Biomimetic Prosthetic Hand Enabled by Liquid Crystal Elastomer Tendons
Abstract
:1. Introduction
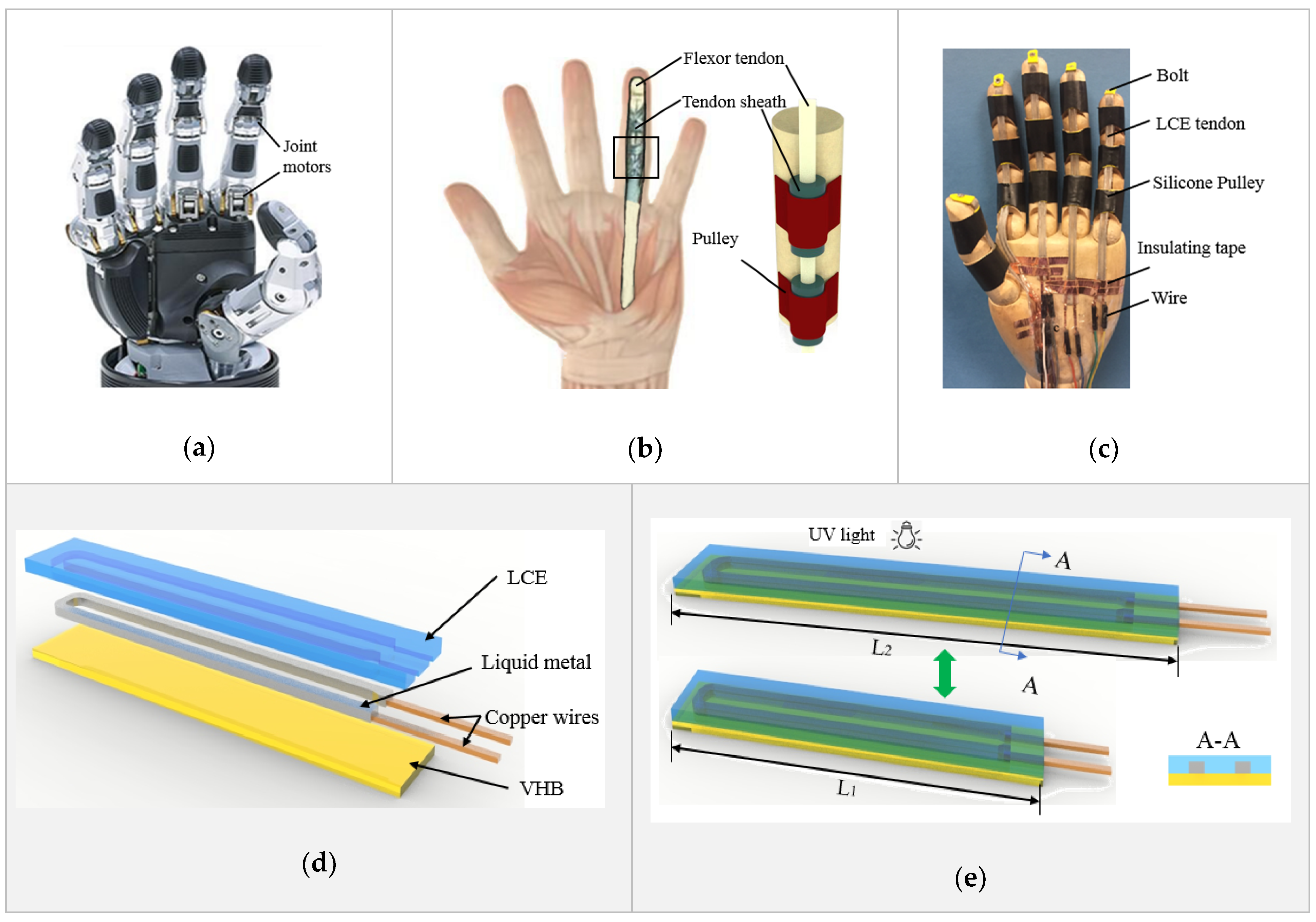
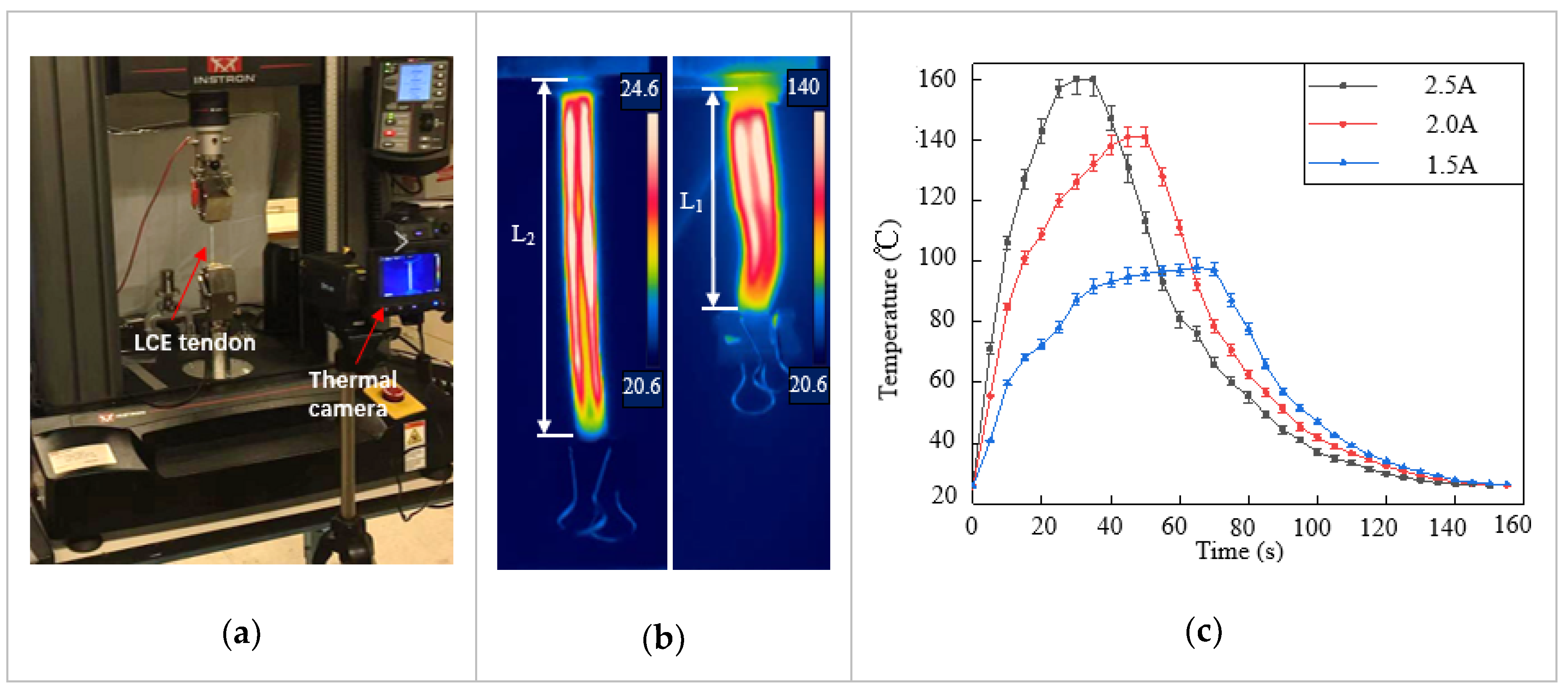
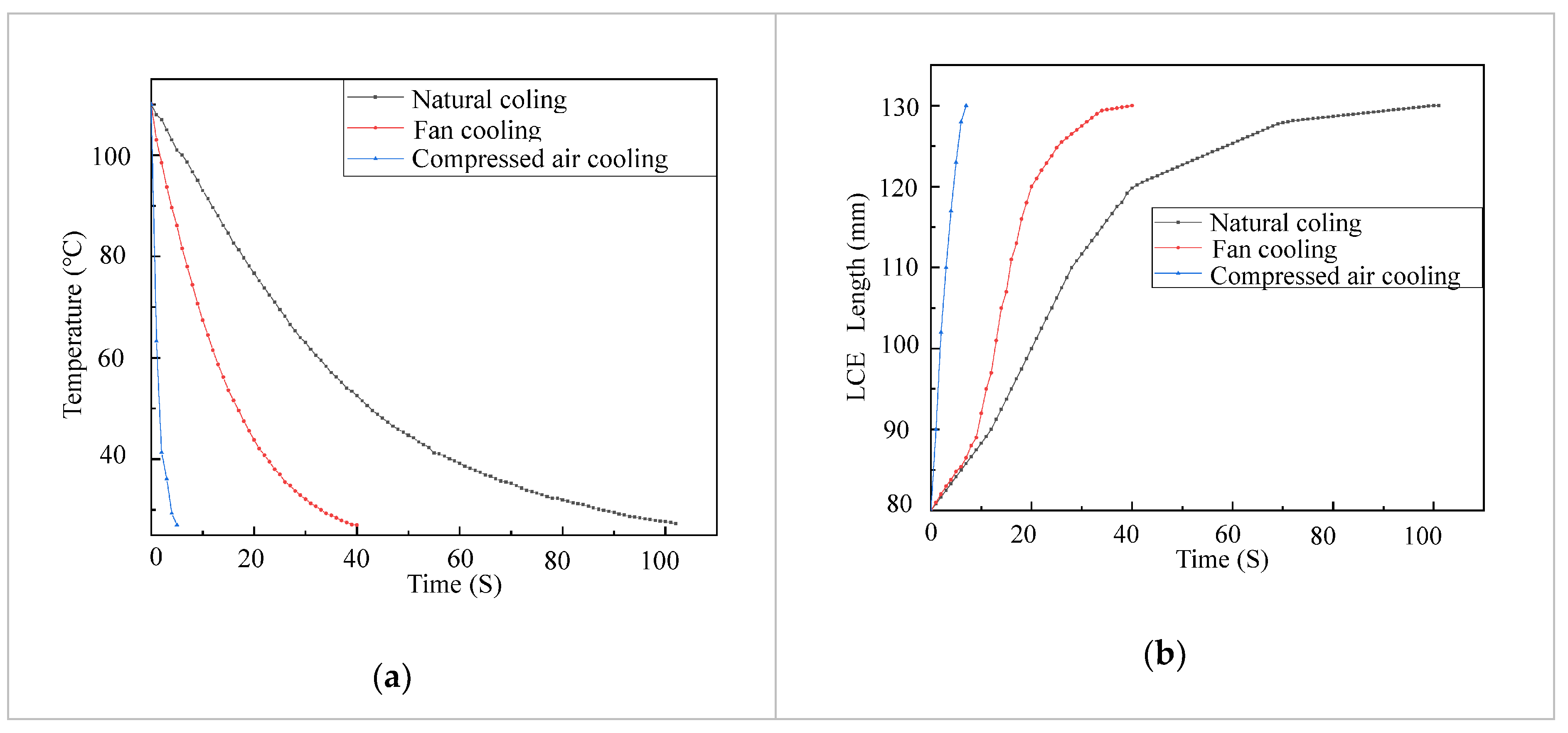
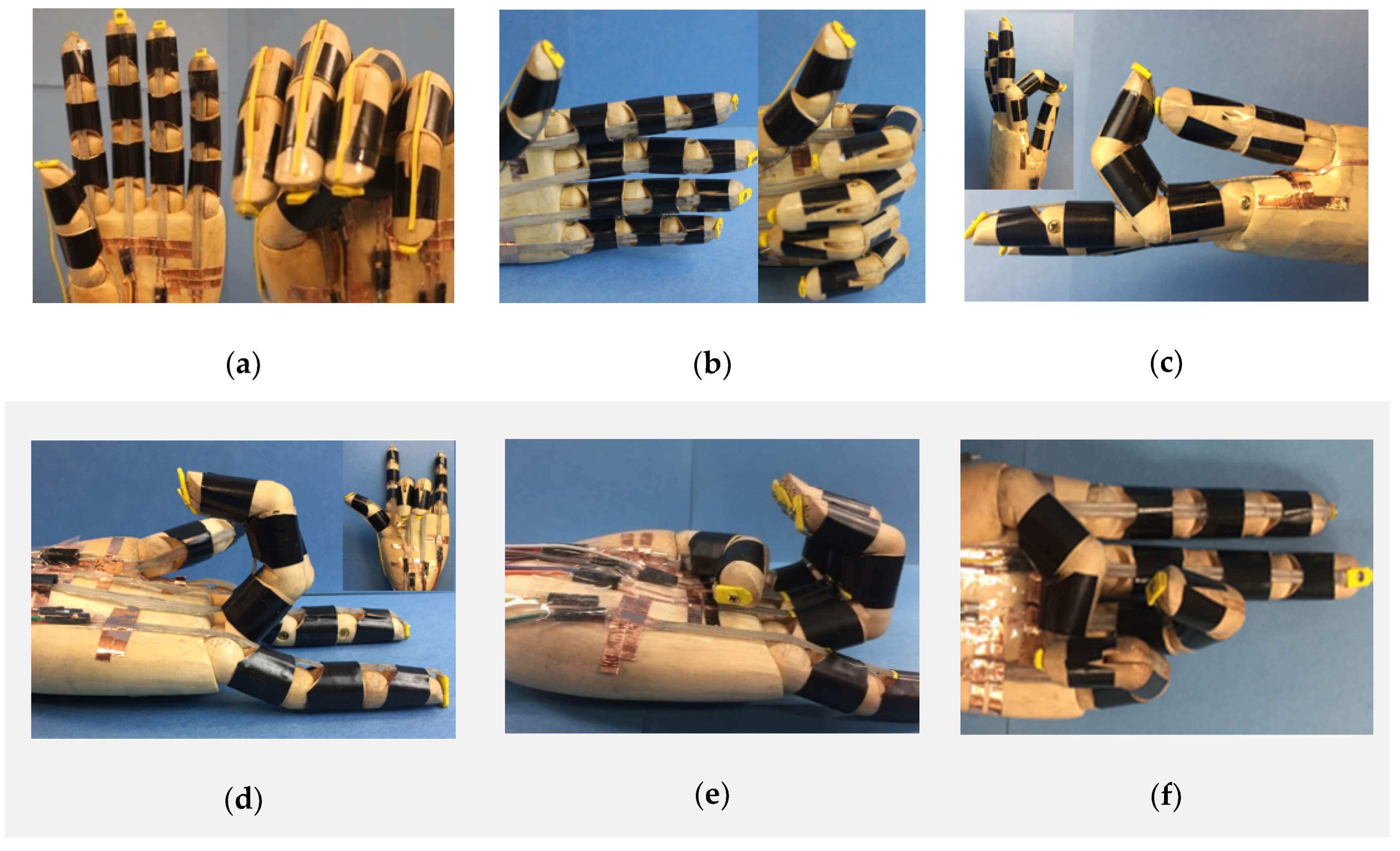
2. Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Han, Z.; Zhang, H.; Shang, X.; Wang, T.; Guo, W.; Gruver, W.A. Design and control of the BUAA four-fingered hand. In Proceedings of the IEEE International Conference on Robotics and Automation, Seoul, Korea, 21–26 May 2001; Volume 3, pp. 2517–2522. [Google Scholar]
- Clement, R.; Bugler, K.; Oliver, C.W. Bionic prosthetic hands: A review of present technology and future aspirations. Surgeon 2011, 9, 336–340. [Google Scholar] [CrossRef]
- Geethanjali, P. Myoelectric control of prosthetic hands: State-of-the-art review. Med. Devices 2016, 2016, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belter, J.T.; Dollar, A.M. Performance characteristics of anthropomorphic prosthetic hands. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar]
- Controzzi, M.; Cipriani, C.; Carrozza, M.C. Design of Artificial Hands: A Review. In The Human Hand as an Inspiration for Robot Hand Development; Balasubramanian, R., Santos, V.J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 219–246. [Google Scholar]
- Vincent Systems GmbH. Available online: https://vincentsystems.de/en/ (accessed on 24 February 2019).
- I-Limb Ultra Touch Bionics. Available online: https://touchbionics.com/products/active-prostheses/i-limb-ultra (accessed on 24 February 2019).
- Life Changing Myoelectric Hand Packed with the Latest Technology-Bebionic. Available online: http://bebionic.com/the_hand (accessed on 24 February 2019).
- Michelangelo Prosthetic Hand. Available online: https://www.ottobockus.com/prosthetics/upper-limb-prosthetics/solution-overview/michelangelo-prosthetic-hand/ (accessed on 24 February 2019).
- Chang, J.H. Design and control of a robotic finger for prosthetic hands. In Proceedings of the 1999 IEEE/RSJ International Conference on Intelligent Robots and Systems. Human and Environment Friendly Robots with High Intelligence and Emotional Quotients (Cat. No.99CH36289), Kyongju, Korea, 17–21 October 1999; Volume 1, pp. 113–117. [Google Scholar]
- Zecca, M.; Micera, S.; Carrozza, M.C.; Dario, P. Control of Multifunctional Prosthetic Hands by Processing the Electromyographic Signal. Crit. Rev. Biomed. Eng. 2002, 30, 459–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Gao, X.; Andrew, T.C. A Magnetically Coupled Dielectric Elastomer Pump for Soft Robotics. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef]
- Mitchell, S.K.; Xingrui, W.; Eric, A.; Trent, M.; Khoi, L.; Nicholas, K.; Gopaluni, V.V.; Christoph, K. An Easy-to-Implement Toolkit to Create Versatile and High-Performance HASEL Actuators for Untethered Soft Robots. Adv. Sci. 2019, 6, 1900178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, C.; Liang, X.; Cai, S. Bioinspired Design of Light-Powered Crawling, Squeezing, and Jumping Untethered Soft Robot. Adv. Mater. Technol. 2019, 4, 4. [Google Scholar] [CrossRef]
- Acome, E.; Mitchell, S.K.; Morrissey, T.G.; Emmett, M.B.; Benjamin, C.; King, M.; Radakovitz, M.; Keplinger, C. Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 2018, 359, 6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Wang, X.; Fei, D.; Zhe, Y.; Zeming, L.; Yingbo, Y.; Liangliang, Z.; Yilun, L.; Hang, X.; Xi, C. Elementary Slender Soft Robots Inspired by Skeleton Joint System of Animals. Soft Robot. 2019, 6, 377–388. [Google Scholar] [CrossRef]
- Huang, X.; Kumar, K.; Jawed, M.K.; Nasab, A.M.; Ye, Z.; Shan, W.; Majidi, C. Highly Dynamic Shape Memory Alloy Actuator for Fast Moving Soft Robots. Adv. Mater. Technol. 2019, 4, 4. [Google Scholar] [CrossRef]
- Majidi, C. Soft-Matter Engineering for Soft Robotics. Adv. Mater. Technol. 2019, 4, 1800477. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, B.; Milana, E.; Baeyens, A.; Broeders, E.; Christiaens, J.; Collin, K.; Reynaerts, D.; Volder, M. Hardware Sequencing of Inflatable Nonlinear Actuators for Autonomous Soft Robots. Adv. Mater. 2019, 31, e1804598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, H.; Tsujiuchi, N.; Koizumi, T.; Kan, H.; Hirano, M.; Nakamura, Y. Development of prosthetic arm with pneumatic prosthetic hand and tendon-driven wrist. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5048–5051. [Google Scholar]
- Schorger, K.; Simon, J.P.; Clark, D.; Williams, A. Pneumatic Hand Prosthesis Project. 2018. Available online: https://scholarworks.calstate.edu/downloads/5d86p1034?locale=en (accessed on 22 June 2021).
- Ellen, R.; Seppe, T.; Joost, B.; Robrecht, V.; Guy, V.; Bram, V. Additive Manufacturing for Self-Healing Soft Robots. Soft Robot. 2020. [Google Scholar] [CrossRef]
- Nikolaos, V.; Andrew, G.J.; Soifer, S.; Johannes, O.; Katia, B. Harnessing Viscous Flow to Simplify the Actuation of Fluidic Soft Robots. Soft Robot. 2020, 7. [Google Scholar] [CrossRef]
- Zeguang, P.; Xiong, X.; Jian, H.; Yan, Z. Highly Stretchable and Durable Conductive Knitted Fabrics for the Skins of Soft Robots. Soft Robot. 2019, 6. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, C.; Wang, W.; Pan, M.; Yang, H.; Zou, J. Reconfigurable Soft Robots: Advanced Artificial Muscle for Flexible Material-Based Reconfigurable Soft Robots. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Matthew, I.; Takuya, U.; Tadahiro, T.; Yoshihiro, K. Exploring Behaviors of Caterpillar-Like Soft Robots with a Central Pattern Generator-Based Controller and Reinforcement Learning. Soft Robot. 2019, 6. [Google Scholar] [CrossRef]
- Lau, J.H. Overview and Outlook of Three-Dimensional Integrated Circuit Packaging, Three-Dimensional Si Integration, and Three-Dimensional Integrated Circuit Integration. J. Electron. Packag. 2014, 136, 040801. [Google Scholar] [CrossRef] [Green Version]
- Andrianesis, K.; Tzes, A. Development and Control of a Multifunctional Prosthetic Hand with Shape Memory Alloy Actuators. J. Intell. Robot. Syst. 2015, 78, 257–289. [Google Scholar] [CrossRef]
- Le, B.; McVary, K.; McKenna, K.; Colombo, A. A Novel Thermal-activated Shape Memory Penile Prosthesis: Comparative Mechanical Testing. Urology 2017, 99, 136–141. [Google Scholar] [CrossRef]
- Wang, H.; Totaro, M.; Beccai, L. Toward Perceptive Soft Robots: Progress and Challenges. Adv. Sci. 2018, 5, 1800541. [Google Scholar] [CrossRef]
- Jiachen, Z.; Eric, D. Untethered Miniature Soft Robots: Modeling and Design of a Millimeter-Scale Swimming Magnetic Sheet. Soft Robot. 2018. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, J. Design of Multifunctional Soft Doming Actuator for Soft Machines. Adv. Mater. Technol. 2018, 3, 1800069. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Deng, J.; Liu, R.; Hsiao, Y.; Zhang, S.L.; Peng, W.; Wu, H.; Wang, X.; Wang, Z.L. Actively Perceiving and Responsive Soft Robots Enabled by Self-Powered, Highly Extensible, and Highly Sensitive Triboelectric Proximity- and Pressure-Sensing Skins. Adv. Mater. 2018, 30, e1801114. [Google Scholar] [CrossRef]
- Wang, C.; Sim, K.; Chen, J.; Kim, H.; Rao, Z.; Li, Y.; Chen, W.; Song, J.; Verduzco, R.; Yu, C. Soft Ultrathin Electronics Innervated Adaptive Fully Soft Robots. Adv. Mater. 2018, 30, 1706695. [Google Scholar] [CrossRef] [Green Version]
- Gerratt, A.P.; Michaud, H.O.; Lacour, S.P. Elastomeric Electronic Skin for Prosthetic Tactile Sensation. Adv. Funct. Mater. 2015, 25, 2287–2295. [Google Scholar] [CrossRef]
- Mehta, J.; Chandra, Y.; Tewari, R. The Use of Dielectric Elastomer Actuators for Prosthetic, Orthotic and Bio-Robotic Applications. Procedia Comput. Sci. 2018, 133, 569–575. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, Z.; Tong, X.; Zhao, Y. Biomimetic Locomotion of Electrically Powered “Janus” Soft Robots Using a Liquid Crystal Polymer. Adv. Mater. 2019, 31, e1903452. [Google Scholar] [CrossRef] [PubMed]
- Jiangbei, W.; Yanqiong, F.; Zhaoyu, L. FifoBots: Foldable Soft Robots for Flipping Locomotion. Soft Robot. 2019, 6. [Google Scholar] [CrossRef]
- Jiao, Z.; Ji, C.; Zou, J.; Yang, H.; Pan, M. Vacuum-Powered Soft Pneumatic Twisting Actuators to Empower New Capabilities for Soft Robots. Adv. Mater. Technol. 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Oh, N.; Park, Y.J.; Lee, S.; Lee, H.; Rodrigue, H. Design of Paired Pouch Motors for Robotic Applications. Adv. Mater. Technol. 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Yunquan, L.; Yonghua, C.; Tao, R.; Yingtian, L.; Hong, C.S. Precharged Pneumatic Soft Actuators and Their Applications to Untethered Soft Robots. Soft Robot. 2018, 5, 567–575. [Google Scholar]
- Kim, K.; Cho, K.H.; Jung, H.S.; Yang, S.Y.; Kim, Y.; Park, J.H.; Jang, H.; Nam, J.; Koo, J.C.; Moon, H.; et al. Double Helix Twisted and Coiled Soft Actuator from Spandex and Nylon. Adv. Eng. Mater. 2018, 20. [Google Scholar] [CrossRef]
- Huaroto, J.J.; Suarez, E.; Krebs, H.I.; Marasco, P.D.; Vela, E.A. A Soft Pneumatic Actuator as a Haptic Wearable Device for Upper Limb Amputees: Toward a Soft Robotic Liner. IEEE Robot. Autom. Lett. 2019, 4, 17–24. [Google Scholar] [CrossRef]
- Caldwell, D.; Tsagarakis, N. Biomimetic actuators in prosthetic and rehabilitation applications. Technol. Health Care 2002, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, E.G.; O’Reilly, O.M. Continuous Models for Peristaltic Locomotion with Application to Worms and Soft Robots). Biomech. Model. Mechanobiol. 2020, 20, 5–30. [Google Scholar] [CrossRef]
- Yue, C.; Long, W.; Kevin, G.; Isuru, G.; Nabil, S.; Eric, B. Modal-Based Kinematics and Contact Detection of Soft Robots. Soft Robot. 2020. [Google Scholar] [CrossRef]
- Javier, T.; Espen, K.; Mojmir, M.; Miguel, O.; Moritz, B. MakeSense: Automated Sensor Design for Proprioceptive Soft Robots. Soft Robot. 2020, 7, 332–345. [Google Scholar]
- Nathaniel, G.; Xiaonan, H.; Carmel, M.; Alyssa, N.; O’Reilly Oliver, M.; Paley Derek, A.; Scott William, L. On Planar Discrete Elastic Rod Models for the Locomotion of Soft Robots. Soft Robot. 2019, 6, 595–610. [Google Scholar]
- Zhang, Y.; Ng, C.J.; Chen, Z.; Zhang, W.; Panjwani, S.; Kowsari, K.; Yang, H.Y.; Ge, Q. Miniature Pneumatic Actuators for Soft Robots by High-Resolution Multimaterial 3D Printing. Adv. Mater. Technol. 2019, 4, 4. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol–acrylate reaction. RSC Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- Yuan, C.; Roach, D.J.; Dunn, C.K.; Mu, Q.; Kuang, X.; Yakacki, C.M.; Wang, T.J.; Yu, K.; Qi, H.J. 3D printed reversible shape changing soft actuators assisted by liquid crystal elastomers. Soft Matter 2017, 13, 5558–5568. [Google Scholar] [CrossRef]
- Yu, Y.; Ikeda, T. Soft Actuators Based on Liquid-Crystalline Elastomers. Angew. Chem. Int. Ed. 2006, 45, 5416–5418. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Yang, Y.; Xu, Y.-S.; Qian, X.; Wei, Y.; Ji, Y. Durable liquid-crystalline vitrimer actuators. Chem. Sci. 2019, 10, 3025–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Li, C.; Huang, X. Actuators based on liquid crystalline elastomer materials. Nanoscale 2013, 5, 5225–5240. [Google Scholar] [CrossRef]
- Yin, L.; Han, L.; Ge, F.; Tong, X.; Zhang, W.; Soldera, A.; Zhao, Y. A Novel Side-Chain Liquid Crystal Elastomer Exhibiting Anomalous Reversible Shape Change. Angew. Chem. 2020, 132, 15129–15134. [Google Scholar] [CrossRef] [PubMed]
- Guin, T.; Hinton, H.E.; Burgeson, E.; Bowland, C.C.; Kearney, L.T.; Li, Y.; Ivanov, I.; Ngoc, A.N.; Amit, K.N. Tunable Electromechanical Liquid Crystal Elastomer Actuators. Adv. Intell. Syst. 2020, 2, 2000022. [Google Scholar] [CrossRef] [Green Version]
- Traugutt, A.N.; Mistry, D.; Luo, C.; Yu, K.; Ge, Q.; Yakacki, M.C. Liquid-Crystal-Elastomer-Based Dissipative Structures by Digital Light Processing 3D Printing. Adv. Mater. 2020, 32, 2000797. [Google Scholar] [CrossRef] [PubMed]
- Ziyu, W.; Jasleen, K.L.; Yannick, F.; Hans, Z. Compact tunable mid-infrared Fabry–Pérot filters actuated by liquid crystal elastomers. J. Micromech. Microeng. 2020, 30, 075002. [Google Scholar]
- Wang, Y.; Wang, Z.; He, Q.; Iyer, P.; Cai, S. Electrically Controlled Soft Actuators with Multiple and Reprogrammable Actuation Modes. Adv. Intell. Syst. 2020, 2, 1900177. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, Y.; Wang, H.; Poling-Skutvik, R.; Osuji, O.C.; Yang, S. Shaping and Locomotion of Soft Robots Using Filament Actuators Made from Liquid Crystal Elastomer–Carbon Nanotube Composites. Adv. Intell. Syst. 2020, 2, 1900163. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, N.; Qin, B.; Xu, J.; Yang, W.; Li, C.; Sun, L.; Zhang, J. Ultrasonics Sonochemistry Assisted Preparation of Polysiloxane Main-Chain Liquid-Crystalline Elastomers. Macromol. Chem. Phys. 2020, 221, 221. [Google Scholar] [CrossRef]
- Taras, T.; Jess, K.; Greta, B.; Hao, Y.; Sergij, S.; QiHuo, W.; MinHo, K.; Oleg, L. Topology control of human fibroblast cells monolayer by liquid crystal elastomer. Sci. Adv. 2020, 6, eaaz6485. [Google Scholar]
- Wu, Y.; Yang, Y.; Qian, X.; Chen, Q.; Wei, Y.; Ji, Y. Liquid-Crystalline Soft Actuators with Switchable Thermal Reprogrammability. Angew. Chem. 2020, 132, 4778–4784. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Tang, Y.; Liu, H.; Yin, J. Leveraging Monostable and Bistable Pre-Curved Bilayer Actuators for High-Performance Multitask Soft Robots. Adv. Mater. Technol. 2020, 5, 5. [Google Scholar] [CrossRef]
- Peter, B.; Yoav, M.; Amir, G.D. Fluid Mechanics of Pneumatic Soft Robots. Soft Robot. 2020. [Google Scholar] [CrossRef]
- Galloway, K.C.; Chen, Y.; Templeton, E.; Rife, B.; Godage, I.S.; Barth, E.J. Fiber Optic Shape Sensing for Soft Robotics. Soft Robot. 2019, 6, 5. [Google Scholar] [CrossRef]
- Shih, B.; Shah, D.S.; Li, J.; Thuruthel, T.G.; Park, Y.; Iida, F.; Bao, Z.; Kramer-Bottiglio, R.; Tolley, M. Electronic Skins and Machine Learning for Intelligent Soft Robots. J. Robot. Mach. Learn. 2020, 5, eaaz9239. [Google Scholar] [CrossRef]
- Haiqing, L.; Zhanan, Z.; Xingli, W.; Chuanqian, S.; Jianliang, X. Fabrication and Characterization of Highly Deformable Artificial Muscle Fibers Based on Liquid Crystal Elastomers. J. Appl. Mech. 2021, 88, 041003. [Google Scholar]
- Emuna, N.; Cohen, N. Inflation-Induced Twist in Geometrically Incompatible Isotropic Tubes. J. Appl. Mech. 2021, 88, 1–20. [Google Scholar] [CrossRef]
- Wang, Y.; Loh, L.Y.W.; Gupta, U.; Foo, C.C.; Zhu, J. Bio-Inspired Soft Swim Bladders of Large Volume Change Using Dual Dielectric Elastomer Membranes. J. Appl. Mech. 2020, 87, 1–27. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Zhu, F.; Huang, Y. Thermal and Mechanical Analyses of Compliant Thermoelectric Coils for Flexible and Bio-integrated Devices. J. Appl. Mech. 2021, 88, 021011. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Linghu, C.; Wang, S.; Song, J. Mechanics Strategies for Implantation of Flexible Neural Probes. J. Appl. Mech. 2021, 88, 010801. [Google Scholar] [CrossRef]
- Vinnikova, S.; Fang, H.; Wang, S. Mechanics of Regular-Shape Nanomeshes for Transparent and Stretchable Devices. J. Appl. Mech. 2020, 87, 101010. [Google Scholar] [CrossRef]
- Tang, R.; Fu, H. Mechanics of Buckled Kirigami Membranes for Stretchable Interconnects in Island-Bridge Structures. J. Appl. Mech. 2020, 87, 051002. [Google Scholar] [CrossRef]
- Gonzalez, K.; Xue, J.; Chu, A.; Kirane, K. Fracture and Energetic Strength Scaling of Soft, Brittle, and Weakly Nonlinear Elastomers. J. Appl. Mech. 2020, 87, 1–38. [Google Scholar] [CrossRef]
- Ardebili, H. A Perspective on the Mechanics Issues in Soft Solid Electrolytes and the Development of Next-Generation Batteries. J. Appl. Mech. 2020, 87, 040801. [Google Scholar] [CrossRef]
- Zhang, Y.; Lü, C.; Lu, B.; Feng, X.; Wang, J. Theoretical Modeling on Monitoring Left Ventricle Deformation Using Conformal Piezoelectric Sensors. J. Appl. Mech. 2020, 87, 011007. [Google Scholar] [CrossRef]
- Shi, C.; Zou, Z.; Lei, Z.; Wu, X.; Liu, Z.; Lu, H.; Zhang, W.; Xiao, J. Investigating the Self-Healing of Dynamic Covalent Thermoset Polyimine and Its Nanocomposites. J. Appl. Mech. 2019, 86, 101005. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, P.; Singh, A.; Joglekar, D.M.; Joglekar, M.M. Electromechanical Instability of Dielectric Elastomer Actuators with Active and Inactive Electric Regions. J. Appl. Mech. 2019, 86, 061008. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Li, X.; Shi, M. An Improved Design of the Substrate of Stretchable Gallium Arsenide Photovoltaics. J. Appl. Mech. 2019, 86, 031009. [Google Scholar] [CrossRef]
- Sanchez-Ferrer, A. Light-induced disorder in liquid-crystalline elastomers for actuation. In Proceedings of the Conference on Nano-Opto-Mechanical Systems (NOMS), San Diego, CA, USA, 21 August 2011. [Google Scholar]
- Mori, T.; Cukelj, R.; Prévôt, M.E.; Ustunel, S.; Story, A.; Gao, Y.; Diabre, K.; McDonough, J.A.; Freeman, E.J.; Hegmann, E.; et al. 3D Porous Liquid Crystal Elastomer Foams Supporting Long-term Neuronal Cultures. Macromol. Rapid Commun. 2020, 41, 1900585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, P.M.; Leonardo, S.; Raffaele, C.; Lorenzo, S.; Matteo, L.; Elisabetta, C.; Corrado, P.; Cecilia, F.; Camilla, P. Liquid Crystalline Networks toward Regenerative Medicine and Tissue Repair. Martella Daniele Paoli Paolo Small 2017, 46. [Google Scholar] [CrossRef] [Green Version]
- Hojin, K.; Bohan, Z.; Huiying, C.; Oluwatomiyin, A.; Aditya, A.; Pulickel, A.; Jeffrey, G.; Rafael, V. Preparation of Monodomain Liquid Crystal Elastomers and Liquid Crystal Elastomer Nanocomposites. J. Vis. Exp. 2016, 108, e53688. [Google Scholar]
- Saed Mohand, O.; Torbati Amir, H.; Nair Devatha, P.; Yakacki, C.M. Synthesis of Programmable Main-chain Liquid-crystalline Elastomers Using a Two-stage Thiol-acrylate Reaction. J. Vis. Exp. 2016, 107, 53546. [Google Scholar]
- Kondo, K.; Kobayashi, F.; Fukuda, A.; Kuze, E. Preparation of Monodomain Cells of Ferroelectric Liquid Crystals and Their Evaluation with an Optical Microscope. Jpn. J. Appl. Phys. 1981, 20, 1773–1777. [Google Scholar] [CrossRef]
- Bai, G.Y.; Zhang, Z.Z.; Xiong, Y. A preliminary study of visualization of anatomical structures of the hand. Chin. J. Clin. Anat. 2005, 3, 227–229. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zou, Z.; Wu, X.; Shi, C.; Liu, Y.; Xiao, J. Biomimetic Prosthetic Hand Enabled by Liquid Crystal Elastomer Tendons. Micromachines 2021, 12, 736. https://doi.org/10.3390/mi12070736
Lu H, Zou Z, Wu X, Shi C, Liu Y, Xiao J. Biomimetic Prosthetic Hand Enabled by Liquid Crystal Elastomer Tendons. Micromachines. 2021; 12(7):736. https://doi.org/10.3390/mi12070736
Chicago/Turabian StyleLu, Haiqing, Zhanan Zou, Xingli Wu, Chuanqian Shi, Yimeng Liu, and Jianliang Xiao. 2021. "Biomimetic Prosthetic Hand Enabled by Liquid Crystal Elastomer Tendons" Micromachines 12, no. 7: 736. https://doi.org/10.3390/mi12070736
APA StyleLu, H., Zou, Z., Wu, X., Shi, C., Liu, Y., & Xiao, J. (2021). Biomimetic Prosthetic Hand Enabled by Liquid Crystal Elastomer Tendons. Micromachines, 12(7), 736. https://doi.org/10.3390/mi12070736





