmental monitoring. RSC Adv. 2013, 3, 18216–18227. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; De Ferrari, F.; Zhang, Y.S.; Nabavinia, M.; Mohammad, N.B.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Sadeghian, R.B.; Nadhman, A.; et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 760–793. [Google Scholar]
- Nock, V.; Blaikie, R.J.; David, T. Patterning, integration and characterisation of polymer optical oxygen sensors for microfluidic device. Lab Chip 2008, 8, 1300–1307. [Google Scholar] [CrossRef]
- Zhu, J.M.; Han, G.W.; Hu, X.J.; Zuo, Y.F.; Chen, L.F.; Wang, F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; et al. A Portable and Accurate Phosphate Sensor Using a Gradient Fabry-Pérot Array. ACS Sens. 2020, 5, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, J.M.; Hu, X.J.; Chen, L.F.; Zuo, Y.F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Tian, H.X. Rapid nitrate determination with a portable lab-on-chip device based on double microstructured assisted reactors. Lab Chip 2021. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.F.; Chen, L.F.; Hu, X.J.; Wang, F. Silver Nanoprism Enhanced Colorimetry for Precise Detection of Dissolved Oxygen. Micromachines 2020, 11, 383. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, B. Nanoscale LnMOF-functionalized nonwoven fibers protected by a polydimethylsiloxane coating layer as a highly sensitive ratiometric oxygen sensor. J. Mater. Chem. C 2016, 4, 8514–8521. [Google Scholar] [CrossRef]
- Sánchez-Barragán, I.; Costa-Fernández, J.M.; Valledor, M.; Campo, J.C.; Sanz-Medel, A. Room-temperature phosphorescence (RTP) for optical sensing. TrAC Trends Anal. Chem. 2006, 25, 958–967. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, T.; Chen, H.; Huang, D.; Chen, X. A dissolved oxygen sensor based on composite fluorinated xerogel doped with platinum porphyrin dye. Luminescence 2011, 26, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.J.; Burger, T.; Borisov, S.M.; Klimant, I. High performance optical trace oxygen sensors based on NIR-emitting benzoporphyrins covalently coupled to silicone matrixes. Sens. Actuators B Chem. 2015, 216, 527–534. [Google Scholar] [CrossRef]
- Yao, L.; Ou, G.; Liu, W.; Zhao, X.H.; Nishijima, H.; Pan, W. Fabrication of high performance oxygen sensors using multilayer oxides with high interfacial conductivity. J. Mater. Chem. A 2016, 4, 11422–11429. [Google Scholar] [CrossRef]
- Luo, J.; Eitel, R. An Integrated Low Temperature Co-Fired Ceramic-Based Clark-Type Oxygen Sensor. IEEE Sens. J. 2017, 17, 1590–1595. [Google Scholar] [CrossRef]

| Dissolved Oxygen Detection | Electrochemical Sensor [42] | Optofluidic Sensor [36] | Optical Sensor [9] | Commercial Sensor [11] | Intelligent Senor |
|---|---|---|---|---|---|
| Principle | Electrode polarography | Colorimetry | Phosphorescence-quenching | Fluorescence-quenching | Phosphorescence-quenching |
| Sensitivity | 64.6 nA·L·mg−1 | 7.5 nm·L·mg−1 | I0/I > 4.6 | - | I0/I = 16.9 |
| Detection range | 0.3 to 8.4 mg L−1 | 0 to 16 mg L−1 | 0 to 11.6 mg L−1 | 0 to 20 mg L−1 | 0 to 14.4 mg L−1 |
| Detection Limit | 0.2 mg L−1 | 3.52 ug·L−1 | 0.03 mg L−1 | 0.01 mg L−1 | 0.01 mg L−1 |
| Response time | 7.5 s | - | < 1min | 40 s | 22 s |
| User Interface | - | - | PC | Customized display panel | Smartphone APP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chen, L.; Zhu, J.; Hu, X.; Yang, Y. A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform. Micromachines 2021, 12, 281. https://doi.org/10.3390/mi12030281
Wang F, Chen L, Zhu J, Hu X, Yang Y. A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform. Micromachines. 2021; 12(3):281. https://doi.org/10.3390/mi12030281
Chicago/Turabian StyleWang, Fang, Longfei Chen, Jiaomeng Zhu, Xuejia Hu, and Yi Yang. 2021. "A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform" Micromachines 12, no. 3: 281. https://doi.org/10.3390/mi12030281
APA StyleWang, F., Chen, L., Zhu, J., Hu, X., & Yang, Y. (2021). A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform. Micromachines, 12(3), 281. https://doi.org/10.3390/mi12030281





