Facile Preparation of Mesoporous MCM-48 Containing Silver Nanoparticles with Fly Ash as Raw Materials for CO Catalytic Oxidation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Synthesis of Silica Resource
2.1.2. Synthesis of Mesoporous MCM-48 Support
2.1.3. Preparation of Ag/MCM-48 by Post-Reduction Method
2.2. Catalytic Activity
3. Results and Discussion
3.1. X-Ray Diffraction Analysis
3.2. Transmission Electron Micrographs Characterization
3.3. N2 Adsorption/Desorption Isotherms and Pore Size Distributions
3.4. Catalytic Oxidation of CO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, Y.X.; Zhang, L.M.; An, X.; Han, C.Y.; Luo, Y.M. Microwave assistant rapid synthesis MCM-41-NH2 from fly ash and Cr(VI) removal performance. Environ. Sci. Pollut. R 2019, 26, 31463–31477. [Google Scholar] [CrossRef] [PubMed]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C.E. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture. J. CO2 Util. 2017, 22, 81–90. [Google Scholar] [CrossRef]
- Li, D.D.; Min, H.Y.; Jiang, X.; Ran, X.Q.; Zou, L.Y.; Fan, J.W. One-pot synthesis of Aluminum-containing ordered mesoporous silica MCM-41 using coal fly ash for phosphate adsorption. J. Colloid Interf. Sci. 2013, 404, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, S.P.; Sabharwal, S.; Kumar, J.V.; Reddy, K.H.P.; Rao, K.S.R.; Prasad, P.S.S. Advantage of radiolysis over impregnation method for the synthesis of SiO2 supported nano-Ag catalyst for direct decomposition of N2O. Catal. Commun. 2008, 9, 756–761. [Google Scholar] [CrossRef]
- Manzoor, D.; Pal, S. Reactivity and Catalytic Activity of Hydrogen Atom Chemisorbed Silver Clusters. J. Phys. Chem. A 2015, 119, 6162–6170. [Google Scholar] [CrossRef] [PubMed]
- Salaev, M.A.; Poleshchuk, O.K.; Vodyankina, O.V. Ethylene glycol oxidation over Ag-containing catalysts: A theoretical study. J. Mol. Catal. Chem. 2015, 396, 61–67. [Google Scholar] [CrossRef]
- Qu, Z.P.; Cheng, M.J.; Huang, W.X.; Bao, X.H. Formation of subsurface oxygen species and its high activity toward CO oxidation over silver catalysts. J. Catal. 2005, 229, 446–458. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal Silver Nanoparticles: Photochemical Preparation and Interaction with O2, CCl4, and Some Metal Ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Sajkowski, D.J.; Boudart, M. Structure Sensitivity of the Catalytic Oxidation of Ethene by Silver. Catal. Rev. 1987, 29, 325–360. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Dai, W.-L.; Fan, K. Ag/MCM-41 as a highly efficient mesostructured catalyst for the chemoselective synthesis of methyl glycolate and ethylene glycol. Appl. Catal. B Environ. 2011, 108–109, 90–99. [Google Scholar] [CrossRef]
- Wang, D.S.; Xie, T.; Peng, Q.; Li, Y.D. Ag, Ag2S, and Ag2Se nanocrystals: Synthesis, assembly, and construction of mesoporous structures. J. Am. Chem. Soc. 2008, 130, 4016–4022. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Choudrey, A.; Yang, P.D. Ag nanowire formation within mesoporous silica. Chem. Commun. 2000, 1063–1064. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.Q.; Ma, Y.L.; Xu, G.; Ma, J.X.; Yin, J.Z.; Wang, A.Q.; Gao, J.J. Synthesis of highly dispersed silver nanoparticles or nano-network modified KIT-6 using supercritical carbon dioxide. J. Mater. Sci. 2015, 50, 855–862. [Google Scholar] [CrossRef]
- Zhang, X.D.; Dong, H.; Gu, Z.J.; Wang, G.; Zuo, Y.H.; Wang, Y.G.; Cui, L.F. Preferential carbon monoxide oxidation on Ag/Al-SBA-15 catalysts: Effect of the Si/Al ratio. Chem. Eng. J. 2015, 269, 94–104. [Google Scholar] [CrossRef]
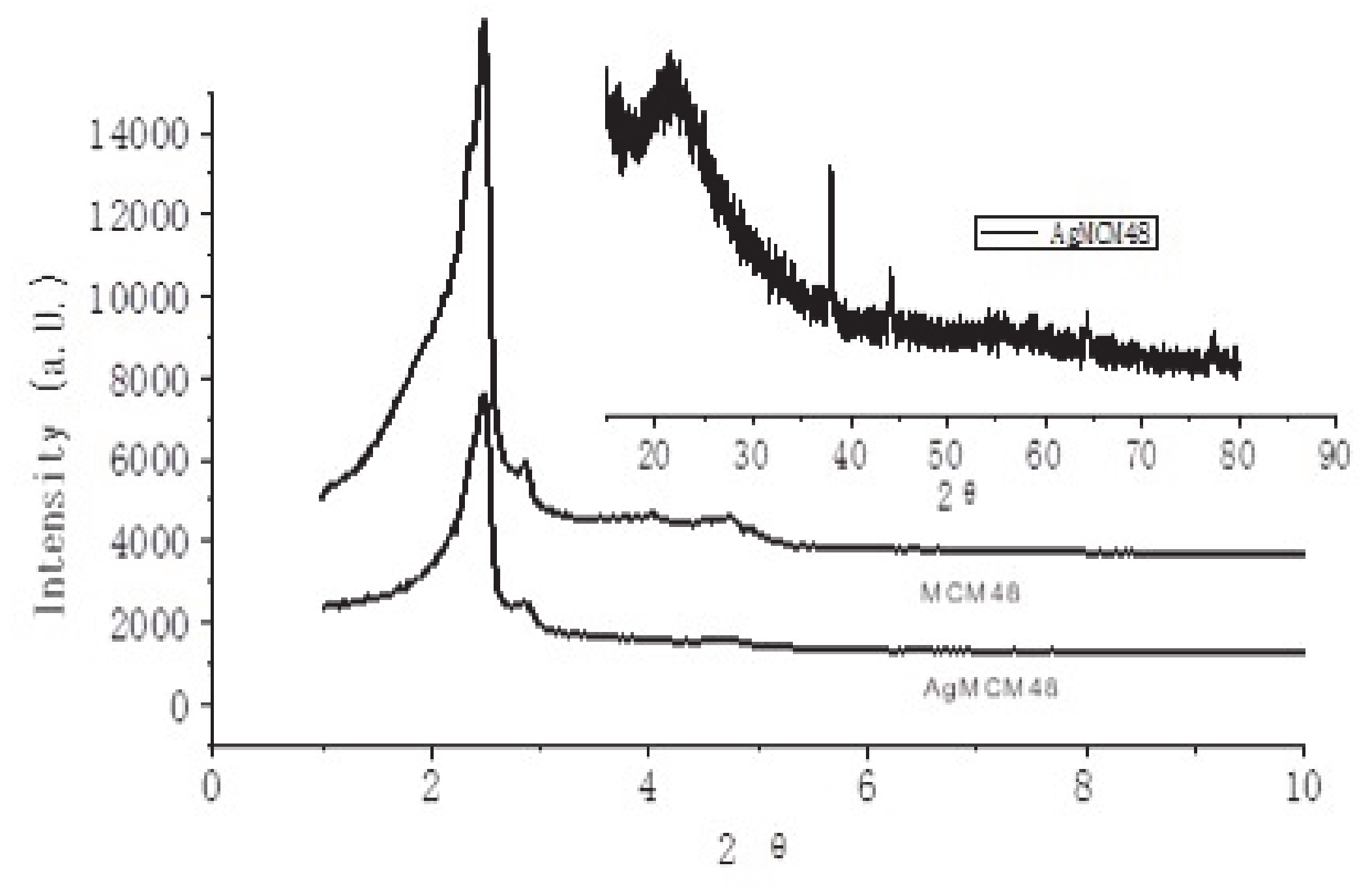
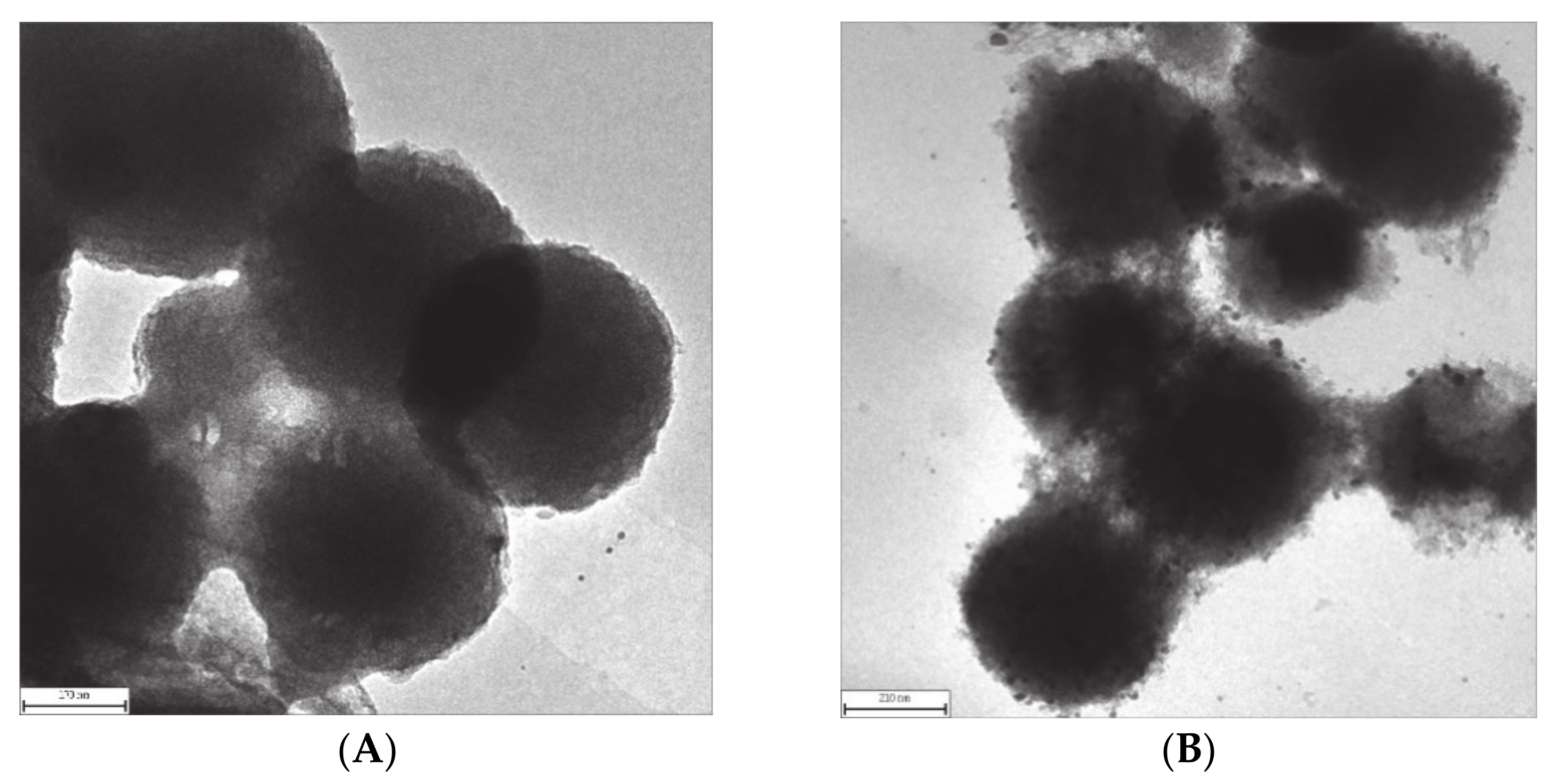
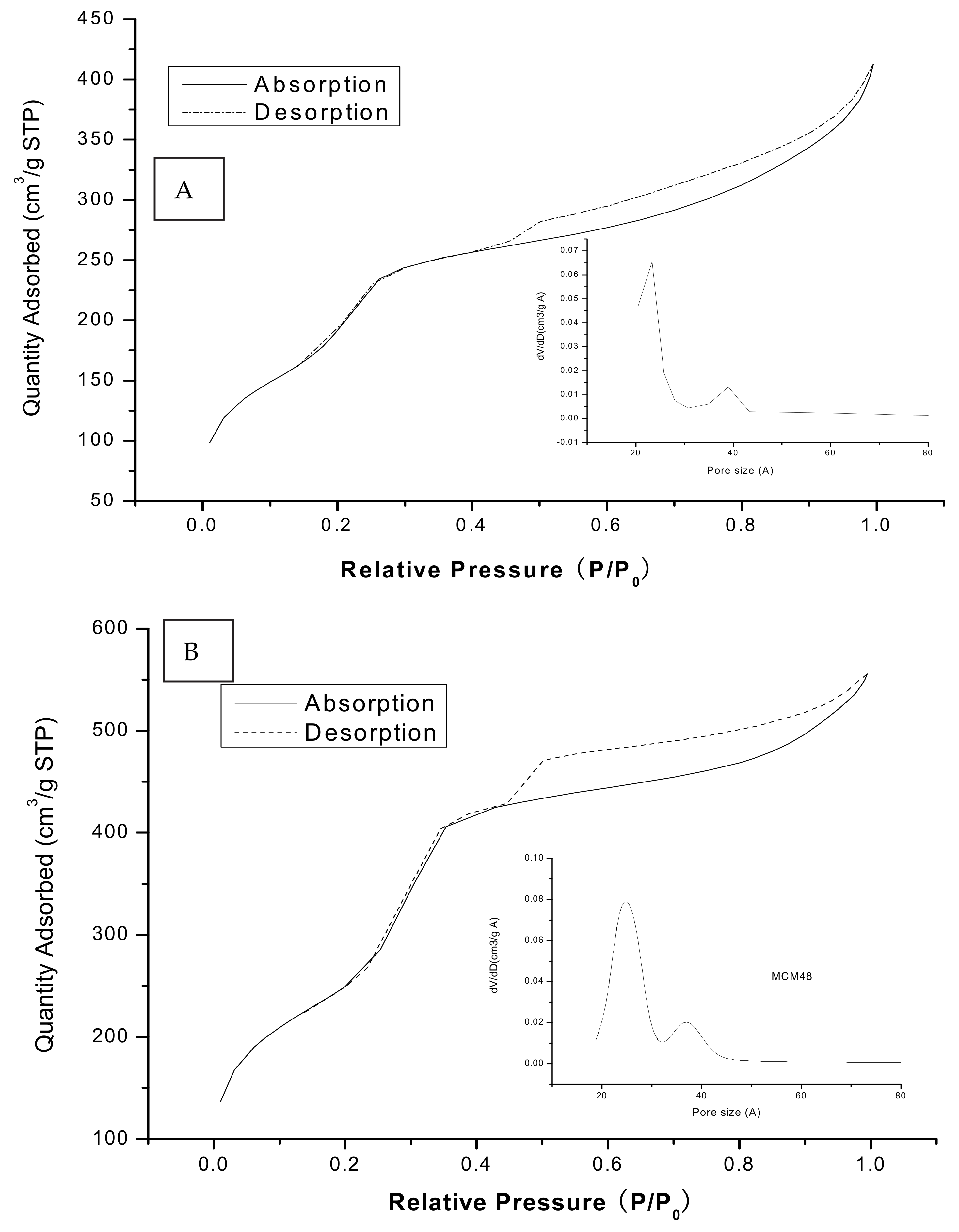

| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | Na2O |
|---|---|---|---|---|---|---|
| 62% | 25% | 3.7% | 2.7% | 1.5% | 0.6% | 0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Chen, Y.; Lu, X.; Ling, Y.; Lin, B. Facile Preparation of Mesoporous MCM-48 Containing Silver Nanoparticles with Fly Ash as Raw Materials for CO Catalytic Oxidation. Micromachines 2021, 12, 841. https://doi.org/10.3390/mi12070841
Tian D, Chen Y, Lu X, Ling Y, Lin B. Facile Preparation of Mesoporous MCM-48 Containing Silver Nanoparticles with Fly Ash as Raw Materials for CO Catalytic Oxidation. Micromachines. 2021; 12(7):841. https://doi.org/10.3390/mi12070841
Chicago/Turabian StyleTian, Dong, Yonghong Chen, Xiaoyong Lu, Yihan Ling, and Bin Lin. 2021. "Facile Preparation of Mesoporous MCM-48 Containing Silver Nanoparticles with Fly Ash as Raw Materials for CO Catalytic Oxidation" Micromachines 12, no. 7: 841. https://doi.org/10.3390/mi12070841
APA StyleTian, D., Chen, Y., Lu, X., Ling, Y., & Lin, B. (2021). Facile Preparation of Mesoporous MCM-48 Containing Silver Nanoparticles with Fly Ash as Raw Materials for CO Catalytic Oxidation. Micromachines, 12(7), 841. https://doi.org/10.3390/mi12070841







