Lung on a Chip Development from Off-Stoichiometry Thiol–Ene Polymer
Abstract
1. Introduction
2. Materials and Methods
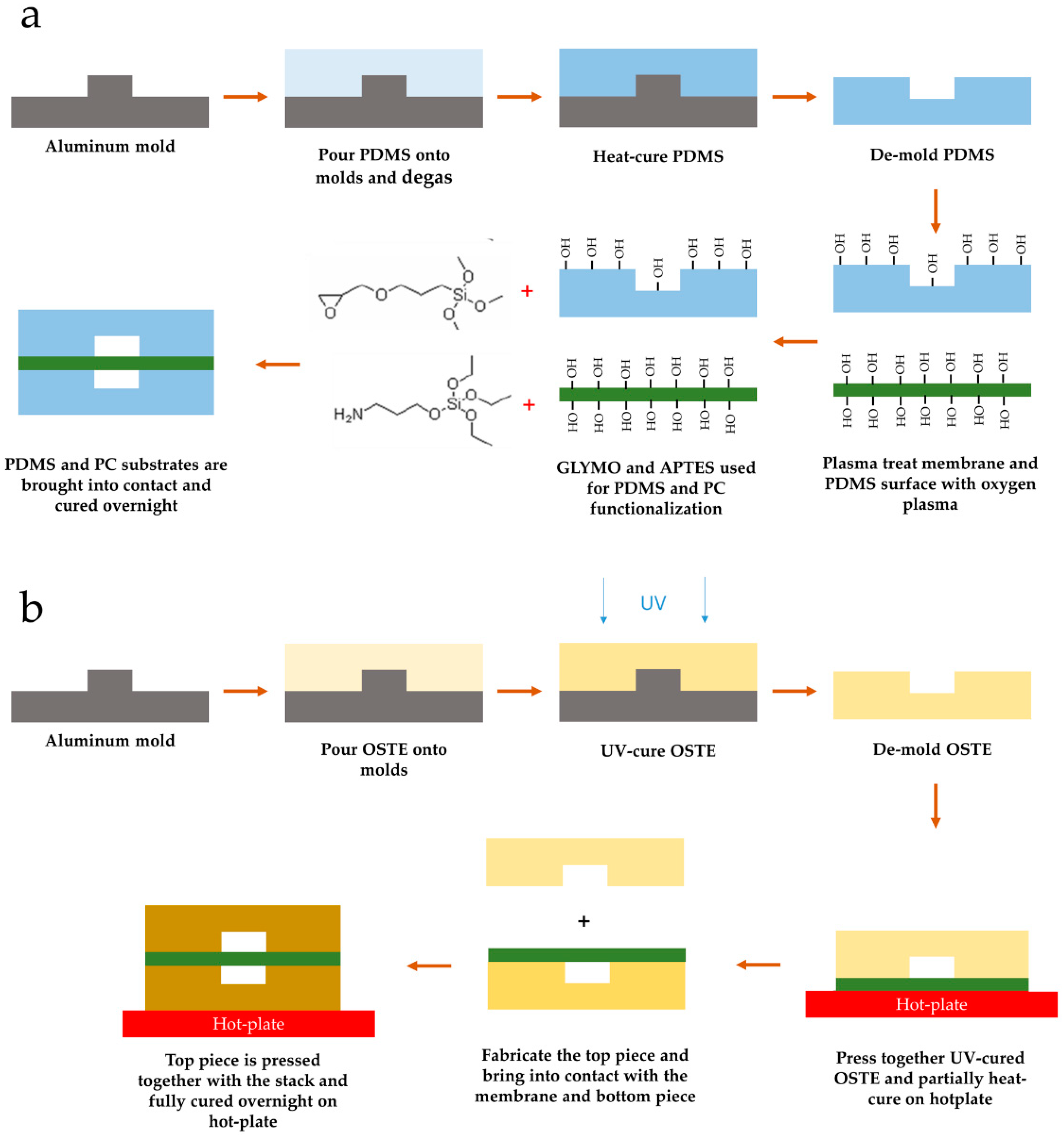
2.1. PDMS and OSTE Test Piece Fabrication for Optical and Biocompatibility Property Evaluation
2.2. PDMS and OSTE Comparison of Light Transmission
2.3. PDMS and OSTE Comparison of Rhodamine Absorption
2.4. PDMS and OSTE Polymer Effect on RNA Isolation, cDNA Synthesis, and Quantitative Reverse Transcription (qRT)-PCR Reaction
2.5. PDMS and OSTE Single-Channel Device Preparation
2.6. Membrane Particle Absorption of PDMS and OSTE
2.7. CellVue Absorption of PDMS and OSTE Materials
2.8. LOAC Device Microfabrication from PDMS and OSTE Polymers
2.9. LOAC Device Preparation and Cell Cultivation
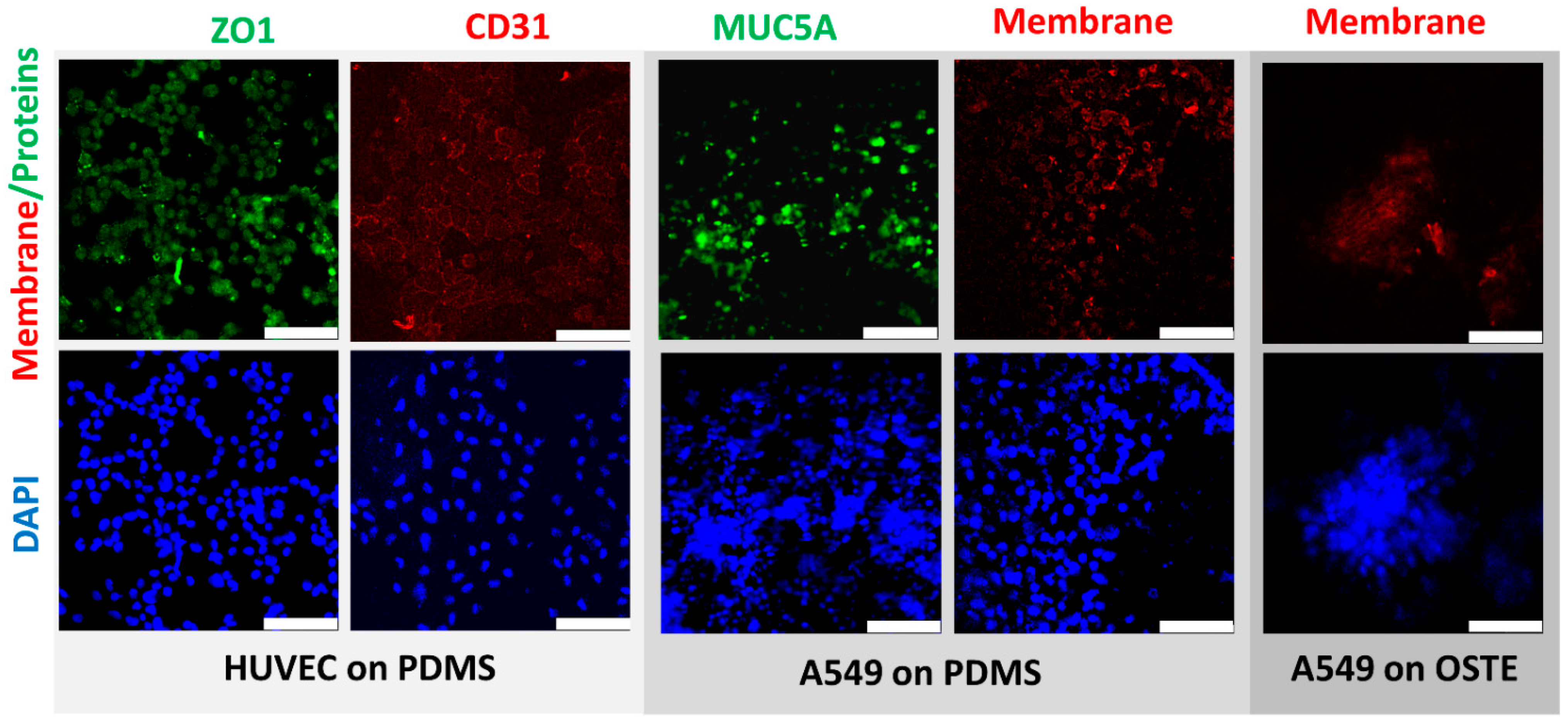
2.10. Immunofluorescence
2.11. Confocal Microscopy and Image Processing
3. Results
3.1. Comparison of OSTE and PDMS Characteristics Crucial for LOAC Development
3.2. Engineering and Cell Culturing on OSTE vs. PDMS LOAC Devices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothan, H.A.; Byrareddy, S.N. The Epidemiology and Pathogenesis of Coronavirus Disease (COVID-19) Outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, Impacts, and Mitigation. In Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed.; The World Bank: San Francisco, CA, USA, 2017; Volume 9, pp. 315–345. ISBN 9781464805271. [Google Scholar]
- Nuzzo, J.B.; Mullen, L.; Snyder, M.; Cicero, A.; Inglesby, T.V. Preparedness for a High-Impact Respiratory Pathogen Pandemic; Johns Hopkins Center for Health Security: Baltimore, MD, USA, 2019. [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 30 March 2021).
- Search of: Covid19—List Results—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results?recrs=&cond=Covid19&term=&cntry=&state=&city=&dist= (accessed on 30 March 2021).
- Ramani, S.; Crawford, S.E.; Blutt, S.E.; Estes, M.K. Human Organoid Cultures: Transformative New Tools for Human Virus Studies. Curr. Opin. Virol. 2018, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.Y.; Chan, M.C.W.; Nicholls, J.M.; Malik Peiris, J.S. Use of Ex Vivo and in Vitro Cultures of the Human Respiratory Tract to Study the Tropism and Host Responses of Highly Pathogenic Avian Influenza A (H5N1) and Other Influenza Viruses. Virus Res. 2013, 178, 133–145. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A Biomimetic Multicellular Model of the Airways Using Primary Human Cells. Lab Chip 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. Human Organ Chip-Enabled Pipeline to Rapidly Repurpose Therapeutics during Viral Pandemics. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stucki, J.D.; Hobi, N.; Galimov, A.; Stucki, A.O.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Frick, M.; Funke-Chambour, M.; Geiser, T.; et al. Medium Throughput Breathing Human Primary Cell Alveolus-on-Chip Model. Sci. Rep. 2018, 8, 14359. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic Devices Fabricated in Poly(Dimethylsiloxane) for Biological Studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability Thickness Dependence of Polydimethylsiloxane (PDMS) Membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- Camou, S.; Fujita, H.; Fujii, T. PDMS 2D Optical Lens Integrated with Microfluidic Channels: Principle and Characterization. Lab Chip 2003, 3, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.S.; De Santiago, G.T.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between Materials and Microfluidics. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of Human Organs-on-Chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human Gut-on-a-Chip Inhabited by Microbial Flora That Experiences Intestinal Peristalsis-like Motions and Flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing Human and Cross-Species Drug Toxicities Using a Liver-Chip. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Björnmalm, M.; Yan, Y.; Caruso, F. Engineering and Evaluating Drug Delivery Particles in Microfluidic Devices. J. Control. Release 2014, 190, 139–149. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Didier, M.; Calamari, E.; Ng, C.F.; Choe, Y.; Clauson, S.L.; Nestor, B.A.; Puerta, J.; Fleming, R.; Firoozinezhad, S.J.; et al. Scalable Fabrication of Stretchable, Dual Channel, Microfluidic Organ Chips. JoVE 2018, e58151. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small Molecule Absorption by PDMS in the Context of Drug Response Bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef]
- Domansky, K.; Sliz, J.D.; Wen, N.; Hinojosa, C.; Thompson, G.; Fraser, J.P.; Hamkins-Indik, T.; Hamilton, G.A.; Levner, D.; Ingber, D.E. SEBS Elastomers for Fabrication of Microfluidic Devices with Reduced Drug Absorption by Injection Molding and Extrusion. Microfluid. Nanofluidics 2017, 21, 107. [Google Scholar] [CrossRef]
- Sasaki, H.; Onoe, H.; Osaki, T.; Kawano, R.; Takeuchi, S. Parylene-Coating in PDMS Microfluidic Channels Prevents the Absorption of Fluorescent Dyes. Sens. Actuators B Chem. 2010, 150, 478–482. [Google Scholar] [CrossRef]
- Gomez-Sjoberg, R.; Leyrat, A.A.; Houseman, B.T.; Shokat, K.; Quake, S.R. Biocompatibility and Reduced Drug Absorption of Sol-Gel-Treated Poly(Dimethyl Siloxane) for Microfluidic Cell Culture Applications. Anal. Chem. 2010, 82, 8954–8960. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative Prediction of Human Pharmacokinetic Responses to Drugs via Fluidically Coupled Vascularized Organ Chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A Complex Human Gut Microbiome Cultured in an Anaerobic Intestine-on-a-Chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Haraldsson, T.; Öberg, K.; Malkoch, M.; van der Wijngaart, W. Beyond PDMS: Off-Stoichiometry Thiol–Ene (OSTE) Based Soft Lithography for Rapid Prototyping of Microfluidic Devices. Lab Chip 2011, 11, 3136. [Google Scholar] [CrossRef]
- Sticker, D.; Geczy, R.; Häfeli, U.O.; Kutter, J.P. Thiol–Ene Based Polymers as Versatile Materials for Microfluidic Devices for Life Sciences Applications. ACS Appl. Mater. Interfaces 2020, 12, 10080–10095. [Google Scholar] [CrossRef]
- Zandi Shafagh, R.; Vastesson, A.; Guo, W.; Van Der Wijngaart, W.; Haraldsson, T. E-Beam Nanostructuring and Direct Click Biofunctionalization of Thiol-Ene Resist. ACS Nano 2018. [Google Scholar] [CrossRef] [PubMed]
- Bourg, S.; d’Orlyé, F.; Griveau, S.; Bedioui, F.; da Silva, J.A.F.; Varenne, A. Multiple Zones Modification of Open Off-Stoichiometry Thiol-Ene Microchannel by Aptamers: A Methodological Study & a Proof of Concept. Chemosensors 2020, 8, 24. [Google Scholar] [CrossRef]
- Sandström, N.; Shafagh, R.Z.; Vastesson, A.; Carlborg, C.F.; van der Wijngaart, W.; Haraldsson, T. Reaction Injection Molding and Direct Covalent Bonding of OSTE+ Polymer Microfluidic Devices. J. Micromech. Microeng. 2015, 25, 075002. [Google Scholar] [CrossRef]
- Hansson, J.; Karlsson, J.M.; Carlborg, C.F.; van der Wijngaart, W.; Haraldsson, T. Low Gas Permeable and Non-Absorbent Rubbery OSTE+ for Pneumatic Microvalves. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 987–990. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS Absorption of Small Molecules and Consequences in Microfluidic Applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Endzeliņs, E.; Abols, A.; Bušs, A.; Zandberga, E.; Palviainen, M.; Siljander, P.; Line, A. Extracellular Vesicles Derived from Hypoxic Colorectal Cancer Cells Confer Metastatic Phenotype to Non-Metastatic Cancer Cells. Anticancer Res. 2018, 38, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Popěna, I.; Abols, A.; Saulite, L.; Pleiko, K.; Zandberga, E.; Jěkabsons, K.; Endzeliņš, E.; Llorente, A.; Lině, A.; Riekstiņa, U. Effect of Colorectal Cancer-Derived Extracellular Vesicles on the Immunophenotype and Cytokine Secretion Profile of Monocytes and Macrophages. Cell Commun. Signal. 2018, 16, 17. [Google Scholar] [CrossRef]
- Tang, L.; Lee, N.Y. A Facile Route for Irreversible Bonding of Plastic-PDMS Hybrid Microdevices at Room Temperature. Lab Chip 2010, 10, 1274–1280. [Google Scholar] [CrossRef]
- Gong, W.; Das, P.; Samanta, S.; Xiong, J.; Pan, W.; Gu, Z.; Zhang, J.; Qu, J.; Yang, Z. Redefining the Photo-Stability of Common Fluorophores with Triplet State Quenchers: Mechanistic Insights and Recent Updates. Chem. Commun. 2019, 55, 8695–8704. [Google Scholar] [CrossRef]
- Makurvet, F.D. Biologics vs. Small Molecules: Drug Costs and Patient Access. Med. Drug Discov. 2021, 9, 100075. [Google Scholar] [CrossRef]
- Zhou, X.C.; Sjöberg, R.; Druet, A.; Schwenk, J.M.; van der Wijngaart, W.; Haraldsson, T.; Carlborg, C.F. Thiol-Ene-Epoxy Thermoset for Low-Temperature Bonding to Biofunctionalized Microarray Surfaces. Lab Chip 2017, 17, 3672–3681. [Google Scholar] [CrossRef]
- Kodzius, R.; Xiao, K.; Wu, J.; Yi, X.; Gong, X.; Foulds, I.G.; Wen, W. Inhibitory Effect of Common Microfluidic Materials on PCR Outcome. Sens. ActuatorsB: Chem. 2012, 161, 349–358. [Google Scholar] [CrossRef]
- Nolte-’t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular Vesicles and Viruses: Are They Close Relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Zhou, X.; Calborg, C.F.; Sandström, N.; Haleem, A.; Vastesson, A.; Saharil, F.; Van Der Wijngaart, W.; Haraldsson, T. Rapid Fabrication of OSTE+ Microfluidic Devices with Lithographically Defined Hydrophobic/ Hydrophilic Patterns and Biocompatible Chip Sealing. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2013, Freiburg, Germany, 27–31 October 2013; pp. 134–136. [Google Scholar]
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.T.; Ertl, P. Multi-Layered, Membrane-Integrated Microfluidics Based on Replica Molding of a Thiol-Ene Epoxy Thermoset for Organ-on-a-Chip Applications. Lab Chip 2015, 15, 4542–4554. [Google Scholar] [CrossRef] [PubMed]
- Ejserholm, F.; Stegmayr, J.; Bauer, P.; Johansson, F.; Wallman, L.; Bengtsson, M.; Oredsson, S. Biocompatibility of a Polymer Based on Off-Stoichiometry Thiol-Enes + Epoxy (OSTE+) for Neural Implants. Biomater. Res. 2015, 19, 19. [Google Scholar] [CrossRef]
- Chang, C.W.; Cheng, Y.J.; Tu, M.; Chen, Y.H.; Peng, C.C.; Liao, W.H.; Tung, Y.C. A Polydimethylsiloxane-Polycarbonate Hybrid Microfluidic Device Capable of Generating Perpendicular Chemical and Oxygen Gradients for Cell Culture Studies. Lab Chip 2014, 14, 3762–3772. [Google Scholar] [CrossRef]
- Bergemann, C.; Quade, A.; Kunz, F.; Ofe, S.; Klinkenberg, E.-D.; Laue, M.; Schröder, K.; Weissmann, V.; Hansmann, H.; Weltmann, K.-D.; et al. Ammonia Plasma Functionalized Polycarbonate Surfaces Improve Cell Migration Inside an Artificial 3D Cell Culture Module. Plasma Process. Polym. 2012, 9, 261–272. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Park, K.S.; Khang, G.; Lee, Y.M.; Lee, H.B. Response of MG63 Osteoblast-like Cells onto Polycarbonate Membrane Surfaces with Different Micropore Sizes. Biomaterials 2004, 25, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.H.; Thomée, E.K.; Dellaquila, A.; Nassman, H.; Segura, T.; Lesher-Pérez, S.C. Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices. Micromachines 2020, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Carterson, A.J.; Höner Zu Bentrup, K.; Ott, C.M.; Clarke, M.S.; Pierson, D.L.; Vanderburg, C.R.; Buchanan, K.L.; Nickerson, C.A.; Schurr, M.J. A549 Lung Epithelial Cells Grown as Three-Dimensional Aggregates: Alternative Tissue Culture Model for Pseudomonas Aeruginosa Pathogenesis. Infect. Immun. 2005, 73, 1129–1140. [Google Scholar] [CrossRef]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-Chips with Integrated Electrodes for Trans-Epithelial Electrical Resistance (TEER) Measurements of Human Epithelial Barrier Function. Lab Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef]
- ORCHID. Organ-on-Chip in Development. Available online: https://h2020-orchid.eu/ (accessed on 30 March 2021).
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Rimsa, R.; Smith, A.J.; Wälti, C.; Wood, C.D. A Planar Surface Acoustic Wave Micropump for Closed-Loop Microfluidics. Appl. Phys. Lett. 2017, 111, 234102. [Google Scholar] [CrossRef]
- Nguyen, T.; Chidambara, V.A.; Andreasen, S.Z.; Golabi, M.; Huynh, V.N.; Linh, Q.T.; Bang, D.D.; Wolff, A. Point-of-Care Devices for Pathogen Detections: The Three Most Important Factors to Realise towards Commercialization. Trac Trends Anal. Chem. 2020, 131, 116004. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.W.; Wang, Y.; Yao, X.; Mu, X.; Lu, X.; Zhou, J.; Cai, Z.; Ren, K. Reliable and Reusable Whole Polypropylene Plastic Microfluidic Devices for a Rapid, Low-Cost Antimicrobial Susceptibility Test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef]
- Ogończyk, D.; Wgrzyn, J.; Jankowski, P.; Dąbrowski, B.; Garstecki, P. Bonding of Microfluidic Devices Fabricated in Polycarbonate. Lab Chip 2010, 10, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection Molding and Characterization of PMMA-Based Microfluidic Devices. Microsyst. Technol. 2020, 26, 1317–1324. [Google Scholar] [CrossRef]
- Guillemette, M.D.; Roy, E.; Auger, F.A.; Veres, T. Rapid Isothermal Substrate Microfabrication of a Biocompatible Thermoplastic Elastomer for Cellular Contact Guidance. Acta Biomater. 2011, 7, 2492–2498. [Google Scholar] [CrossRef]
- Domansky, K.; Leslie, D.C.; McKinney, J.; Fraser, J.P.; Sliz, J.D.; Hamkins-Indik, T.; Hamilton, G.A.; Bahinski, A.; Ingber, D.E. Clear Castable Polyurethane Elastomer for Fabrication of Microfluidic Devices. Lab Chip 2013, 13, 3956–3964. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Thach, H.; Roy, E.; Huynh, K.; Perrault, C. Low-Cost, Accessible Fabrication Methods for Microfluidics Research in Low-Resource Settings. Micromachines 2018, 9, 461. [Google Scholar] [CrossRef]
- MacLean-Blevins, M.T. Process Selection—Which Plastics Process to Use? Des. Success. Prod. Plast. 2018, 51–77. [Google Scholar] [CrossRef]
- Xie, P.; Chang, L.; Song, L.; Cai, T.; Ding, Y.; Yang, W. The Research of UV Curing Injection Molding. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1664, p. 110005. [Google Scholar]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; Fitzgerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with Combined Multi-Electrode Array and Transepithelial Electrical Resistance Measurement Capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Parfejevs, V.; Sagini, K.; Buss, A.; Sobolevska, K.; Llorente, A.; Riekstina, U.; Abols, A. Adult Stem Cell-Derived Extracellular Vesicles in Cancer Treatment: Opportunities and Challenges. Cells 2020, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef]
- Sobrino, A.; Phan, D.T.T.; Datta, R.; Wang, X.; Hachey, S.J.; Romero-López, M.; Gratton, E.; Lee, A.P.; George, S.C.; Hughes, C.C.W. 3D Microtumors in Vitro Supported by Perfused Vascular Networks. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Allwardt, V.; Ainscough, A.J.; Viswanathan, P.; Sherrod, S.D.; McLean, J.A.; Haddrick, M.; Pensabene, V. Translational Roadmap for the Organs-on-a-Chip Industry toward Broad Adoption. Bioengineering 2020, 7, 112. [Google Scholar] [CrossRef]
- Karg, T.J.; Golic, K.G. Photoconversion of DAPI and Hoechst Dyes to Green and Red-Emitting Forms after Exposure to UV Excitation. Chromosoma 2018, 127, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Geczy, R.; Sticker, D.; Bovet, N.; Häfeli, U.O.; Kutter, J.P. Chloroform Compatible, Thiol-Ene Based Replica Molded Micro Chemical Devices as an Alternative to Glass Microfluidic Chips. Lab Chip 2019, 19, 798–806. [Google Scholar] [CrossRef]
- Gökaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(Dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(Dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS Membranes with Tunable Gas Permeability for Microfluidic Applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]



| Property | PDMS | OSTE |
|---|---|---|
| Light transmission in the 300–800 nm range | Good, comparable to glass | Intermediate, multilayer chips suffer from significant light scattering |
| Cell viability | Good cell viability [23] | Acceptable cell viability [48] |
| Bonding to PC membrane | Intermediate steps necessary to functionalize PDMS and membrane materials [40] | Readily bondable via epoxy groups available prior to thermal treatment [47] |
| Surface modification | Intermediate steps necessary [76] | Readily available –OH or –SH groups [31] |
| Stability in chloroform and ethanol, solvents used for cell fixing | Medium–poor [77] | Good [75] |
| Gas permeability | High [78] | Low [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimsa, R.; Galvanovskis, A.; Plume, J.; Rumnieks, F.; Grindulis, K.; Paidere, G.; Erentraute, S.; Mozolevskis, G.; Abols, A. Lung on a Chip Development from Off-Stoichiometry Thiol–Ene Polymer. Micromachines 2021, 12, 546. https://doi.org/10.3390/mi12050546
Rimsa R, Galvanovskis A, Plume J, Rumnieks F, Grindulis K, Paidere G, Erentraute S, Mozolevskis G, Abols A. Lung on a Chip Development from Off-Stoichiometry Thiol–Ene Polymer. Micromachines. 2021; 12(5):546. https://doi.org/10.3390/mi12050546
Chicago/Turabian StyleRimsa, Roberts, Artis Galvanovskis, Janis Plume, Felikss Rumnieks, Karlis Grindulis, Gunita Paidere, Sintija Erentraute, Gatis Mozolevskis, and Arturs Abols. 2021. "Lung on a Chip Development from Off-Stoichiometry Thiol–Ene Polymer" Micromachines 12, no. 5: 546. https://doi.org/10.3390/mi12050546
APA StyleRimsa, R., Galvanovskis, A., Plume, J., Rumnieks, F., Grindulis, K., Paidere, G., Erentraute, S., Mozolevskis, G., & Abols, A. (2021). Lung on a Chip Development from Off-Stoichiometry Thiol–Ene Polymer. Micromachines, 12(5), 546. https://doi.org/10.3390/mi12050546







