Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use
Abstract
:1. Introduction
2. Materials and Methods
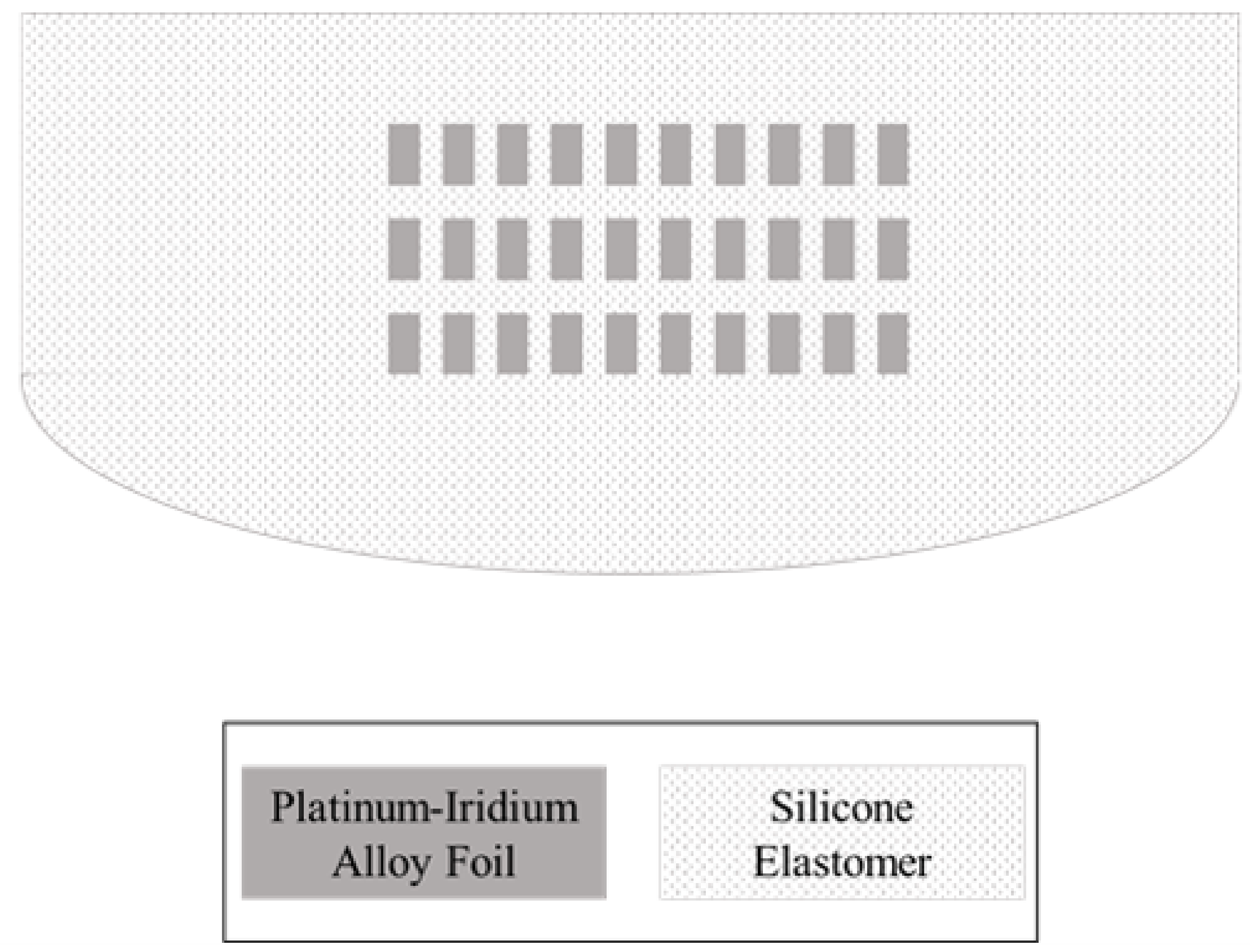
2.1. Design and Structure
2.2. Fabrication
2.3. Electrochemical Evaluation
2.4. Stiffness Measurement
2.5. Insertion and Extraction Force Measurement
3. Results
3.1. Thirty-Two-Channel Cochlear Electrode Array
3.2. Electrochemical Evaluation
3.3. Stiffness Measurement
3.4. Insertion and Extraction Force Measurement
4. Discussion
4.1. Cost and Feasibility
4.2. Design Challenges for High-Density CI Electrode Array
4.3. Mechanical Property
4.4. Future Research Direction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magro, I.; Emmett, S.D.; Saunders, J. Cost-effectiveness of CI in developing countries. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Emmett, S.D.; Tucci, D.L.; Bento, R.F.; Garcia, J.M.; Juman, S.; Chiossone-Kerdel, J.A.; Liu, T.J.; De Muñoz, P.C.; Ullauri, A.; Letort, J.J.; et al. Moving beyond GDP: Cost effectiveness of cochlear implantation and deaf education in Latin America. Otol. Neurotol. 2016, 37, 1040–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, J.E.; Barrs, D.M.; Gong, W.; Wilson, B.S.; Mojica, K.; Tucci, D.L. Cost effectiveness of childhood cochlear implantation and deaf education in Nicaragua: A disability adjusted life year model. Otol. Neurotol. 2015, 36, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Bento, R.F.; Bahmad, F.; Hippolyto, M.A.; Da Costa, S.S. Overcoming developing-world challenges in cochlear implantation: A South American perspective. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 200–208. [Google Scholar] [CrossRef]
- Kumar, R.; Kameswaran, M. Cochlear implantation in the developing world: Perspectives from the Indian subcontinent. ENT Audiol News 2017, 26, 88–89. [Google Scholar]
- Sampath Kumar, R.; Kameswaran, M. A sustainable model for cochlear implantation in the developing world: Perspectives from the Indian subcontinent. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 196–199. [Google Scholar] [CrossRef]
- Li, J.N.; Chen, S.; Zhai, L.; Han, D.Y.; Eshraghi, A.A.; Feng, Y.; Yang, S.M.; Liu, X. The advances in hearing rehabilitation and cochlear implants in China. Ear Hear. 2017, 38, 647. [Google Scholar] [CrossRef]
- Liu, C.M.; Lee, C.T.C. Association of hearing loss with dementia. JAMA Netw. Open 2019, 2, e198112. [Google Scholar] [CrossRef] [Green Version]
- An, S.K.; Park, S.I.; Jun, S.B.; Lee, C.J.; Byun, K.M.; Sung, J.H.; Wilson, B.S.; Rebscher, S.J.; Oh, S.H.; Kim, S.J. Design for a simplified cochlear implant system. IEEE Trans. Biomed. Eng. 2007, 54, 973–982. [Google Scholar] [CrossRef]
- Fishman, K.E.; Shannon, R.V.; Slattery, W.H. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J. Speech Lang. Hear. Res. 1997, 40, 1201–1215. [Google Scholar] [CrossRef]
- Friesen, L.M.; Shannon, R.V.; Baskent, D.; Wang, X. Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. J. Acoust. Soc. Am. 2001, 110, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- Garnham, C.; O’Driscoll, M.; Ramsden, R.; Saeed, S. Speech understanding in noise with a Med-El COMBI 40+ cochlear implant using reduced channel sets. Ear Hear. 2002, 23, 540–552. [Google Scholar] [CrossRef]
- Corbett, S.; Ketterl, J.; Johnson, T. Polymer-based microelectrode arrays. MRS Online Proceed. Library 2006, 926, 1–6. [Google Scholar] [CrossRef]
- Min, K.S.; Oh, S.H.; Park, M.H.; Jeong, J.; Kim, S.J. A polymer-based multichannel cochlear electrode array. Otol. Neurotol. 2014, 35, 1179–1186. [Google Scholar] [CrossRef]
- Gwon, T.M. A Polymer Cochlear Electrode Array: Atraumatic Deep Insertion, Tripolar Stimulation, and Long-Term Reliability; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kim, J.H.; Min, K.S.; An, S.K.; Jeong, J.S.; Jun, S.B.; Cho, M.H.; Son, Y.D.; Cho, Z.H.; Kim, S.J. Magnetic resonance imaging compatibility of the polymer-based cochlear implant. Clin. Experiment. Otorhinolaryngol. 2012, 5, S19. [Google Scholar] [CrossRef]
- Lee, S.W.; Min, K.S.; Jeong, J.; Kim, J.; Kim, S.J. Monolithic encapsulation of implantable neuroprosthetic devices using liquid crystal polymers. IEEE Trans. Biomed. Eng. 2011, 58, 2255–2263. [Google Scholar]
- Jiang, G.; Zhou, D.D. Technology advances and challenges in hermetic packaging for implantable medical devices. In Implantable Neural Prostheses 2; Springer: Berlin/Heidelberg, Germany, 2009; pp. 27–61. [Google Scholar]
- Croghan, N.B.; Duran, S.I.; Smith, Z.M. Re-examining the relationship between number of cochlear implant channels and maximal speech intelligibility. J. Acoust. Soc. Am. 2017, 142, EL537–EL543. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.A.; Noble, J.H.; Dawant, B.M.; Dwyer, R.T.; Labadie, R.F.; Gifford, R.H. Speech recognition as a function of the number of channels in perimodiolar electrode recipients. J. Acoust. Soc. Am. 2019, 145, 1556–1564. [Google Scholar] [CrossRef]
- Kontorinis, G.; Paasche, G.; Lenarz, T.; Stöver, T. The effect of different lubricants on cochlear implant electrode insertion forces. Otol. Neurotol. 2011, 32, 1050–1056. [Google Scholar] [CrossRef]
- Rebscher, S.J.; Hetherington, A.; Bonham, B.; Wardrop, P.; Whinney, D.; Leake, P.A. Considerations for the design of future cochlear implant electrode arrays: Electrode array stiffness, size and depth of insertion. J. Rehabil. Res. Dev. 2008, 45, 731. [Google Scholar] [CrossRef]
- Zeng, F.G.; Rebscher, S.; Harrison, W.; Sun, X.; Feng, H. Cochlear implants: System design, integration, and evaluation. IEEE Rev. Biomed. Eng. 2008, 1, 115–142. [Google Scholar] [CrossRef] [Green Version]
- Gollan, P.J.; Kaufman, B.E.; Taras, D.; Wilkinson, A. Voice and Involvement at Work: Experience with Non-Union Representation; Routledge: London, UK, 2014; p. 76. [Google Scholar]
- Cochlear. 2019 Annual Report; Cochlear Limited: Sydney, Australia, 2019. [Google Scholar]
- Clark, G.M.; Shepherd, R.; Patrick, J.F.; Black, R.; Tong, Y. Design and fabrication of the banded electrode array. Sci. Pub. 1983, 405, 191–201. [Google Scholar] [CrossRef]
- Merrill, D.R. The electrochemistry of charge injection at the electrode/tissue interface. In Implantable Neural Prostheses 2; Springer: Berlin/Heidelberg, Germany, 2010; pp. 85–138. [Google Scholar]
- Shepherd, R.K.; Carter, P.M.; Dalrymple, A.N.; Enke, Y.L.; Wise, A.K.; Nguyen, T.; Firth, J.; Thompson, A.; Fallon, J.B. Platinum dissolution and tissue response following long-term electrical stimulation at high charge densities. J. Neur. Eng. 2021, 18, 036021. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Klein, E.; Gossler, C.; Paul, O.; Ruther, P. High-density μLED-based optical cochlear implant with improved thermomechanical behavior. Front. Neurosci. 2018, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, J.Y.; Kim, Y.C.; Kim, S.; Chou, N.; Lee, S.; Choung, Y.H.; Kim, S.; Brugger, J.; Choi, H.; et al. A 3D microscaffold cochlear electrode array for steroid elution. Adv. Healthc. Mater. 2019, 8, 1900379. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, P.; Beek-King, V.; Sharpe, A.; Crawford, J.; Tridandapani, S.; McKinnon, B.; Blake, D. Highly flexible silicone coated neural array for intracochlear electrical stimulation. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iverson, K.C.; Bhatti, P.T.; Falcone, J.; Figueroa, R.; McKinnon, B.J. Cochlear implantation using thin-film array electrodes. Otolaryngol. Head Neck Surg. 2011, 144, 934–939. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 14708-7: Implants for Surgery—Active Implantable Medical Devices—Part 7: Particular Requirements for Cochlear Implant Systems, 1st ed.; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Min, K.S. A Study on the Liquid Crystal Polymer-Based Intracochlear Electrode Array. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2014. [Google Scholar]
- Bodington, E.; Saeed, S.R.; Smith, M.C.; Stocks, N.G.; Morse, R.P. A narrative review of the logistic and economic feasibility of cochlear implants in lower-income countries. Cochlear Implants Int. 2021, 22, 7–16. [Google Scholar] [CrossRef]
- Reeve, L.; Baldrick, P. Biocompatibility assessments for medical devices–evolving regulatory considerations. Expert Rev. Med. Dev. 2017, 14, 161–167. [Google Scholar] [CrossRef]
- Gwon, T.M.; Min, K.S.; Kim, J.H.; Oh, S.H.; Lee, H.S.; Park, M.H.; Kim, S.J. Fabrication and evaluation of an improved polymer-based cochlear electrode array for atraumatic insertion. Biomed. Microdev. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Moteki, H.; Nishio, S.Y.; Miyagawa, M.; Tsukada, K.; Noguchi, Y.; Usami, S.I. Feasibility of hearing preservation for residual hearing with longer cochlear implant electrodes. Acta Oto-Laryngol. 2018, 138, 1080–1085. [Google Scholar] [CrossRef] [Green Version]









| Name | Vertical | Horizontal | Mean | |
|---|---|---|---|---|
| of | Stiffness | Stiffness | Stiffness | V/H Ratio |
| Manufacturer | (mN) | (mN) | (mN) | |
| TODOC Co., Ltd. | 19.8 | 15.7 | 17.8 | 1.26 |
| Nurobiosys | 25.2 | 21.4 | 23.3 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Ha, Y.; Choi, G.; Hyun, J.; Kim, S.; Oh, S.-H.; Min, K.-S. Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use. Micromachines 2021, 12, 778. https://doi.org/10.3390/mi12070778
Shin S, Ha Y, Choi G, Hyun J, Kim S, Oh S-H, Min K-S. Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use. Micromachines. 2021; 12(7):778. https://doi.org/10.3390/mi12070778
Chicago/Turabian StyleShin, Soowon, Yoonhee Ha, Gwangjin Choi, Junewoo Hyun, Sangwoo Kim, Seung-Ha Oh, and Kyou-Sik Min. 2021. "Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use" Micromachines 12, no. 7: 778. https://doi.org/10.3390/mi12070778
APA StyleShin, S., Ha, Y., Choi, G., Hyun, J., Kim, S., Oh, S. -H., & Min, K. -S. (2021). Manufacturable 32-Channel Cochlear Electrode Array and Preliminary Assessment of Its Feasibility for Clinical Use. Micromachines, 12(7), 778. https://doi.org/10.3390/mi12070778






