Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Selection and Sorting of Studies
2.4. Active Electrode Yield
3. Results
4. Discussion
4.1. Intended Application and Abiotic Considerations
4.2. Biological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.P.; Simeral, J.D.; Hochberg, L.R.; Donoghue, J.P.; Black, M.J. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J. Neural Eng. 2008, 5, 455–476. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; Mcfarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain—Computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Li, Y.; Wang, Y.; Zhu, J.; Zhang, S.; Zheng, X. Corrigendum: Long-term decoding stability of local field potentials from silicon arrays in primate motor cortex during a 2D center out task. J. Neural Eng. 2014, 11, 036009. [Google Scholar] [CrossRef]
- Bouton, C.E.; Shaikhouni, A.; Annetta, N.V.; Bockbrader, M.A.; Friedenberg, D.A.; Nielson, D.M.; Sharma, G.; Sederberg, P.B.; Glenn, B.C.; Mysiw, W.J.; et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016, 533, 247–250. [Google Scholar] [CrossRef]
- Collinger, J.L.; Wodlinger, B.; Downey, J.E.; Wang, W.; Tyler-Kabara, E.C.; Weber, D.J.; McMorland, A.J.C.; Velliste, M.; Boninger, M.L.; Schwartz, A.B. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 2013, 381, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Ganzer, P.D.; Colachis, S.C.; Schwemmer, M.A.; Weber, D.J.; Bockbrader, M.A.; Ganzer, P.D.; Colachis, S.C.; Schwemmer, M.A.; Friedenberg, D.A.; Dunlap, C.F. Restoring the Sense of Touch Using a Sensorimotor Demultiplexing Neural Interface. Cell 2020, 181, 763–773.e12. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; Van Der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Donoghue, J.P.; Nurmikko, A.; Black, M.; Hochberg, L.R. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J. Physiol. 2007, 579, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Dobelle, W.H.; Mladejovsky, M.G.; Girvin, J.P. Artificial Vision for the Blind: Electrical Stimulation of Visual Cortex Offers Hope for a Functional Prosthesis. Science 1974, 183, 440–444. [Google Scholar] [CrossRef]
- Vargas-Irwin, C.E.; Feldman, J.M.; King, B.; Simeral, J.D.; Sorice, B.L.; Oakley, E.M.; Cash, S.S.; Eskandar, E.N.; Friehs, G.M.; Hochberg, L.R.; et al. Watch, imagine, attempt: Motor cortex single-unit activity reveals context-dependent movement encoding in humans with tetraplegia. Front. Hum. Neurosci. 2018, 12, 450. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015, 12, 011001. [Google Scholar] [CrossRef]
- Simon, D.M.; Charkhkar, H.; St. John, C.; Rajendran, S.; Kang, T.; Reit, R.; Arreaga-Salas, D.; McHail, D.G.; Knaack, G.L.; Sloan, A.; et al. Design and demonstration of an intracortical probe technology with tunable modulus. J. Biomed. Mater. Res.-Part A 2017, 105, 159–168. [Google Scholar] [CrossRef]
- Stiller, A.M.; Usoro, J.; Frewin, C.L.; Danda, V.R.; Ecker, M.; Joshi-Imre, A.; Musselman, K.C.; Voit, W.; Modi, R.; Pancrazio, J.J.; et al. Chronic Intracortical Recording and Electrochemical Stability of Thiol-ene/Acrylate Shape Memory Polymer Electrode Arrays. Micromachines 2018, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Rihani, R.T.; Stiller, A.M.; Usoro, J.O.; Lawson, J.; Kim, H.; Black, B.J.; Danda, V.R.; Maeng, J.; Varner, V.D.; Ware, T.H.; et al. Deployable, liquid crystal elastomer-based intracortical probes. Acta Biomater. 2020, 111, 54–64. [Google Scholar] [CrossRef]
- Shen, W.; Das, S.; Vitale, F.; Richardson, A.; Ananthakrishnan, A.; Struzyna, L.A.; Brown, D.P.; Song, N.; Ramkumar, M.; Lucas, T.; et al. Microfabricated intracortical extracellular matrix-microelectrodes for improving neural interfaces. Microsystems Nanoeng. 2018, 4, 30. [Google Scholar] [CrossRef]
- Pancrazio, J.J.; Deku, F.; Ghazavi, A.; Stiller, A.M.; Rihani, R.; Frewin, C.L.; Varner, V.D.; Gardner, T.J.; Cogan, S.F. Thinking Small: Progress on Microscale Neurostimulation Technology. Neuromodulation Technol. Neural Interface 2017, 2017, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Daryl, R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2013, 11, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 2018, 15, 016007. [Google Scholar] [CrossRef]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Callanan, M.E.; Sunil, S.; Capadona, J.R. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials 2013, 34, 7001–7015. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Dexamethasone Coated Neural Probes Elicit Attenuated Inflammatory Response and Neuronal Loss Compared to Uncoated Neural Probes. Brain Res. 2007, 23, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Golabchi, A.; Wu, B.; Li, X.; Carlisle, D.L.; Kozai, T.D.Y.; Friedlander, R.M.; Cui, X.T. Melatonin improves quality and longevity of chronic neural recording. Biomaterials 2018, 180, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Du, Z.; Gugel, Z.V.; Smith, M.A.; Chase, S.M.; Bodily, L.M.; Caparosa, E.M.; Friedlander, R.M.; Cui, X.T. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays. J. Neurosci. Methods 2015, 242, 15–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.A.; Miriani, R.M.; Langhals, N.B.; Joseph, M.D.; Anderson, D.J.; Kipke, D.R. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. J. Neurophysiol. 2009, 101, 1679–1689. [Google Scholar] [CrossRef]
- Gabran, S.R.I.; Salam, M.T.; Dian, J.; El-hayek, Y.; Velazquez, J.L.P.; Genov, R.; Member, S.; Carlen, P.L.; Salama, M.M.A.; Mansour, R.R. 3-D Flexible Nano-Textured High-Density Microelectrode Arrays for High-Performance. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Usoro, J.O.; Shih, E.; Black, B.J.; Rihani, R.T.; Abbott, J.; Chakraborty, B.; Pancrazio, J.J.; Cogan, S.F. Chronic stability of local field potentials from standard and modified Blackrock microelectrode arrays implanted in the rat motor cortex. Biomed. Phys. Eng. Express 2019, 5, 065017. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Catt, K.; Du, Z.; Na, K.; Srivannavit, O.; Haque, R.U.M.; Seymour, J.; Wise, K.D.; Yoon, E.; Cui, X.T. Chronic In Vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE Trans. Biomed. Eng. 2016, 63, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Mols, K.; Musa, S.; Nuttin, B.; Lagae, L.; Bonin, V. In vivo characterization of the electrophysiological and astrocytic responses to a silicon neuroprobe implanted in the mouse neocortex. Sci. Rep. 2017, 7, 15642. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, A.; Rajan, S.D.; Muthuswamy, J. Long-term changes in the material properties of brain tissue at the implant-tissue interface. J. Neural Eng. 2013, 10, 066001. [Google Scholar] [CrossRef] [Green Version]
- Hascup, K.N.; Hascup, E.R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Second-by-Second Measure of L-Glutamate in the Prefontal Cortex and Striatum of Freely Moving Mice. J. Pharmacol. Exp. Ther. 2008, 2, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Dzirasa, K.; Fuentes, R.; Kumar, S.; Potes, J.M.; Nicolelis, M.A.L. Chronic in vivo multi-circuit neurophysiological recordings in mice. J. Neurosci. Methods 2011, 195, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, A.J.; Kyriakides, T.R. Nanoparticle-based evaluation of blood-brain barrier leakage during the foreign body response. J. Neural Eng. 2013, 10, 016013. [Google Scholar] [CrossRef] [Green Version]
- Hammer, D.X.; Lozzi, A.; Abliz, E.; Greenbaum, N.; Turner, K.P.; Pfefer, T.J.; Agrawal, A.; Krauthamer, V.; Welle, C.G. Optical coherence microscopy of mouse cortical vasculature surrounding implanted electrodes. Opt. Tech. Neurosurg. Neurophotonics Optogenetics 2014, 8928, 892804. [Google Scholar] [CrossRef]
- Potter-Baker, K.A.; Ravikumar, M.; Burke, A.A.; Meador, W.D.; Householder, K.T.; Buck, A.C.; Sunil, S.; Stewart, W.G.; Anna, J.P.; Tomaszewski, W.H.; et al. A comparison of neuroinflammation to implanted microelectrodes in rat and mouse models. Biomaterials 2014, 35, 5637–5646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T.D.Y.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.M.; Hong, G.; Zhou, T.; Schuhmann, T.G.; Viveros, R.D.; Lieber, C.M. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 2016, 13, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Eles, J.R.; Vazquez, A.L.; Cui, X.T. Two-photon imaging of chronically implanted neural electrodes: Sealing methods and new insights. J. Neurosci. Methods 2016, 258, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording. Adv. Sci. 2018, 5, 1700625. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Kozai, T.D.Y. In vivo spatiotemporal dynamics of NG2 glia activity caused by neural electrode implantation. Biomaterials 2018, 164, 121–133. [Google Scholar] [CrossRef]
- Golabchi, A.; Woeppel, K.M.; Li, X.; Lagenaur, C.F.; Cui, X.T. Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain. Biosens. Bioelectron. 2020, 155, 112096. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, S.; Grena, B.J.B.; Kimbrough, I.F.; Thompson, E.G.; Fink, Y.; Sontheimer, H.; Yoshinobu, T.; Jia, X. Polymer Composite with Carbon Nanofibers Aligned during Thermal Drawing as a Microelectrode for Chronic Neural Interfaces. ACS Nano 2017, 11, 6574–6585. [Google Scholar] [CrossRef]
- Guan, S.; Wang, J.; Gu, X.; Zhao, Y.; Hou, R.; Fan, H.; Zou, L.; Gao, L.; Du, M.; Li, C.; et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 2019, 5, eaav2842. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Wang, J.; Guan, S.; Du, M.; Wu, K.; Xu, K.; Zou, L.; Tian, H.; Fang, Y. Magnetic Actuation of Flexible Microelectrode Arrays for Neural Activity Recordings. Nano Lett. 2019, 19, 8032–8039. [Google Scholar] [CrossRef]
- Haiss, F.; Butovas, S.; Schwarz, C. A miniaturized chronic microelectrode drive for awake behaving head restrained mice and rats. J. Neurosci. Methods 2010, 187, 67–72. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. Biostability Assessment of Flexible Parylene C-based Implantable Sensor in Wireless Chronic Neural Recording. Procedia Eng. 2016, 168, 189–192. [Google Scholar] [CrossRef]
- Okun, M.; Lak, A.; Carandini, M.; Harris, K.D. Long term recordings with immobile silicon probes in the mouse cortex. PLoS ONE 2016, 11, e0151180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Lai, H.Y.; Lin, S.H.; Cho, C.W.; Chao, W.H.; Liao, C.H.; Tsang, S.; Chen, Y.F.; Lin, S.Y. Design and fabrication of a polyimide-based microelectrode array: Application in neural recording and repeatable electrolytic lesion in rat brain. J. Neurosci. Methods 2009, 182, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kong, C.; Chang, J.W.; Jun, S.B. Carbon-fiber based microelectrode array embedded with a biodegradable silk support for in vivo neural recording. J. Korean Med. Sci. 2019, 34, e24. [Google Scholar] [CrossRef] [PubMed]
- Mercanzini, A.; Colin, P.; Bensadoun, J.C.; Bertsch, A.; Renaud, P. In vivo electrical impedance spectroscopy of tissue reaction to microelectrode arrays. IEEE Trans. Biomed. Eng. 2009, 56, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.; Linsmeier, C.E.; Schouenborg, J. The density difference between tissue and neural probes is a key factor for glial scarring. Sci. Rep. 2013, 3, 2942. [Google Scholar] [CrossRef]
- Márton, G.; Orbán, G.; Kiss, M.; Fiáth, R.; Pongrácz, A.; Ulbert, I. A multimodal, SU-8-Platinum-Polyimide microelectrode array for chronic in vivo neurophysiology. PLoS ONE 2015, 10, e0145307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigosa, J.; Panarese, A.; Dominici, N.; Friedli, L.; Van Den Brand, R.; Carpaneto, J.; Digiovanna, J.; Courtine, G.; Micera, S. Decoding bipedal locomotion from the rat sensorimotor cortex. J. Neural Eng. 2015, 12, 056014. [Google Scholar] [CrossRef] [PubMed]
- Ceyssens, F.; Bovet Carmona, M.; Kil, D.; Deprez, M.; Tooten, E.; Nuttin, B.; Takeoka, A.; Balschun, D.; Kraft, M.; Puers, R. Chronic neural recording with probes of subcellular cross-section using 0.06 mm2 dissolving microneedles as insertion device. Sens. Actuators B Chem. 2019, 284, 369–376. [Google Scholar] [CrossRef]
- Nicolelis, M.A.L.; Ghazanfar, A.A.; Faggin, B.M.; Votaw, S.; Oliveira, L.M.O. Reconstructing the engram: Simultaneous, multisite, many single neuron recordings. Neuron 1997, 18, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Hetke, J.F.; Williams, J.C.; Pellinen, D.S.; Vetter, R.J.; Kipke, D.R. 3-D silicon probe array with hybrid polymer interconnect for chronic cortical recording. In Proceedings of the First International IEEE EMBS Conference on Neural Engineering, Capri, Italy, 20–22 March 2003; pp. 181–184. [Google Scholar] [CrossRef]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Turner, J.N.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Moxon, K.A.; Leiser, S.C.; Gerhardt, G.A.; Barbee, K.A.; Chapin, J.K. Ceramic-Based Multisite Electrode Arrays for Chronic Single-Neuron Recording. IEEE Trans. Biomed. Eng. 2004, 51, 647–656. [Google Scholar] [CrossRef]
- Moxon, K.A.; Kalkhoran, N.M.; Markert, M.; Sambito, M.A.; McKenzie, J.L.; Webster, J.T. Nanostructured surface modification of ceramic-based microelectrodes to enhance biocompatibility for a direct brain-machine interface. IEEE Trans. Biomed. Eng. 2004, 51, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Rennaker, R.L.; Ruyle, A.M.; Street, S.E.; Sloan, A.M. An economical multi-channel cortical electrode array for extended periods of recording during behavior. J. Neurosci. Methods 2005, 142, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Holecko, M.M.; Williams, J.C.; Massia, S.P. Visualization of the intact interface between neural tissue and implanted microelectrode arrays. J. Neural Eng. 2005, 2, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef]
- Cheung, K.C.; Renaud, P.; Tanila, H.; Djupsund, K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens. Bioelectron. 2007, 22, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Otto, K.J.; Johnson, M.D.; Kipke, D.R. and Improve Unit Recordings With Chronically Implanted Microelectrodes. IEEE Trans. Biomed. Eng. 2006, 53, 333–340. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [Green Version]
- He, W.; McConnell, G.C.; Bellamkonda, R.V. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J. Neural Eng. 2006, 3, 316. [Google Scholar] [CrossRef]
- Stice, P.; Gilletti, A.; Panitch, A.; Muthuswamy, J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J. Neural Eng. 2007, 4, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Musallam, S.; Bak, M.J.; Troyk, P.R.; Andersen, R.A. A floating metal microelectrode array for chronic implantation. J. Neurosci. Methods 2007, 160, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, E.C.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007, 102, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J.; Shain, W.; Kipke, D.R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410–423. [Google Scholar] [CrossRef]
- Hascup, E.R.; af Bjerkén, S.; Hascup, K.N.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009, 1291, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, G.C.; Butera, R.J.; Bellamkonda, R.V. Bioimpedance modeling to monitor astrocytic response to chronically implanted electrodes. J. Neural Eng. 2009, 6, 055005. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Hendricks, J.; Richardson-Burns, S.; Jan, E.; Martin, D.; Carmena, J.M. PEDOT coated microelectrode arrays for chronic neural recording and stimulation. In Proceedings of the 2009 4th International IEEE/EMBS Conference on Neural Engineering, Antalya, Turkey, 29 April–2 May 2009; Volume 4, pp. 383–386. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 056003. [Google Scholar] [CrossRef] [PubMed]
- Azemi, E.; Lagenaur, C.F.; Cui, X.T. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials 2011, 32, 681–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wagner, F.; Diagne, M.; Borton, D.A.; Zhang, J.; Ozden, I.; Burwell, R.D.; Nurmikko, A.V.; van Wagenen, R.; Diester, I.; et al. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J. Neural Eng. 2012, 13, 016001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef]
- Prasad, A.; Sanchez, J.C. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J. Neural Eng. 2012, 9, 026028. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuo, J.T.W.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, A.; Kondaveeti, P.; Nissanov, J.; Barbee, K.; Shewokis, P.; Rioux, L.; Moxon, K.A. Preventing neuronal damage and inflammation in vivo during cortical microelectrode implantation through the use of Poloxamer P-188. J. Neural Eng. 2013, 10, 016011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, T.; Karumbaiah, L.; Gaupp, E.A.; Patkar, R.; Patil, K.; Betancur, M.; Stanley, G.B.; Bellamkonda, R.V. The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials 2013, 34, 4703–4713. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.K.; Park, D.J.; Skousen, J.L.; Hess-Dunning, A.E.; Tyler, D.J.; Rowan, S.J.; Weder, C.; Capadona, J.R. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014, 11, 056014. [Google Scholar] [CrossRef] [Green Version]
- Mandal, H.S.; Knaack, G.L.; Charkhkar, H.; McHail, D.G.; Kastee, J.S.; Dumas, T.C.; Peixoto, N.; Rubinson, J.F.; Pancrazio, J.J. Improving the performance of poly(3,4-ethylenedioxythiophene) for brain-machine interface applications. Acta Biomater. 2014, 10, 2446–2454. [Google Scholar] [CrossRef]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.Y.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015, 12, 046009. [Google Scholar] [CrossRef] [Green Version]
- Alba, N.A.; Du, Z.J.; Catt, K.A.; Kozai, T.D.Y.; Cui, X.T. In vivo electrochemical analysis of a PEDOT/MWCNT neural electrode coating. Biosensors 2015, 5, 618–646. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Tien, L.W.; Chen, F.; Berke, J.D.; Kaplan, D.L.; Yoon, E. Silk-backed structural optimization of high-density flexible intracortical neural probes. J. Microelectromechan. Syst. 2015, 24, 62–69. [Google Scholar] [CrossRef]
- Charkhkar, H.; Knaack, G.L.; Mchail, D.G.; Mandal, H.S.; Peixoto, N.; Rubinson, J.F.; Dumas, T.C.; Pancrazio, J.J. Chronic intracortical neural recordings using microelectrode arrays coated with PEDOT-TFB. Acta Biomater. 2016, 32, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.R.; Zhang, H.; Robbins, M.T.; Nofar, J.B.; Marshall, S.P.; Kobylarek, M.J.; Kozai, T.D.Y.; Kotov, N.A.; Chestek, C.A. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J. Neural Eng. 2016, 13, 066002. [Google Scholar] [CrossRef] [Green Version]
- Hara, S.A.; Kim, B.J.; Kuo, J.T.W.; Lee, C.D.; Meng, E.; Pikov, V. Long-term stability of intracortical recordings using perforated and arrayed Parylene sheath electrodes. J. Neural Eng. 2016, 13, 066020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.J.; Kuzum, D.; Hwang, S.W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G.; et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Knaack, G.L.; McHail, D.G.; Borda, G.; Koo, B.; Peixoto, N.; Cogan, S.F.; Dumas, T.C.; Pancrazio, J.J. In vivo Characterization of Amorphous Silicon Carbide As a Biomaterial for Chronic Neural Interfaces. Front. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Goss-Varley, M.; Dona, K.R.; McMahon, J.A.; Shoffstall, A.J.; Ereifej, E.S.; Lindner, S.C.; Capadona, J.R. Microelectrode implantation in motor cortex causes fine motor deficit: Implications on potential considerations to Brain Computer Interfacing and Human Augmentation. Sci. Rep. 2017, 7, 15254. [Google Scholar] [CrossRef] [Green Version]
- Black, B.J.; Kanneganti, A.; Joshi-Imre, A.; Rihani, R.; Chakraborty, B.; Abbott, J.; Pancrazio, J.J.; Cogan, S.F. Chronic recording and electrochemical performance of utah microelectrode arrays implanted in rat motor cortex. J. Neurophysiol. 2018, 120, 2083–2090. [Google Scholar] [CrossRef]
- Cody, P.A.; Eles, J.R.; Lagenaur, C.F.; Kozai, T.D.Y.; Cui, X.T. Unique electrophysiological and impedance signatures between encapsulation types: An analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials 2018, 161, 117–128. [Google Scholar] [CrossRef]
- Lo, M.C.; Wang, S.; Singh, S.; Damodaran, V.B.; Ahmed, I.; Coffey, K.; Barker, D.; Saste, K.; Kals, K.; Kaplan, H.M.; et al. Evaluating the in vivo glial response to miniaturized parylene cortical probes coated with an ultra-fast degrading polymer to aid insertion. J. Neural Eng. 2018, 15, 036002. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Polei, M.D.; Skousen, J.L.; Tresco, P.A. An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex. Biomaterials 2018, 154, 1–11. [Google Scholar] [CrossRef]
- Winter, B.M.; Daniels, S.R.; Salatino, J.W.; Purcell, E.K. Genetic modulation at the neural microelectrode interface: Methods and applications. Micromachines 2018, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Joshi-Imre, A.; Black, B.J.; Abbott, J.; Kanneganti, A.; Rihani, R.; Chakraborty, B.; Danda, V.R.; Maeng, J.; Sharma, R.; Rieth, L.; et al. Chronic recording and electrochemical performance of amorphous silicon carbide-coated Utah electrode arrays implanted in rat motor cortex. J. Neural Eng. 2019, 16, 046006. [Google Scholar] [CrossRef]
- Chuapoco, M.R.; Choy, M.; Schmid, F.; Duffy, B.A.; Lee, H.J.; Lee, J.H. Carbon monofilament electrodes for unit recording and functional MRI in same subjects. Neuroimage 2019, 186, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Cassar, I.R.; Yu, C.; Sambangi, J.; Lee, C.D.; Whalen, J.J.; Petrossians, A.; Grill, W.M. Electrodeposited platinum-iridium coating improves in vivo recording performance of chronically implanted microelectrode arrays. Biomaterials 2019, 205, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Stiller, A.M.; Usoro, J.O.; Lawson, J.; Araya, B.; González-González, M.A.; Danda, V.R.; Voit, W.E.; Black, B.J.; Pancrazio, J.J. Mechanically robust, softening shape memory polymer probes for intracortical recording. Micromachines 2020, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Welle, E.J.; Patel, P.R.; Woods, J.E.; Petrossians, A.; Della Valle, E.; Vega-Medina, A.; Richie, J.M.; Cai, D.; Weiland, J.D.; Chestek, C.A. Ultra-small carbon fiber electrode recording site optimization and improved in vivo chronic recording yield. J. Neural Eng. 2020, 17, 026037. [Google Scholar] [CrossRef]
- Guitchounts, G.; Cox, D. 64-Channel Carbon Fiber Electrode Arrays for Chronic Electrophysiology. Sci. Rep. 2020, 10, 3830. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Liu, J.; Fu, T.M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Eldawlatly, S.; Oweiss, K.G. Temporal precision in population—But not individual neuron—Dynamics reveals rapid experience-dependent plasticity in the rat barrel cortex. Front. Comput. Neurosci. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovalekic, A.; Cavé-Lopez, S.; Canopoli, A.; Ondracek, J.M.; Nager, A.; Vyssotski, A.L.; Hahnloser, R.H.R. A lightweight feedback-controlled microdrive for chronic neural recordings. J. Neural Eng. 2017, 14, 026006. [Google Scholar] [CrossRef] [Green Version]
- Henry, K.S.; Neilans, E.G.; Abrams, K.S.; Idrobo, F.; Carney, L.H. Neural correlates of behavioral amplitude modulation sensitivity in the budgerigar midbrain. J. Neurophysiol. 2016, 115, 1905–1916. [Google Scholar] [CrossRef] [Green Version]
- Maynard, E.M.; Fernandez, E.; Normann, R.A. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J. Neurosci. Methods 2000, 97, 93–101. [Google Scholar] [CrossRef]
- McCreery, D.; Lossinsky, A.; Pikov, V.; Liu, X. Microelectrode array for chronic deep-brain microstimulation and recording. IEEE Trans. Biomed. Eng. 2006, 53, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Witte, R.S.; Rousche, P.J.; Kipke, D.R. Fast wave propagation in auditory cortex of an awake cat using a chronic microelectrode array. J. Neural Eng. 2007, 4, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Davis, T.S.; House, P.A.; Normann, R.A.; Greger, B. The Functional Consequences of Chronic, Physiologically Effective Intracortical Microstimulation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 194, ISBN 9780444538154. [Google Scholar]
- Kane, S.R.; Cogan, S.F.; Ehrlich, J.; Plante, T.D.; McCreery, D.B.; Troyk, P.R. Electrical performance of penetrating microelectrodes chronically implanted in cat cortex. IEEE Trans. Biomed. Eng. 2013, 60, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- McCreery, D.; Cogan, S.; Kane, S.; Pikov, V. Correlations between histology and neuronal activity recorded by microelectrodes implanted chronically in the cerebral cortex. J. Neural Eng. 2016, 13, 036012. [Google Scholar] [CrossRef]
- Carriero, G.; Arcieri, S.; Cattalini, A.; Corsi, L.; Gnatkovsky, V.; De Curtis, M. A guinea pig model of mesial temporal lobe epilepsy following nonconvulsive status epilepticus induced by unilateral intrahippocampal injection of kainic acid. Epilepsia 2012, 53, 1917–1927. [Google Scholar] [CrossRef]
- Hoogerwerf, A.C.; Wise, K.D. A Three-Dimensional Microelectrode Array for Chronic Neural Recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 2000, 47, 911–918. [Google Scholar] [CrossRef]
- Perkins, L.N.; Semu, D.; Shen, J.; Boas, D.A.; Gardner, T.J. High-density microfibers as a potential optical interface to reach deep brain region. J. Neural Eng. 2018, 15, 066002. [Google Scholar] [CrossRef] [Green Version]
- Yin, M.; Borton, D.A.; Aceros, J.; Patterson, W.R.; Nurmikko, A. A 100-Channel Hermetically Sealed Implantable Device for Chronic Wireless Neurosensing Applications. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, H.S.; Jackson, A.; Jackson, R.; Clowry, G.J.; Vassilevski, K.; O’Neill, A.; Baker, S.N. The sinusoidal probe: A new approach to improve electrode longevity. Front. Neuroeng. 2014, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogan, S.F.; Edell, D.J.; Guzelian, A.A.; Liu, Y.P.; Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. Part A 2003, 67, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Swadlow, H.A.; Bereshpolova, Y.; Bezdudnaya, T.; Cano, M.; Stoelzel, C.R. A multi-channel, implantable microdrive system for use with sharp, ultra-fine “Reitboeck” microelectrodes. J. Neurophysiol. 2005, 93, 2959–2965. [Google Scholar] [CrossRef]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical flexibility reduces the foreign body response to long-term implanted microelectrodes in rabbit cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef]
- Ghosh, S.; Putrino, D.; Burro, B.; Ring, A. Patterns of spatio-temporal correlations in the neural activity of the cat motor cortex during trained forelimb movements. Somatosens. Mot. Res. 2009, 26, 31–49. [Google Scholar] [CrossRef]
- Schultz, R.L.; Willey, T.J. The ultrastructure of the sheath around chronically implanted electrodes in brain. J. Neurocytol. 1976, 5, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Agnew, W.F.; Yuen, T.G.H.; McCreery, D.B.; Bullara, L.A. Histopathologic evaluation of prolonged intracortical electrical stimulation. Exp. Neurol. 1986, 92, 162–185. [Google Scholar] [CrossRef]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the utah intracortical electrode array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Normann, R.A.; Maynard, E.M.; Rousche, P.J.; Warren, D.J. A neural interface for a cortical vision prosthesis. Vision Res. 1999, 39, 2577–2587. [Google Scholar] [CrossRef] [Green Version]
- Rousche, P.J.; Normann, R.A. Chronic intracortical microstimulation (ICMS) of cat sensory cortex using the utah intracortical electrode array. IEEE Trans. Rehabil. Eng. 1999, 7, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McCreery, D.B.; Carter, R.R.; Bullara, L.A.; Yuen, T.G.H.; Agnew, W.F. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehabil. Eng. 1999, 7, 315–326. [Google Scholar] [CrossRef]
- Lin, W.S.; Tillery, S.H.; He, J. Stability of the chronic multichannel recording neuron signals. Annu. Int. Conf. IEEE Eng. Med. Biol.-Proc. 2003, 3, 2193–2196. [Google Scholar] [CrossRef]
- Suner, S.; Fellows, M.R.; Vargas-Irwin, C.; Nakata, G.K.; Donoghue, J.P. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 524–541. [Google Scholar] [CrossRef]
- Griffith, R.W.; Humphrey, D.R. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci. Lett. 2006, 406, 81–86. [Google Scholar] [CrossRef]
- Santhanam, G.; Linderman, M.D.; Gilja, V.; Afshar, A.; Ryu, S.I.; Meng, T.H.; Shenoy, K.V. HermesB: A continuous neural recording system for freely behaving primates. IEEE Trans. Biomed. Eng. 2007, 54, 2037–2050. [Google Scholar] [CrossRef] [Green Version]
- Eliades, S.J.; Wang, X. Chronic multi-electrode neural recording in free-roaming monkeys. J. Neurosci. Methods 2008, 172, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Dickey, A.S.; Suminski, A.; Amit, Y.; Hatsopoulos, N.G. Single-unit stability using chronically implanted multielectrode arrays. J. Neurophysiol. 2009, 102, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, G.W.; Schwartz, A.B. Recording from the same neurons chronically in motor cortex. J. Neurophysiol. 2012, 107, 1970–1978. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.S.; Parker, R.A.; House, P.A.; Bagley, E.; Wendelken, S.; Normann, R.A.; Greger, B. Spatial and temporal characteristics of V1 microstimulation during chronic implantation of a microelectrode array in a behaving macaque. J. Neural Eng. 2012, 9, 065003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.H.; Dammann, J.F.; Boback, J.L.; Tenore, F.V.; Otto, K.J.; Gaunt, R.A.; Bensmaia, S.J. The effect of chronic intracortical microstimulation on the electrode-tissue interface. J. Neural Eng. 2014, 11, 026004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Willett, F.R.; Taylor, D.M. Relationship between microelectrode array impedance and chronic recording quality of single units and local field potentials. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3045–3048. [Google Scholar] [CrossRef]
- Jackson, A.; Fetz, E.E. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J. Neurophysiol. 2007, 98, 3109–3118. [Google Scholar] [CrossRef] [Green Version]
- Eleryan, A.; Vaidya, M.; Southerland, J.; Badreldin, I.S.; Balasubramanian, K.; Fagg, A.H.; Hatsopoulos, N.; Oweiss, K. Tracking single units in chronic, large scale, neural recordings for brain machine interface applications. Front. Neuroeng. 2014, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Rajan, A.T.; Boback, J.L.; Dammann, J.F.; Tenore, F.V.; Wester, B.A.; Otto, K.J.; Gaunt, R.A.; Bensmaia, S.J. The effects of chronic intracortical microstimulation on neural tissue and fine motor behavior. J. Neural Eng. 2015, 12, 066018. [Google Scholar] [CrossRef]
- Abe, H.; McManus, J.N.J.; Ramalingam, N.; Li, W.; Marik, S.A.; Borgloh, S.M. zum A.; Gilbert, C.D. Adult cortical plasticity studied with chronically implanted electrode arrays. J. Neurosci. 2015, 35, 2778–2790. [Google Scholar] [CrossRef] [Green Version]
- Malaga, K.A.; Schroeder, K.E.; Patel, P.R.; Irwin, Z.T.; Thompson, D.E.; Nicole Bentley, J.; Lempka, S.F.; Chestek, C.A.; Patil, P.G. Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates. J. Neural Eng. 2015, 13, 016010. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.G.; Weigand, P.K.; Sritharan, S.Y.; Lucas, T.H. A chronic neural interface to the macaque dorsal column nuclei. J. Neurophysiol. 2016, 115, 2255–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2016, 13, 026003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sritharan, S.Y.; Richardson, A.G.; Weigand, P.K.; Planell-Mendez, I.; Liu, X.; Zhu, H.; Zhang, M.; Van der Spiegel, J.; Lucas, T.H. Somatosensory Encoding with Cuneate Nucleus Microstimulation: Detection of Artificial Stimuli. IEEE Eng. Med. Biol. 2016, 4719–4722. [Google Scholar] [CrossRef]
- Suresh, A.K.; Winberry, J.E.; Versteeg, C.; Chowdhury, R.; Tomlinson, T.; Rosenow, J.M.; Miller, L.E.; Bensmaia, S.J. Methodological considerations for a chronic neural interface with the cuneate nucleus of macaques. J. Neurophysiol. 2017, 118, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Kyle, C.T.; Permenter, M.R.; Vogt, J.A.; Rapp, P.R.; Barnes, C.A. Behavioral Impact of Long-Term Chronic Implantation of Neural Recording Devices in the Rhesus Macaque. Neuromodulation 2019, 22, 435–440. [Google Scholar] [CrossRef]
- Debnath, S.; Prins, N.W.; Pohlmeyer, E.; Mylavarapu, R.; Geng, S.; Sanchez, J.C.; Prasad, A. Long-term stability of neural signals from microwire arrays implanted in common marmoset motor cortex and striatum. Biomed. Phys. Eng. Express 2018, 4, 055025. [Google Scholar] [CrossRef]
- Hao, Y.; Riehle, A.; Brochier, T.G. Mapping horizontal spread of activity in monkey motor cortex using single pulse microstimulation. Front. Neural Circuits 2016, 10, 104. [Google Scholar] [CrossRef]
- Budoff, S.A.; Yano, K.M.; De Mesquita, F.C.; Doerl, J.G.; De Santana, M.B.; Nascimento, M.S.L.; Kunicki, A.C.B.; De Araújo, M.F.P. Astrocytic response to acutely- and chronically-implanted microelectrode arrays in the marmoset (Callithrix jacchus) brain. Brain Sci. 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Barz, F.; Livi, A.; Lanzilotto, M.; Maranesi, M.; Bonini, L.; Paul, O.; Ruther, P. Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: From design to fabrication, assembly, and functional validation in non-human primates. J. Neural Eng. 2017, 14, 036010. [Google Scholar] [CrossRef] [PubMed]
- Chauviere, L.; Pothof, F.; Gansel, K.S.; Klon-Lipok, J.; Aarts, A.A.A.; Holzhammer, T.; Paul, O.; Singer, W.J.; Ruther, P. In vivo recording quality of mechanically decoupled floating versus skull-fixed silicon-based neural probes. Front. Neurosci. 2019, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Bak, M.J.; McIntosh, J.S. Long-term chronic recording from cortical neurons. Exp. Neurol. 1976, 52, 496–506. [Google Scholar] [CrossRef]
- Loeb, G.E.; Bak, M.J.; Schmidt, E.M.; Salcman, M. Parylene as a Chronically Stable, Reproducible Microelectrode Insulator. IEEE Trans. Biomed. Eng. 1977, BME-24, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Mcintosh, J.S.; Bak, M.J. Long-term implants of Parylene-C coated microelectrodes. Med. Biol. Eng. Comput. 1988, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nicolelis, M.A.L.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Aflalo, T.; Revechkis, B.; Rosario, E.R.; Ouellette, D.; Pouratian, N.; Andersen, R.A. Partially Mixed Selectivity in Human Posterior Parietal Association Cortex. Neuron 2017, 95, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Aflalo, T.; Revechkis, B.; Rosario, E.; Ouellette, D.; Pouratian, N.; Andersen, R.A. Preservation of partially mixed selectivity in human posterior parietal cortex across changes in task context. eNeuro 2020, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Murguialday, A.; Curado, M.R.; Broetz, D.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Garcia-Cossio, E.; Cho, W.; Caria, A.; Cohen, L.G.; et al. Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-up. Neurorehabil. Neural Repair 2019, 33, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef]
- Simeral, J.D.; Kim, S.P.; Black, M.J.; Donoghue, J.P.; Hochberg, L.R. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 2011, 8, 025027. [Google Scholar] [CrossRef] [Green Version]
- Klaes, C.; Kellis, S.; Aflalo, T.; Lee, B.; Pejsa, K.; Shanfield, K.; Hayes-Jackson, S.; Aisen, M.; Heck, C.; Liu, C.; et al. Hand shape representations in the human posterior parietal cortex. J. Neurosci. 2015, 35, 15466–15476. [Google Scholar] [CrossRef] [Green Version]
- Aflalo, T.; Kellis, S.; Klaes, C.; Lee, B.; Shi, Y.; Pejsa, K.; Shanfield, K.; Hayes-Jackson, S.; Aisen, M.; Heck, C.; et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 2015, 348, 906–910. [Google Scholar] [CrossRef] [Green Version]
- Downey, J.E.; Weiss, J.M.; Muelling, K.; Venkatraman, A.; Valois, J.S.; Hebert, M.; Bagnell, J.A.; Schwartz, A.B.; Collinger, J.L. Blending of brain-machine interface and vision-guided autonomous robotics improves neuroprosthetic arm performance during grasping. J. Neuroeng. Rehabil. 2016, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Flesher, S.N.; Collinger, J.L.; Foldes, S.T.; Weiss, J.M.; Downey, J.E.; Tyler-Kabara, E.C.; Bensmaia, S.J.; Schwartz, A.B.; Boninger, M.L.; Gaunt, R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Li, X.; Bodily, L.M.; Caparosa, E.M.; Zenonos, G.A.; Carlisle, D.L.; Friedlander, R.M.; Cui, X.T. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials 2014, 35, 9620–9634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedell, H.; Capadona, J. Anti-inflammatory Approaches to Mitigate the Neuroinflammatory Response to Brain-Dwelling Intracortical Microelectrodes. J. Immunol. 2018, 2, 15. [Google Scholar] [CrossRef]
- Bedell, H.W.; Hermann, J.K.; Ravikumar, M.; Lin, S.; Rein, A.; Li, X.; Molinich, E.; Smith, P.D.; Selkirk, S.M.; Miller, R.H.; et al. Targeting CD14 on blood derived cells improves intracortical microelectrode performance. Biomaterials 2018, 163, 163–173. [Google Scholar] [CrossRef]
- Kilborn, S.H.; Trudel, G.; Uhthoff, H. Review of Growth Plate Closure Compared with Age at Sexual Maturity and Lifespan in Laboratory Animals. Contemp. Top. Lab. Anim. Sci. 2002, 41, 21–26. [Google Scholar] [PubMed]
- Kozai, T. The History and Horizons of Microscale Neural Interfaces. Micromachines 2018, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Xue, Q.; Dieme, R.; Sankar, V.; Mayrand, R.C.; Nishida, T.; Streit, W.J.; Sanchez, J.C. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Samikkannu, M.; Mohammed, F.; Dietrich, W.D.; Rajguru, S.M.; Prasad, A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials 2018, 164, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, M.; Sunil, S.; Black, J.; Barkauskas, D.S.; Haung, A.Y.; Miller, R.H.; Selkirk, S.M.; Capadona, J.R. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted Intracortical microelectrodes. Biomaterials 2014, 35, 8049–8064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresco, P.A.; Winslow, B.D. The challenge of integrating devices into the central nervous system. Crit. Rev. Biomed. Eng. 2011, 39, 29–44. [Google Scholar] [CrossRef]
- Gilletti, A.; Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 2006, 3, 189–195. [Google Scholar] [CrossRef]
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J. Neural Eng. 2005, 2, 103–113. [Google Scholar] [CrossRef]
- Lee, H.; Bellamkonda, R.V.; Sun, W.; Levenston, M.E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2005, 2, 81–89. [Google Scholar] [CrossRef]
- Prodanov, D.; Delbeke, J. Mechanical and Biological Interactions of Implants with the Brain and Their Impact on Implant Design. Front. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Capadona, J.R. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J. Neural Eng. 2012, 9, 046020. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Martini, N.; Hassler, C.; Kirch, R.D.; Stieglitz, T.; Seifert, A.; Hofmann, U.G. In vivo monitoring of glial scar proliferation on chronically implanted neural electrodes by fiber optical coherence tomography. Front. Neuroeng. 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Shoffstall, A.; Capadona, J.R. Prospects for a Robust Cortical Recording Interface, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780128053539. [Google Scholar]
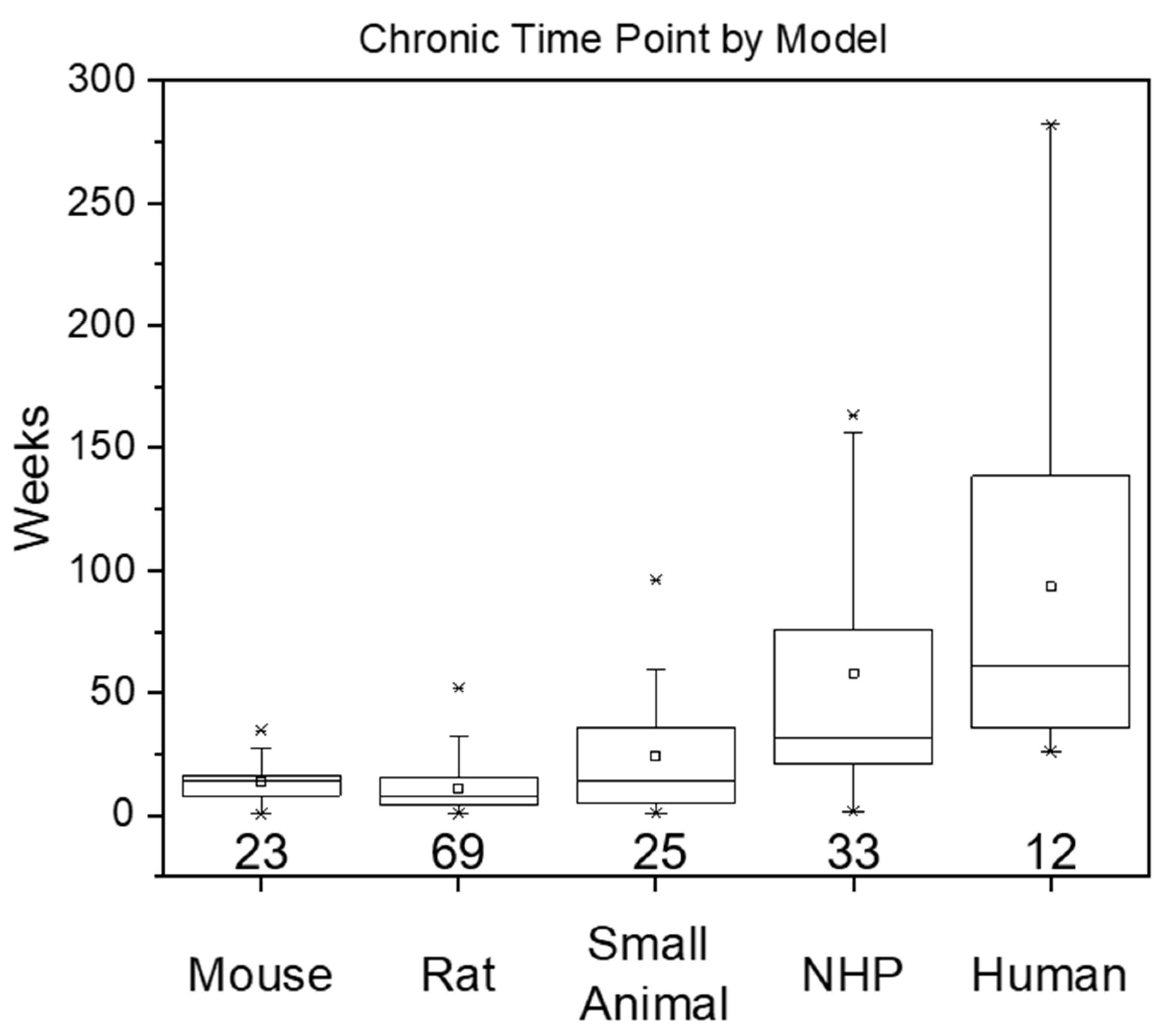
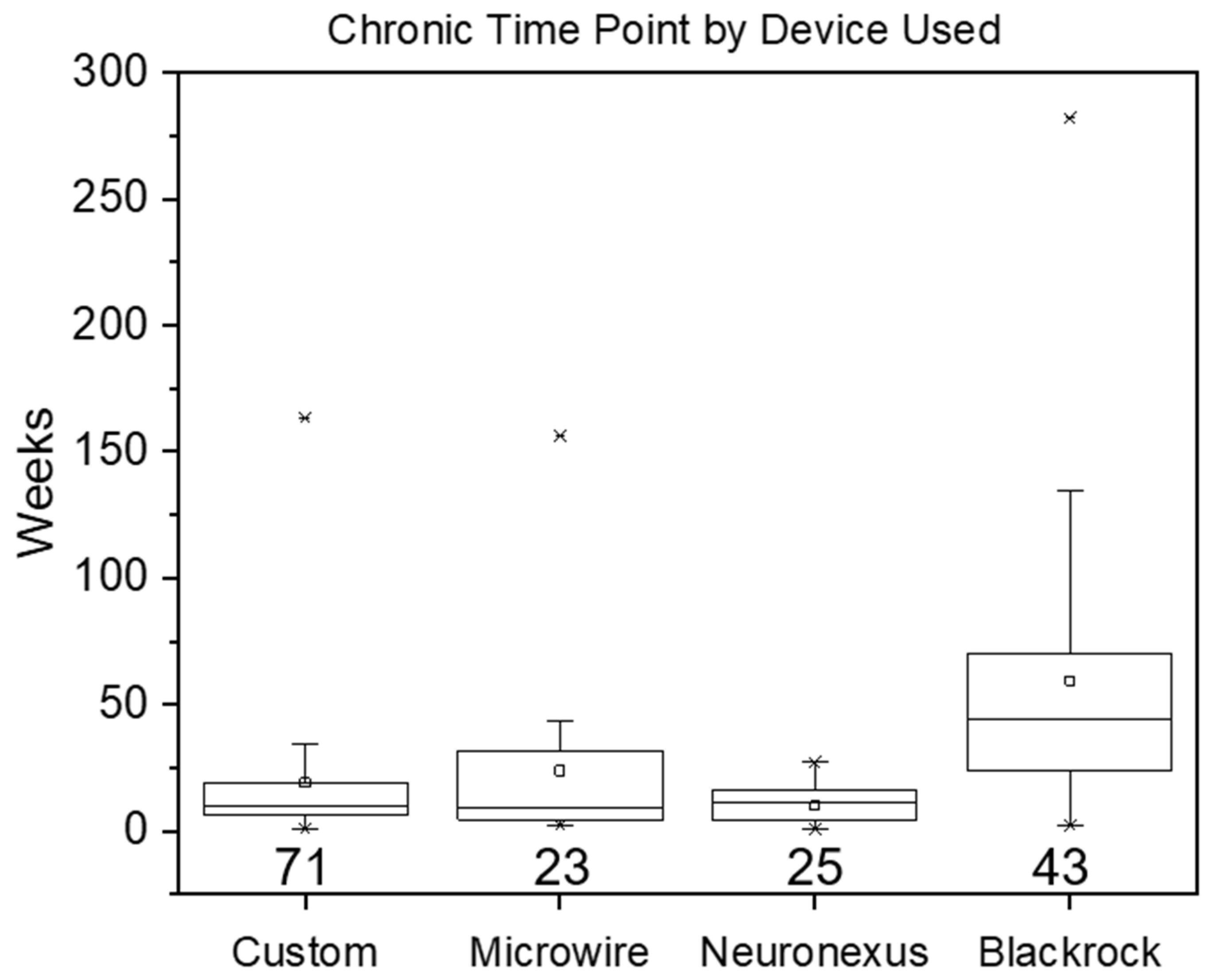
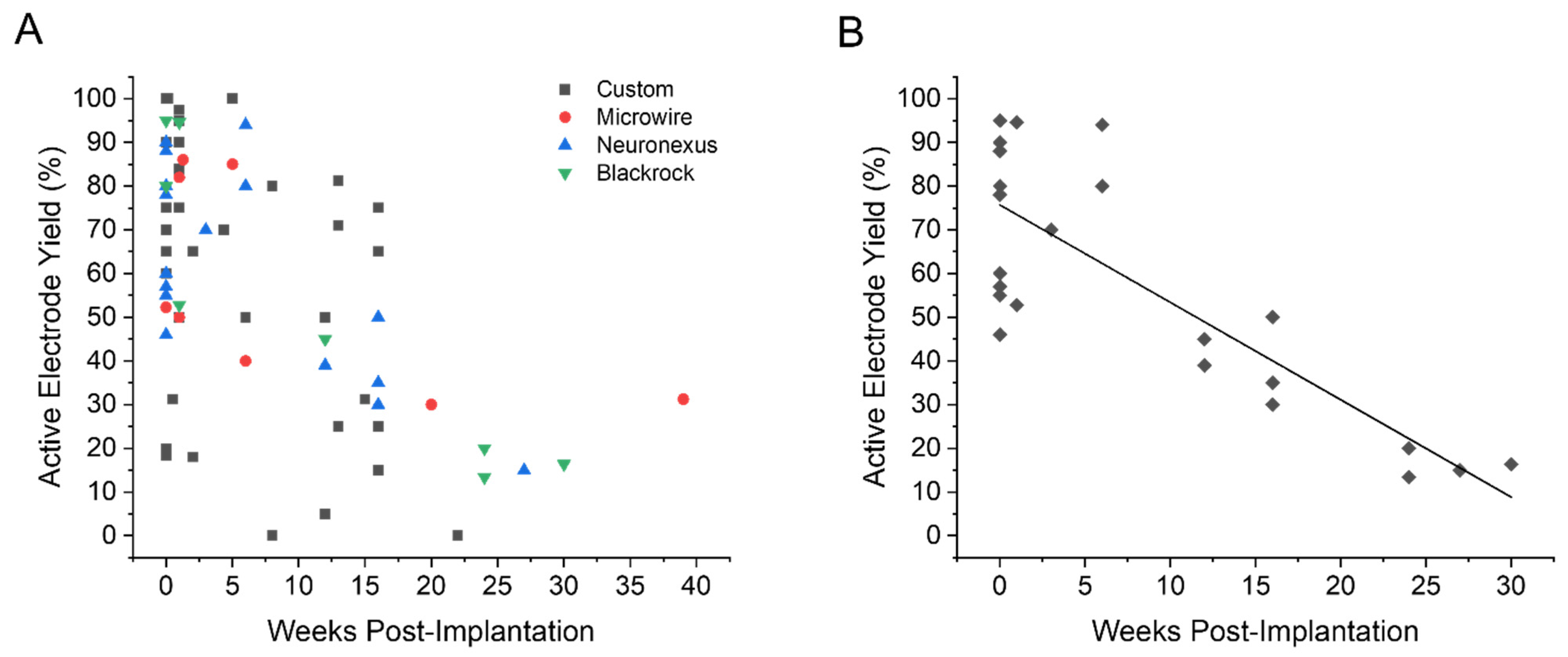
| Metric (in Weeks) | Mouse | Rat | Small Animal | Non-Human Primate | Human |
|---|---|---|---|---|---|
| Number of Studies | 23 | 69 | 25 | 33 | 12 |
| Mean | 13.5 | 10.8 | 25.1 | 58.0 | 93.3 |
| Median | 12.0 | 8.00 | 14.3 | 31.9 | 61.0 |
| Range | 0.429–34.7 | 1.00–52.0 | 0.700–96.0 | 1.43–163 | 26.0–282 |
| Standard Deviation | 8.70 | 9.40 | 23.3 | 53.0 | 77.3 |
| References | [24,25,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] | [16,22,26,27,28,29,33,38,48,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | [111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135] | [3,133,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164] | [1,4,7,165,166,167,168,169,170,171,172,173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usoro, J.O.; Sturgill, B.S.; Musselman, K.C.; Capadona, J.R.; Pancrazio, J.J. Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation. Micromachines 2021, 12, 972. https://doi.org/10.3390/mi12080972
Usoro JO, Sturgill BS, Musselman KC, Capadona JR, Pancrazio JJ. Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation. Micromachines. 2021; 12(8):972. https://doi.org/10.3390/mi12080972
Chicago/Turabian StyleUsoro, Joshua O., Brandon S. Sturgill, Kate C. Musselman, Jeffrey R. Capadona, and Joseph J. Pancrazio. 2021. "Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation" Micromachines 12, no. 8: 972. https://doi.org/10.3390/mi12080972
APA StyleUsoro, J. O., Sturgill, B. S., Musselman, K. C., Capadona, J. R., & Pancrazio, J. J. (2021). Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation. Micromachines, 12(8), 972. https://doi.org/10.3390/mi12080972







