Mechanical Durability of Flexible Printed Circuit Boards Containing Thin Coverlays Fabricated with Poly(Amide-Imide-Urethane)/Epoxy Interpenetrating Networks
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Instrumentation
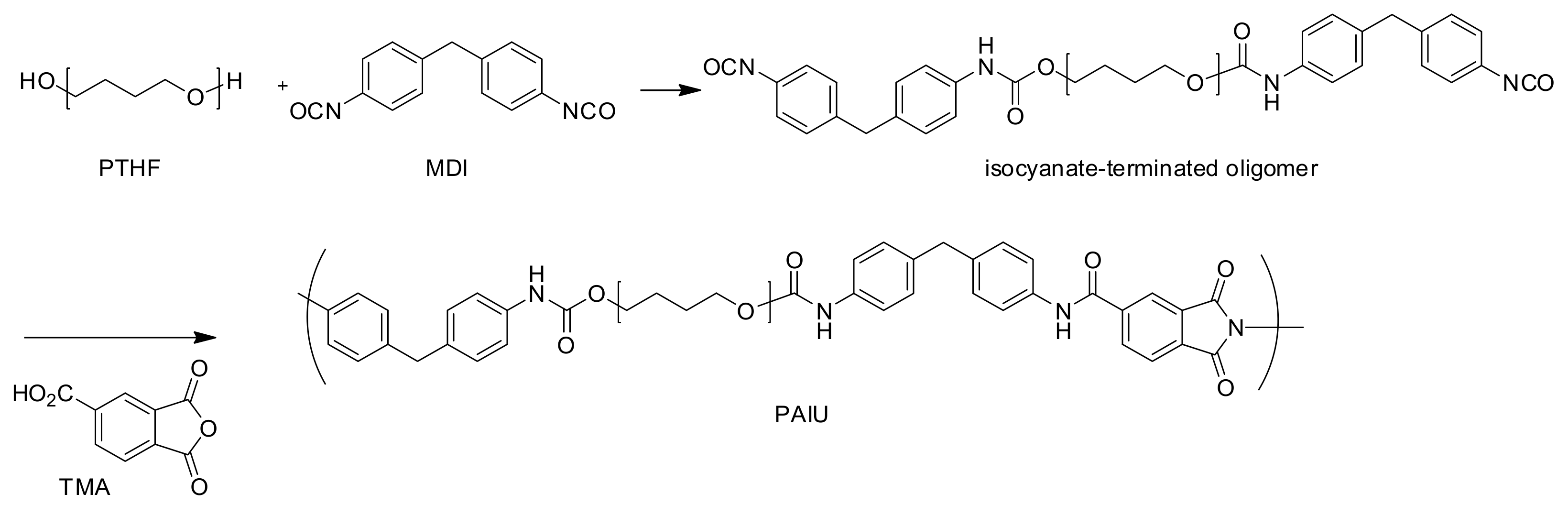
2.3. Synthesis of Poly(Amide-Imide-Urethane) (PAIU)
2.4. Preparation PAIU/Epoxy Blends
2.5. Preparation of Test Specimens
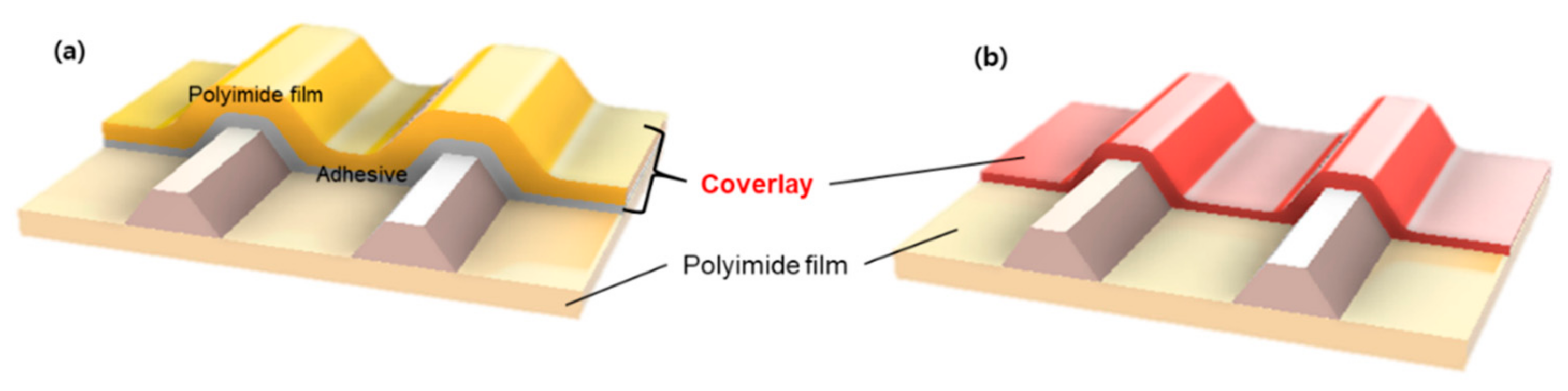
2.6. Fabrication of Flexible Printed Circuit Boards (FPCBs)
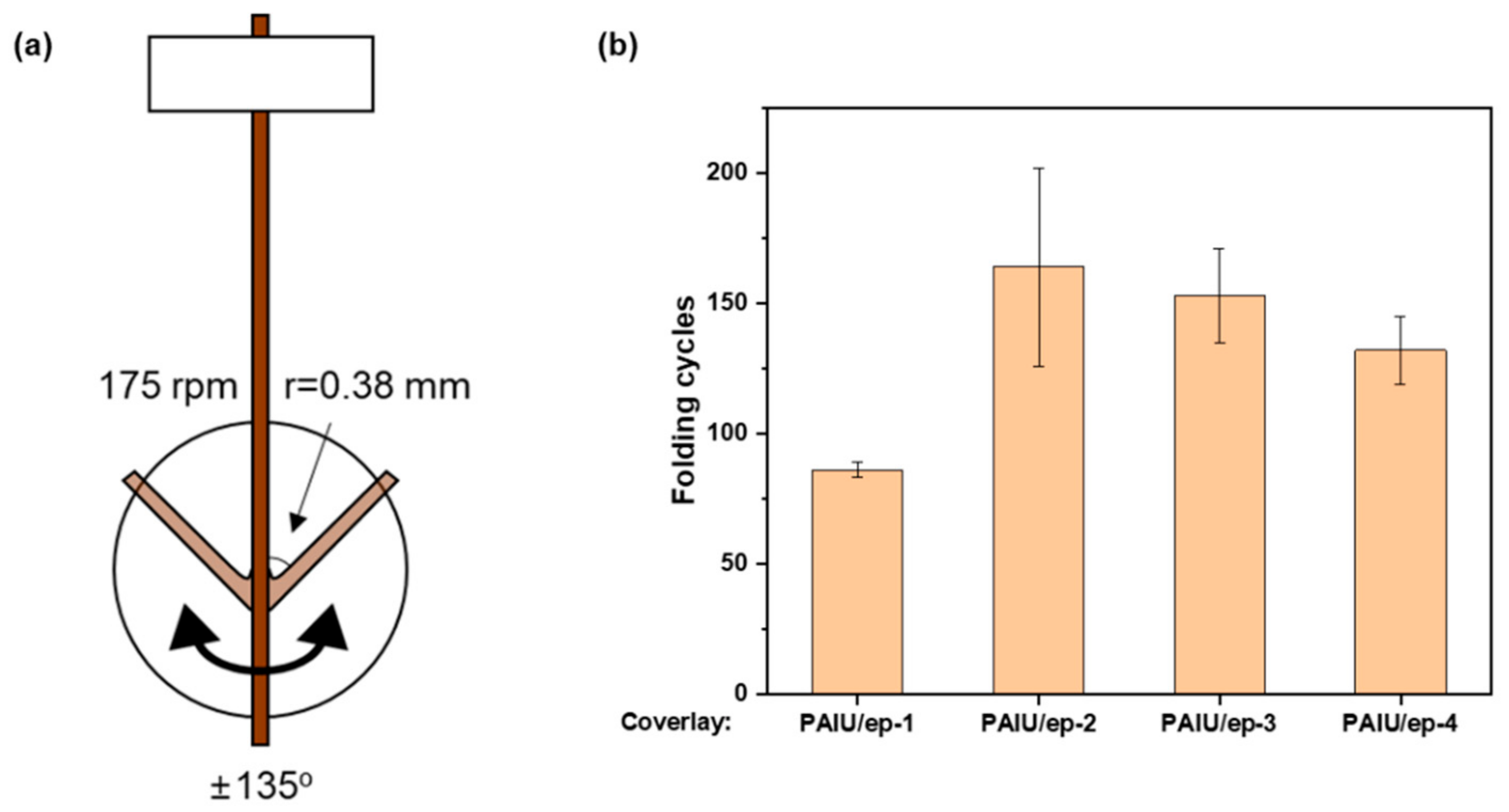
2.7. MIT Folding Endurance Test
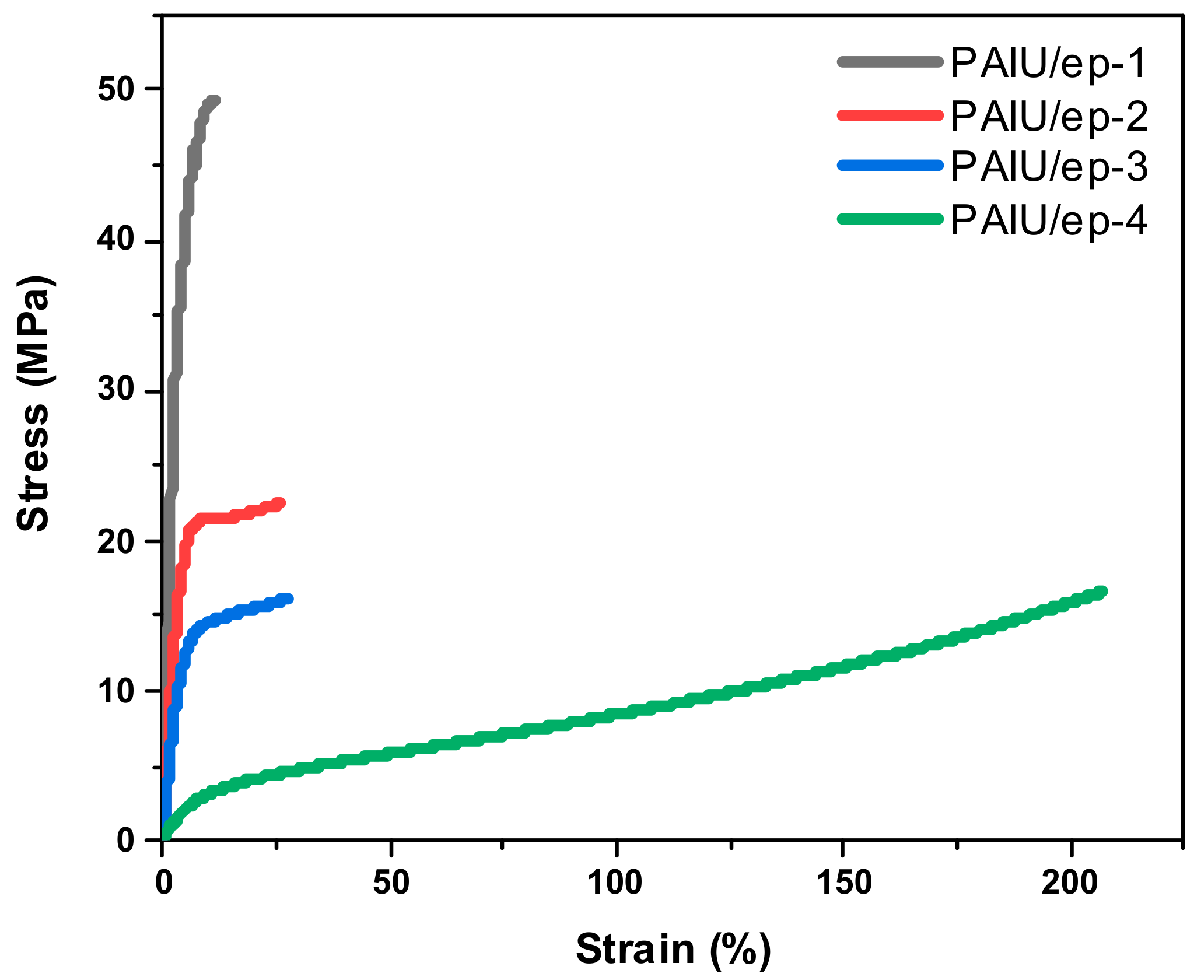
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamiya, S.; Furuta, H.; Omiya, M. Adhesion Energy of Cu/Polyimide Interface in Flexible Printed Circuits. Surf. Coat. Technol. 2007, 202, 1084–1088. [Google Scholar] [CrossRef]
- Ding, R.; Braunisch, H.; Tsang, L.; Chang, W. Simulation and Measurement Correlation of Random Rough Surface Effects in Interconnects. In Proceedings of the 2012 IEEE 21st Conference on Electrical Performance of Electronic Packaging and Systems, Tempe, AZ, USA, 21–24 October 2012; pp. 272–275. [Google Scholar]
- Jeng, J.-L.; Tsai, J.-Y.; Lu, C.-S.; King, J.-S. Photosensitive polyimide for coverlay of flexible printed circuit. In Proceedings of the 2007 International Microsystems, Packaging, Assembly and Circuits Technology, Taipei, Taiwan, 1–3 October 2007; pp. 369–372. [Google Scholar]
- Lee, S.; Yoo, T.; Han, Y.; Kim, H.; Han, H. Polyimide–Epoxy Composites with Superior Bendable Properties for Application in Flexible Electronics. J. Electron. Mater. 2017, 46, 4740–4749. [Google Scholar] [CrossRef]
- Ho, K.S.; Chen, L.W. Kinetic Studies of Polyamide-Imide Synthesis. J. Polym. Sci. A Polym. Chem. 1997, 35, 1703–1710. [Google Scholar] [CrossRef]
- Terney, S.; Keating, J.; Zielinski, J.; Wright, W.H.; Hakala, J.; Sheffer, H. Poly Amide-Imides. Polym. Chem. 1970, 8, 683–692. [Google Scholar]
- Carleton, P.S.; Farrissey, W.J.; Rose, J.S. The Formation of Polyimides from Anhydrides and Isocyanates. J. Appl. Polym. Sci. 1972, 16, 2983–2989. [Google Scholar] [CrossRef]
- Barikani, M.; Ataei, S.M. Preparation and Properties of Polyimides and Polyamide-Imides from Diisocyanates. J. Polym. Sci. A Polym. Chem. 1999, 37, 2245–2250. [Google Scholar] [CrossRef]
- Afsharian-Moghadam, H.; Haddadi-Asl, V. Synthesis and Characterization of a New Semi-Aliphatic Poly(Amide-Imide) and Evaluation of the Effect of Reaction Conditions. Des. Monomers Polym. 2008, 11, 223–234. [Google Scholar] [CrossRef]
- Diaham, S.; Locatelli, M.L.; Lebey, T.; Dinculescu, S. Dielectric and Thermal Properties Of-Imide (PAI) Films. In Proceedings of the 2009 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; pp. 482–485. [Google Scholar]
- López-Badillo, M.; Velasco-Hernández, M.A.; García-Castro, M.A.; Aranda-García, R.J.; Galicia-Aguilar, J.A.; Guevara-Espinosa, M.D.; Carreón-Rodríguez, V.E. Obtaining Kinetic Parameters of Polyamide Imide Reaction. Rev. Mex Ing. Quim. 2020, 19, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.W.; Ho, K.S. Synthesis of Polyamide-Imide by Blocked-Methylene Diisocyanates. J. Polym. Sci. A Polym. Chem. 1997, 35, 1711–1717. [Google Scholar] [CrossRef]
- Tang, Y.F.; Liu, J.; Li, Z.; Chen, B.Y.; Mei, S.F. Study on Blocking and Deblocking Kinetics of Diisocyanate with E-Caprolactam Using Ftir Spectroscopy. Asian J. Chem. 2013, 25, 5703–5706. [Google Scholar] [CrossRef]
- Lee, J.M.; Subramani, S.; Lee, Y.S.; Kim, J.H. Thermal Decomposition Behavior of Blocked Diisocyanates Derived from Mixture of Blocking Agents. Macromol. Res. 2005, 13, 427–434. [Google Scholar] [CrossRef]
- Wang, T.-L.; Huang, J. Synthesis and Properties of Poly(Amide-Imide-Urethane) Thermoplastic Elastomers. Polym. Int. 1998, 46, 280–284. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiemanzelat, F. Synthesis and Characterization of New Optically Active Poly(Amide-Imide-Urethane) Thermoplastic Elastomers, Derived from Bis(p-Amido Benzoic Acid)-N-Trimellitylimido-l-Leucine and Polyoxyethylene-MDI. React. Funct. Polym. 2005, 62, 153–167. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, K.N.; Noh, S.M.; Jung, H.W. The Effect of Thermal Radical Initiator Derived from O-Imino-Isourea on Thermal Curing Characteristics and Properties of Automotive Clearcoats. J. Coat. Technol. Res. 2015, 12, 177–186. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiemanzelat, F. Synthesis and Characterization of Novel Optically Active Poly(Ether-Urethane)s Modified by Copoly(Amide-Imide) Segments Based on Amino Acid through Diisocyanate Route: Influence of Reaction Parameters. Iran. Polym. J. 2006, 15, 79–90. [Google Scholar]
- Prisacariu, C.; Scortanu, E.; Airinei, A.; Agapie, B.; Iurzhenko, M.; Mamunya, Y.P. New Developments in Thermoplastic Polyurethanes of Variable Crystallinity: Sensitivity of Cyclic Stress-Strain Response to Chemical Structure. Procedia Eng. 2011, 10, 446–454. [Google Scholar] [CrossRef]
- Cho, Y.K.; Hwang, S.H. Diisocyanate Type Effects on Flexibility and Coating Performance of UV-Curable Hard Coatings Based on Tetrafunctional Urethane Acrylates. Polym. Bull. 2018, 75, 5795–5807. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A. Synthesis of Thermally and Mechanically Improved Polyurethane-Urea Elastomers by Using Novel Diamines as Chain Extenders. e-Polymer 2016, 16, 411–418. [Google Scholar] [CrossRef]
- Cao, Y.; Morrissey, T.G.; Acome, E.; Allect, S.I.; Wong, B.M.; Keplinger, C.; Wang, C. A Transparent, Self-Healing, Highly Stretchable Ionic Conductor. Adv. Mater. 2017, 29, 1605099. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Allect, S.I.; Wong, B.M.; Nguyen, D.-S.; Wang, C. A Highly Stretchy, Transparent Elastomer with the Capability to Automatically Self-Heal Underwater. Adv. Mater. 2018, 30, 1804602. [Google Scholar] [CrossRef]
- Acome, E.; Mitchell, S.K.; Morrissey, T.G.; Emmett, M.B.; Benjamin, C.; King, M.; Radakovitz, M.; Keplinger, C. Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 2018, 359, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.H.; Duh, J.G. Fatigue Characterization for Flexible Circuit with Polyimide on Adhesiveless Copper. J. Electron. Mater. 2015, 44, 3934–3941. [Google Scholar] [CrossRef]
- Hatano, T.; Kurosawa, Y.; Miyake, J. Effect of Material Processing on Fatigue of FPC Rolled Copper Foil. J. Electron. Mater. 2000, 29, 611–616. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Martin, M.D.; Retegi, A.; Gabilondo, N.; Corcuera, M.A.; Eceiza, A. Synthesis and Characterization of Polyurethanes with High Renewable Carbon Content and Tailored Properties. ACS Sustain. Chem. Eng. 2016, 4, 5684–5692. [Google Scholar] [CrossRef]
- Tan, J.; Brash, J.L. Nonfouling Biomaterials Based on Polyethylene Oxide-Containing Amphiphilic Triblock Copolymers as Surface Modifying Additives: Synthesis and Characterization of Copolymers and Surface Properties of Copolymer-Polyurethane Blends. J. Appl. Polym. Sci. 2008, 108, 1617–1628. [Google Scholar] [CrossRef]
- Badri, K.B.H.; Sien, W.C.; Shahrom, S.B.R.; Hao, L.C.; Baderuliksan, N.Y.; Rabbi, N.; Norzali, A. FTIR spectroscopy analysis of the prepolymerization of palm-based polyurethane. Solid State Technol. 2010, 18, 1–8. [Google Scholar]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR Spectroscopic and Thermal Study of Waterborne Polyurethane-Acrylate Leather Coatings Using Tartaric Acid as an Ionomer. e-Polymers 2016, 16, 463–474. [Google Scholar] [CrossRef]
- Caddeo, S.; Baino, F.; Ferreira, A.M.; Sartori, S.; Novajra, G.; Ciardelli, G.; Vitale-Brovarone, C. Collagen/Polyurethane-Coated Bioactive Glass: Early Achievements towards the Modelling of Healthy and Osteoporotic Bone. Key Eng. Mater. 2014, 631, 184–189. [Google Scholar] [CrossRef]
- Kaewpirom, S.; Kunwong, D. Curing Behavior and Cured Film Performance of Easy-to-Clean UV-Curable Coatings Based on Hybrid Urethane Acrylate Oligomers. J. Polym. Res. 2012, 19, 9995. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Molten Salt-Supported Polycondensation of Optically Active Diacid Monomers with an Aromatic Thiazole-Bearing Diamine Using Microwave Irradiation. J. Adv. Res. 2014, 5, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Zhai, L.; He, M.H.; Wang, C.O.; Mo, S.; Fan, L. Thermal Expansion Behavior of Poly(Amide-Imide) Films with Ultrahigh Tensile Strength and Ultralow CTE. Chin. J. Polym. Sci. 2020, 38, 748–758. [Google Scholar] [CrossRef]
- Xie, Z.; Dao, B.; Hodgkin, J.; Hoang, M.; Hill, A.; Gray, S. Synthesis and Characterization of Hybrid Organic-Inorganic Materials Based on Sulphonated Polyamideimide and Silica. J. Polym. Res. 2011, 18, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.P.; Guiver, M.D.; Yoshikawa, M.; Brownstein, S. Structural Determination of Torlon® 4000T Polyamide-Imide by NMR Spectroscopy. Polymer 2004, 45, 1111–1117. [Google Scholar] [CrossRef]
- Ubaghs, L.; Sharma, B.; Keul, H.; Höcker, H.; van Benthem, R. Synthesis and Characterization of Alternating Poly(Amide Urea)s and Poly(Amide Urethane Urethane)s from ε-Caprolactam, Diamines, and Diphenyl Carbonate or Ethylene Carbonate. e-Polymers 2003, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.I.; Park, H.S.; Park, J.H.; Knowles, J.C.; Gong, M.S. Application of High-Strength Biodegradable Polyurethanes Containing Different Ratios of Biobased Isomannide and Poly (ε-Caprolactone) Diol. J. Bioact. Compat. Polym. 2013, 28, 274–288. [Google Scholar] [CrossRef]
- Tanaka, K.; Allen, S.A.B.; Kohl, P.A. Variable Frequency Microwave Curing of Amide-Epoxy Based Polymers. IEEE Trans. Compon. Packag. Manuf. Technol. 2007, 30, 472–477. [Google Scholar] [CrossRef]
- Park, S.J.; Heo, G.Y.; Lee, J.R.; Hong, Y.T.; Choi, K.Y. Improvement of Toughness of Tetrafunctional Epoxy (TGDDM) Resin Using Polyamideimide (PAI) Resin. Polymer 2002, 26, 599–606. [Google Scholar]
- Park, S.J.; Heo, G.Y.; Jin, F.L. Cure Behaviors and Thermal Stabilities of Tetrafunctional Epoxy Resin Toughened by Polyamideimide. Macromol. Res. 2015, 23, 320–324. [Google Scholar] [CrossRef]
- Kong, X.; Tan, S.; Narine, S.S. Semi-and Full-Interpenetrating Polymer Networks Based on Polyurethane Produced from Canola Oil and Poly(Methyl Methacrylate). J. Appl. Polym. Sci. 2009, 114, 139–148. [Google Scholar] [CrossRef]
- Baker, D.A.; Bellare, A.; Pruitt, L. The Effects of Degree of Crosslinking on the Fatigue Crack Initiation and Propagation Resistance of Orthopedic-Grade Polyethylene. J. Biomed. Mater. Res. A 2003, 66, 146–154. [Google Scholar] [CrossRef]
- Baker, D.A.; Hastings, R.S.; Pruitt, L. Study of Fatigue Resistance of Chemical and Radiation Crosslinked Medical Grade Ultrahigh Molecular Weight Polyethylene. J. Biomed. Mater. Res. A 1999, 46, 573–581. [Google Scholar] [CrossRef]
- Baker, D.A.; Hastings, R.S.; Pruitt, L. Compression and Tension Fatigue Resistance of Medical Grade Ultra High Molecular Weight Polyethylene: The Effect of Morphology, Sterilization, Aging and Temperature. Polymer 2000, 41, 795–808. [Google Scholar] [CrossRef]
- Connelly, G.M.; Rimnac, J.-C.M.; Wright, T.M.; Hertzberg, R.W.; Manson, J.A. Fatigue Crack Propagation Behavior of Ultrahigh Molecular Weight Polyethylene. J. Orthop. Res. 1984, 2, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, C.; Xu, Y.; Jin, F. A Comparative Study of the Different Procedures for Seismic Cracking Analysis of Concrete Dams. Soil Dyn. Earthq. Eng. 2011, 31, 283–310. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical Characteristics of Orally Disintegrating Films: Comparison of Folding Endurance and Tensile Properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef] [PubMed]




| PAIU (g) | Epoxy (g) | 2MZ-A (g) | |
|---|---|---|---|
| PAIU/ep-1 | 30 | 70 | 0.7 |
| PAIU/ep-2 | 50 | 50 | 0.5 |
| PAIU/ep-3 | 70 | 30 | 0.3 |
| PAIU/ep-4 | 90 | 10 | 0.1 |
| PAIU/ep-1 | PAIU/ep-2 | PAIU/ep-3 | PAIU/ep-4 | |
|---|---|---|---|---|
| Tensile strength (MPa) | 50.0 ± 3.2 | 23.1 ± 1.3 | 16.8 ± 1.9 | 16.6 ± 3.7 |
| Elongation at break (%) | 11.4 ± 4.2 | 32.5 ± 9.0 | 33.6 ± 12.3 | 204.7 ± 38.6 |
| Young’s modulus (MPa) | 1120 ± 70 | 410 ± 30 | 320 ± 30 | 70/10 |
| Tg (°C) | 137.8 | 118.3 | 99.3 | 60.7 |
| Weight Loss at 300 °C | Td,95 a (°C) | |
|---|---|---|
| PAIU/ep-1 | 1.88 | 354 |
| PAIU/ep-2 | 2.33 | 331 |
| PAIU/ep-3 | 3.32 | 316 |
| PAIU/ep-4 | 3.36 | 314 |
| PAIU/ep-1 | PAIU/ep-2 | PAIU/ep-3 | PAIU/ep-4 | |
|---|---|---|---|---|
| Weight reduction (%) | 0.62 | 0.36 | 0.59 | 1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, B.-Y.; Park, S.D.; Seo, J.-H.; Lee, C.-J.; Yoo, M.J.; Kim, Y. Mechanical Durability of Flexible Printed Circuit Boards Containing Thin Coverlays Fabricated with Poly(Amide-Imide-Urethane)/Epoxy Interpenetrating Networks. Micromachines 2021, 12, 943. https://doi.org/10.3390/mi12080943
Kim J, Kim B-Y, Park SD, Seo J-H, Lee C-J, Yoo MJ, Kim Y. Mechanical Durability of Flexible Printed Circuit Boards Containing Thin Coverlays Fabricated with Poly(Amide-Imide-Urethane)/Epoxy Interpenetrating Networks. Micromachines. 2021; 12(8):943. https://doi.org/10.3390/mi12080943
Chicago/Turabian StyleKim, Jeongah, Bo-Young Kim, Seong Dae Park, Ji-Hun Seo, Chan-Jae Lee, Myong Jae Yoo, and Youngmin Kim. 2021. "Mechanical Durability of Flexible Printed Circuit Boards Containing Thin Coverlays Fabricated with Poly(Amide-Imide-Urethane)/Epoxy Interpenetrating Networks" Micromachines 12, no. 8: 943. https://doi.org/10.3390/mi12080943





