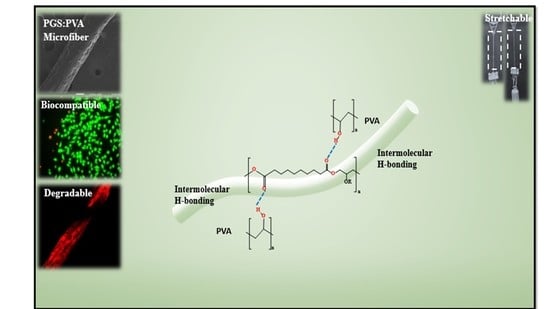
A Composite Microfiber for Biodegradable Stretchable Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Microfiber
2.3. Cytotoxicity Test
2.4. Fabrication of Microfiber-Based Strain Sensors
2.5. Characterization
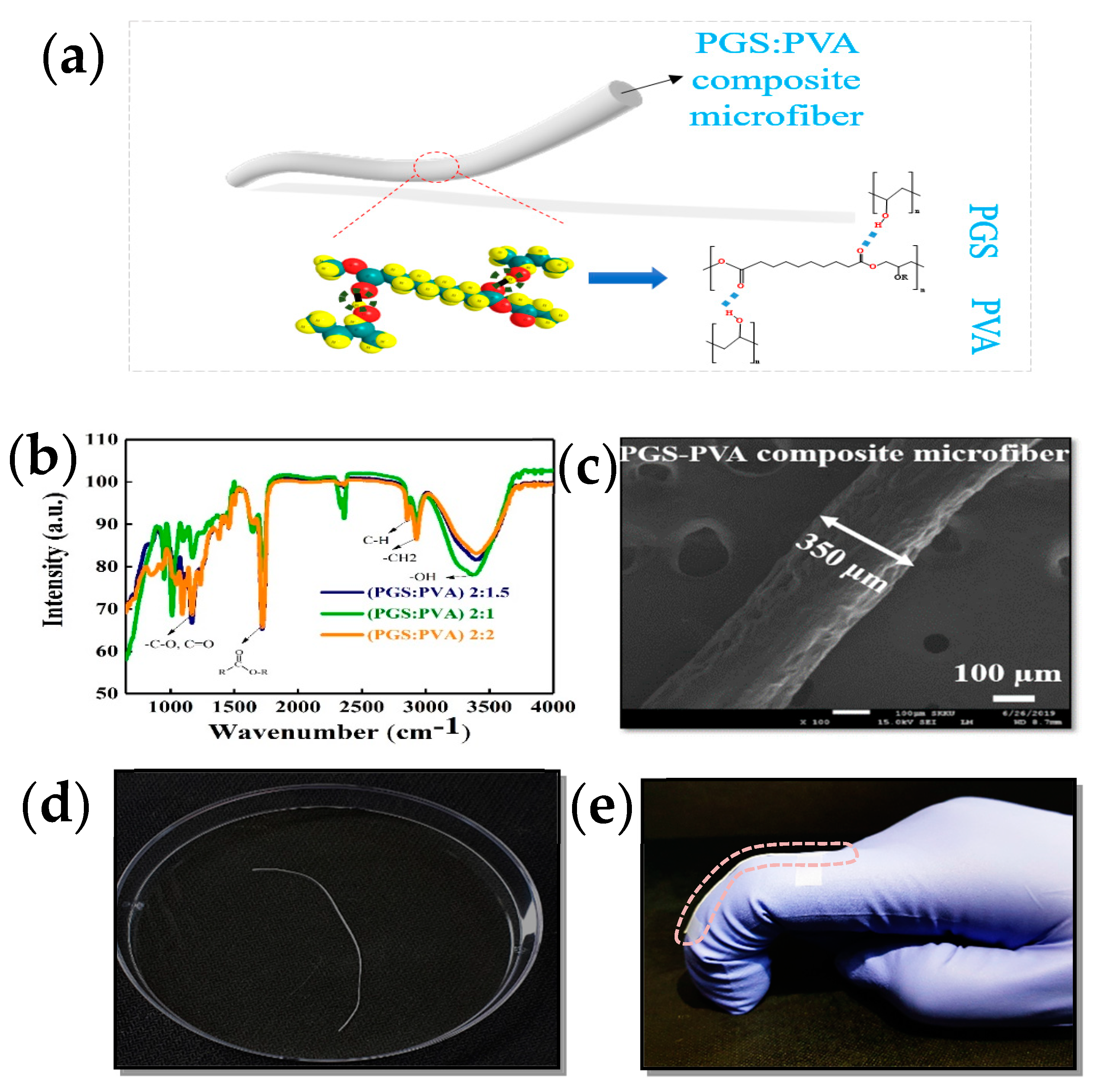
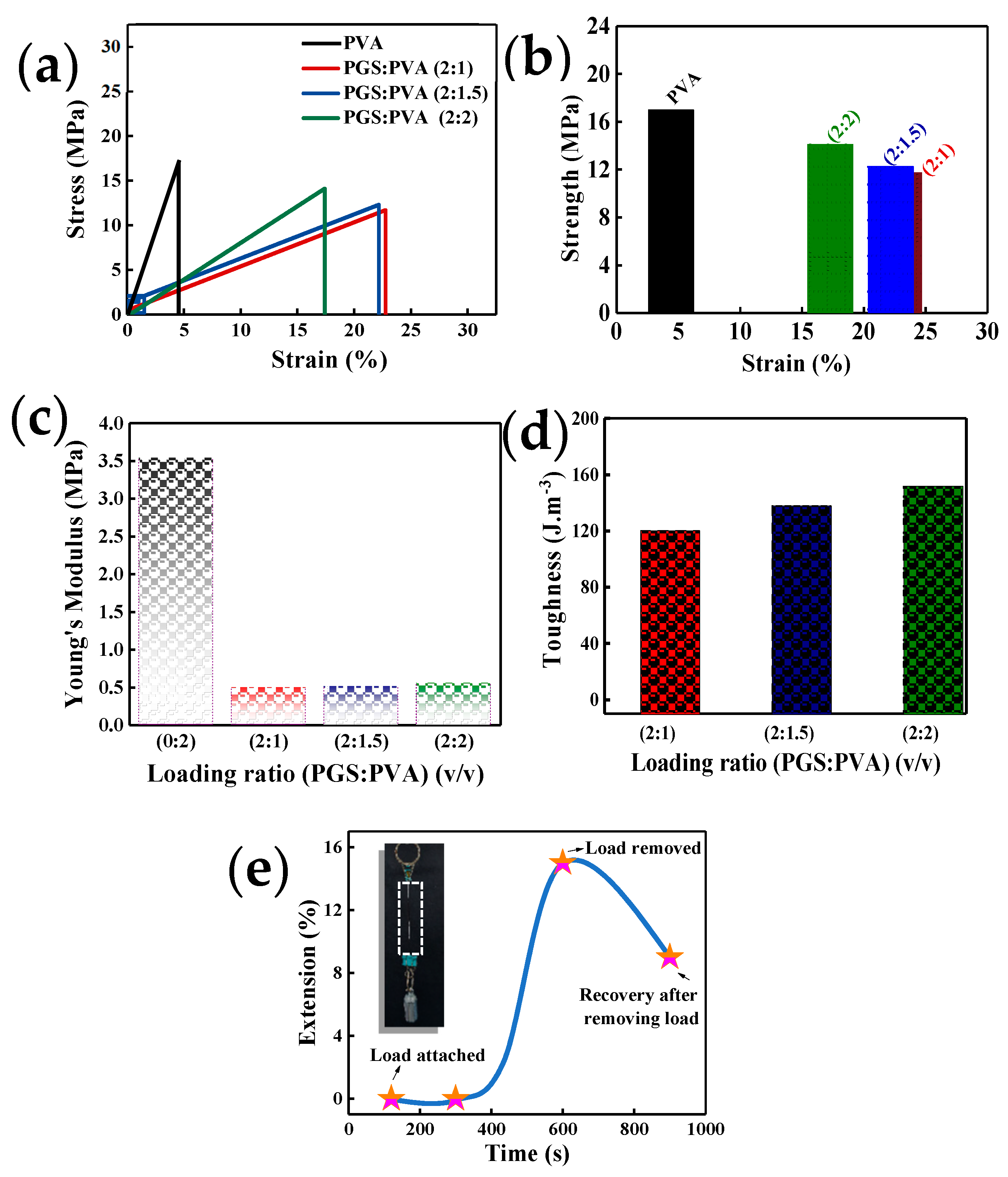
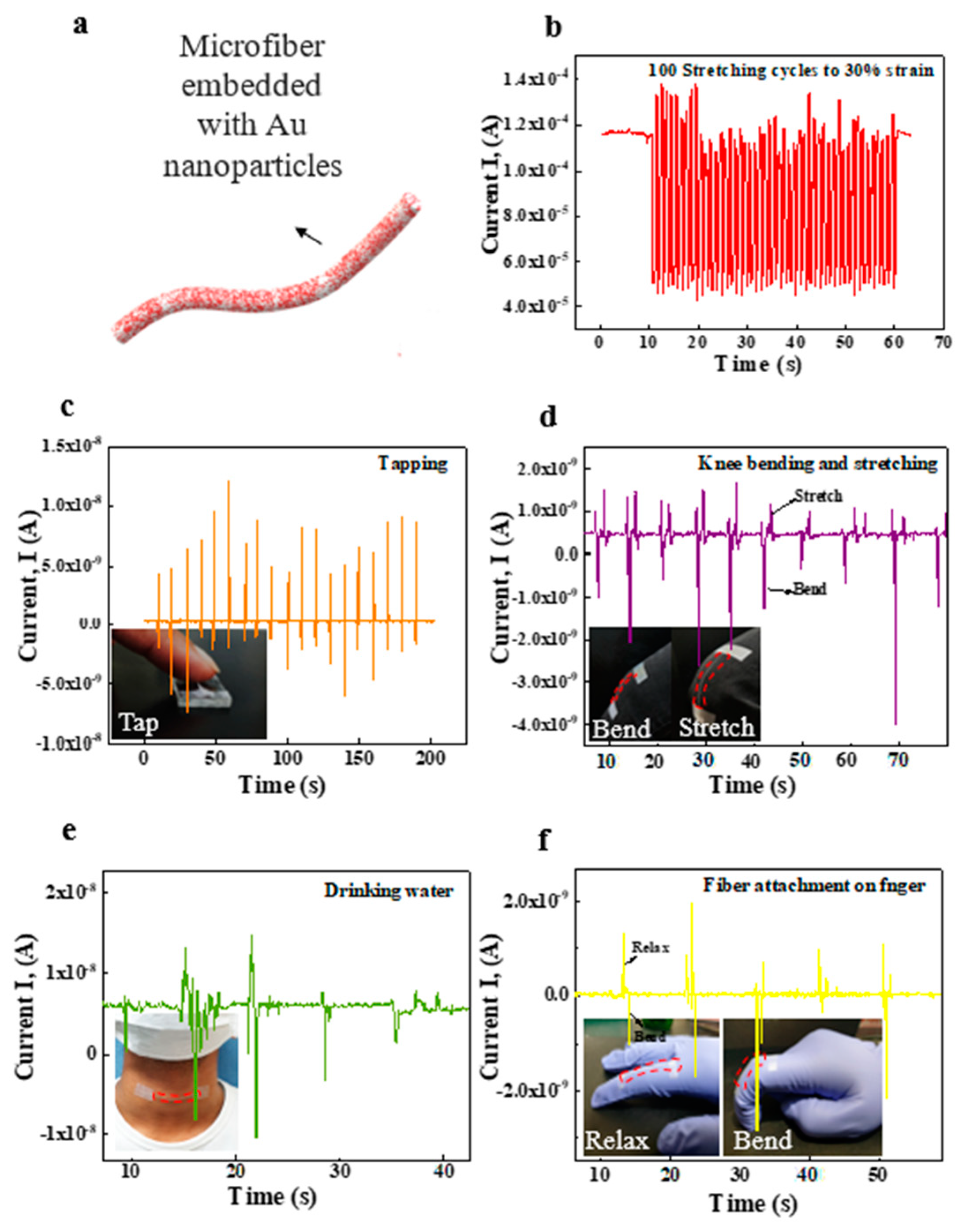
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Wan, S.; Zhu, Z.; Yin, K.; Su, S.; Bi, H.; Xu, T.; Zhang, H.; Shi, Z.; He, L.; Sun, L. A Highly Skin-Conformal and Biodegradable Graphene-Based Strain Sensor. Small Methods 2018, 2, 1700374. [Google Scholar] [CrossRef]
- Trung, T.Q.; Duy, L.T.; Ramasundaram, S.; Lee, N.E. Transparent, Stretchable, and Rapid-Response Humidity Sensor for Body-Attachable Wearable Electronics. Nano Res. 2017, 10, 2021–2033. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-Stretchable Wearable Strain Sensors Based on Skin-Inspired Adhesive, Tough and Conductive Hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Bae, J.Y.; Gwak, E.J.; Hwang, G.S.; Hwang, H.W.; Lee, D.J.; Lee, J.S.; Joo, Y.C.; Sun, J.Y.; Jun, S.H.; Ok, M.R.; et al. Biodegradable Metallic Glass for Stretchable Transient Electronics. Adv. Sci. 2021, 8, 2004029. [Google Scholar] [CrossRef]
- Liu, K.; Tran, H.; Feig, V.R.; Bao, Z. Biodegradable and Stretchable Polymeric Materials for Transient Electronic Devices. MRS Bull. 2020, 45, 96–102. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent Progress on Biodegradable Materials and Transient Electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Yoo, J.; Shim, J.S.; Ryu, Y.I.; Choi, S.; Lee, J.Y.; Lee, H.M.; Koo, J.; Kang, S.K. Biodegradable Molybdenum/Polybutylene Adipate Terephthalate Conductive Paste for Flexible and Stretchable Transient Electronics. Adv. Mater. Technol. 2021, 2001297. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet Entirely Degradable Gelatin-Based Biogels for Soft Robots and Electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y.; Zhou, J.; Wang, T.; Zhang, R.; Cao, J.; Luo, M.; Li, N.; Zhang, N.; Gong, H.; et al. Implantable and Biodegradable Micro-Supercapacitor Based on a Superassembled Three-Dimensional Network Zn@ppy Hybrid Electrode. ACS Appl. Mater. Interfaces 2021, 13, 8285–8293. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Xu, Z.; Qiu, W.; Wu, R.; Wang, Y.; Xu, Q.; Liu, X.Y.; Guo, W. A Biodegradable and Stretchable Protein-Based Sensor as Artificial Electronic Skin for Human Motion Detection. Small 2019, 15, 1805084. [Google Scholar] [CrossRef]
- Kafi, M.A.; Paul, A.; Vilouras, A.; Dahiya, R. Mesoporous Chitosan Based Conformable and Resorbable Biostrip for Dopamine Detection. Biosens. Bioelectron. 2020, 147, 111781. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lou, Z.; Jiang, K.; Shen, G. Bio-Multifunctional Smart Wearable Sensors for Medical Devices. Adv. Intell. Syst. 2019, 1, 1900040. [Google Scholar] [CrossRef] [Green Version]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible Potentiometric PH Sensors for Wearable Systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.K.; Murphy, R.K.J.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable Silicon Electronic Sensors for the Brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Feig, V.R.; Liu, K.; Wu, H.C.; Chen, R.; Xu, J.; Deisseroth, K.; Bao, Z. Stretchable and Fully Degradable Semiconductors for Transient Electronics. ACS Cent. Sci. 2019, 5, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Neng, W.B.; Xie, W.G.; Lu, B.; Zhen, Z.C.; Zhao, J.L.; Wang, G.X.; Ji, J.H. Biodegradable Thermoplastic Copolyester Elastomers: Methyl Branched PBAmT. E-Polymers 2021, 21, 336–345. [Google Scholar] [CrossRef]
- Hartmann, F.; Baumgartner, M.; Kaltenbrunner, M. Becoming Sustainable, The New Frontier in Soft Robotics. Adv. Mater. 2021, 33, 2004413. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.S.; Ha, J.S. Flexible/Stretchable Supercapacitors with Novel Functionality for Wearable Electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef]
- Chiong, J.A.; Tran, H.; Lin, Y.; Zheng, Y.; Bao, Z. Integrating Emerging Polymer Chemistries for the Advancement of Recyclable, Biodegradable, and Biocompatible Electronics. Adv. Sci. 2021, 2101233. [Google Scholar] [CrossRef]
- Chen, S.; Sun, L.; Zhou, X.; Guo, Y.; Song, J.; Qian, S.; Liu, Z.; Guan, Q.; Meade Jeffries, E.; Liu, W.; et al. Mechanically and Biologically Skin-like Elastomers for Bio-Integrated Electronics. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and Microstructural Properties of a Novel Edible Film Synthesized from Balangu Seed Mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Shih, C.C.; Pan, S.Y.; Chueh, C.C.; Chen, W.C. Advances and Challenges of Green Materials for Electronics and Energy Storage Applications: From Design to End-of-Life Recovery. J. Mater. Chem. A 2018, 6, 20546–20563. [Google Scholar] [CrossRef]
- Irimia-Vladu, M. “Green” Electronics: Biodegradable and Biocompatible Materials and Devices for Sustainable Future. Chem. Soc. Rev. 2014, 43, 588–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, J.; Porcarelli, L.; Rödlmeier, T.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hernandez-Sosa, G. Fully Printed Light-Emitting Electrochemical Cells Utilizing Biocompatible Materials. Adv. Funct. Mater. 2018, 28, 1705795. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jürgensen, N.; Morfa, A.J.; Wang, B.; Tekoglu, S.; Hernandez-Sosa, G. Poly(Lactic-Co-Glycolic Acid) (PLGA) as Ion-Conducting Polymer for Biodegradable Light-Emitting Electrochemical Cells. ACS Sustain. Chem. Eng. 2016, 4, 7050–7055. [Google Scholar] [CrossRef]
- Jürgensen, N.; Zimmermann, J.; Morfa, A.J.; Hernandez-Sosa, G. Biodegradable Polycaprolactone as Ion Solvating Polymer for Solution-Processed Light-Emitting Electrochemical Cells. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, W.; Chatterjee, P.; Lv, C.; Popovich, J.; Song, Z.; Dai, L.; Kalani, M.Y.S.; Haydel, S.E.; Jiang, H. Food-Materials-Based Edible Supercapacitors. Adv. Mater. Technol. 2016, 1, 1600059. [Google Scholar] [CrossRef]
- Pietsch, M.; Schlisske, S.; Held, M.; Strobel, N.; Wieczorek, A.; Hernandez-Sosa, G. Biodegradable Inkjet-Printed Electrochromic Display for Sustainable Short-Lifecycle Electronics. J. Mater. Chem. C 2020, 8, 16716–16724. [Google Scholar] [CrossRef]
- Koh, L.D.; Yeo, J.; Lee, Y.Y.; Ong, Q.; Han, M.; Tee, B.C.K. Advancing the Frontiers of Silk Fibroin Protein-Based Materials for Futuristic Electronics and Clinical Wound-Healing (Invited Review). Mater. Sci. Eng. C 2018, 86, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Nur Hanani, Z.A.; McNamara, J.; Roos, Y.H.; Kerry, J.P. Effect of Plasticizer Content on the Functional Properties of Extruded Gelatin-Based Composite Films. Food Hydrocoll. 2013, 31, 264–269. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2020, 33, 2000619. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester Elastomers for Soft Tissue Engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(Lactic Acid) and Its Composites. Polymers. 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.; Nor, F.M.; Lee, H.Y.; Lim, J.Y. Elastic Properties of Polycaprolactone at Small Strains Are Significantly Affected by Strain Rate and Temperature. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 1015–1020. [Google Scholar] [CrossRef]
- Hidalgo-Bastida, L.A.; Barry, J.J.A.; Everitt, N.M.; Rose, F.R.A.J.; Buttery, L.D.; Hall, I.P.; Claycomb, W.C.; Shakesheff, K.M. Cell Adhesion and Mechanical Properties of a Flexible Scaffold for Cardiac Tissue Engineering. Acta Biomater. 2007, 3, 457–462. [Google Scholar] [CrossRef]
- Yu, X.; Shou, W.; Mahajan, B.K.; Huang, X.; Pan, H. Materials, Processes, and Facile Manufacturing for Bioresorbable Electronics: A Review. Adv. Mater. 2018, 30, 1707624. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric Biomaterials for Tissue Engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Kang, S.K.; Koo, J.; Lee, Y.K.; Rogers, J.A. Advanced Materials and Devices for Bioresorbable Electronics. Acc. Chem. Res. 2018, 51, 988–998. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Ouyang, L.; Highley, C.B.; Burdick, J.A. Norbornene-Modified Poly(Glycerol Sebacate) as a Photocurable and Biodegradable Elastomer. Polym. Chem. 2017, 8, 5091–5099. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A Highly Bioactive and Biodegradable Poly(Glycerol Sebacate)-Silica Glass Hybrid Elastomer with Tailored Mechanical Properties for Bone Tissue Regeneration. J. Mater. Chem. B 2015, 3, 3222–3233. [Google Scholar] [CrossRef]
- Gultekinoglu, M.; Öztürk, Ş.; Chen, B.; Edirisinghe, M.; Ulubayram, K. Preparation of Poly(Glycerol Sebacate) Fibers for Tissue Engineering Applications. Eur. Polym. J. 2019, 121, 109297. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Mikael, P.E.; Lin, L.; Dong, W.; Zheng, Y.; Simmons, T.J.; Zhang, F.; Linhardt, R.J. Biodegradable and Bioactive PCL-PGS Core-Shell Fibers for Tissue Engineering. ACS Omega 2017, 2, 6321–6328. [Google Scholar] [CrossRef]
- Rostamian, M.; Kalaee, M.R.; Dehkordi, S.R.; Panahi-Sarmad, M.; Tirgar, M.; Goodarzi, V. Design and Characterization of Poly(Glycerol-Sebacate)-Co-Poly(Caprolactone) (PGS-Co-PCL) and Its Nanocomposites as Novel Biomaterials: The Promising Candidate for Soft Tissue Engineering. Eur. Polym. J. 2020, 138, 109985. [Google Scholar] [CrossRef]
- Yan, Y.; Sencadas, V.; Jin, T.; Huang, X.; Chen, J.; Wei, D.; Jiang, Z. Tailoring the Wettability and Mechanical Properties of Electrospun Poly(L-Lactic Acid)-Poly(Glycerol Sebacate) Core-Shell Membranes for Biomedical Applications. J. Colloid Interface Sci. 2017, 508, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and Characterization of Novel Electrically Conductive PANI-PGS Composites for Cardiac Tissue Engineering Applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef]
- Frydrych, M.; Román, S.; Green, N.H.; MacNeil, S.; Chen, B. Thermoresponsive, Stretchable, Biodegradable and Biocompatible Poly(Glycerol Sebacate)-Based Polyurethane Hydrogels. Polym. Chem. 2015, 6, 7974–7987. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Gaharwar, A.K.; Iviglia, G.; Zhang, H.; Mukundan, S.; Mihaila, S.M.; Demarchi, D.; Khademhosseini, A. Highly Elastomeric Poly(Glycerol Sebacate)-Co-Poly(Ethylene Glycol) Amphiphilic Block Copolymers. Biomaterials 2013, 34, 3970–3983. [Google Scholar] [CrossRef] [Green Version]
- Tadayyon, G.; Krukiewicz, K.; Britton, J.; Larrañaga, A.; Vallejo-Giraldo, C.; Fernandez-Yague, M.; Guo, Y.; Orpella-Aceret, G.; Li, L.; Poudel, A.; et al. In Vitro Analysis of a Physiological Strain Sensor Formulated from a PEDOT:PSS Functionalized Carbon Nanotube-Poly(Glycerol Sebacate Urethane) Composite. Mater. Sci. Eng. C 2021, 121, 111857. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Tawk, C.; Alici, G. Environmentally Friendly and Biodegradable Ultrasensitive Piezoresistive Sensors for Wearable Electronics Applications. ACS Appl. Mater. Interfaces 2020, 12, 8761–8772. [Google Scholar] [CrossRef]
- Yan, Y.; Sencadas, V.; Jin, T.; Huang, X.; Lie, W.; Wei, D.; Jiang, Z. Effect of Multi-Walled Carbon Nanotubes on the Cross-Linking Density of the Poly(Glycerol Sebacate) Elastomeric Nanocomposites. J. Colloid Interface Sci. 2018, 521, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym.-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, Properties and Biomedical Applications of Poly(Glycerol Sebacate) (PGS): A Review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Joodaki, H.; Panzer, M.B. Skin Mechanical Properties and Modeling: A Review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric Scaling of Skin Thickness, Elasticity, Viscoelasticity to Mass for Micro-Medical Device Translation: From Mice, Rats, Rabbits, Pigs to Humans. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Wakhlu, A.; Chowdhury, A.C.; Mohindra, N.; Tripathy, S.R.; Misra, D.P.; Agarwal, V. Assessment of Extent of Skin Involvement in Scleroderma Using Shear Wave Elastography. Indian J. Rheumatol. 2017, 12, 194–198. [Google Scholar]
- Sun, Z.; Gepner, B.D.; Cottler, P.S.; Lee, S.H.; Kerrigan, J.R. In Vitro Mechanical Characterization and Modeling of Subcutaneous Adipose Tissue: A Comprehensive Review. J. Biomech. Eng. 2021, 143, 070803. [Google Scholar] [CrossRef] [PubMed]
- Grego, A.V.; Mingrone, G. Dicarboxylic Acids, an Alternate Fuel Substrate in Parenteral Nutrition: An Update. Clin. Nutr. 1995, 14, 143–148. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a Soft Elastomer Poly(Glycerol Sebacate) Designed to Match the Mechanical Properties of Myocardial Tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Khalaji, S.; Golshan Ebrahimi, N.; Hosseinkhani, H. Enhancement of Biocompatibility of PVA/HTCC Blend Polymer with Collagen for Skin Care Application. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 459–468. [Google Scholar] [CrossRef]
- Byrne, D.; Boeije, G.; Croft, I.; Hüttmann, G.; Luijkx, G.; Meier, F.; Parulekar, Y.; Stijntjes, G. Biodegradability of Polyvinyl Alcohol Based Film Used for Liquid Detergent Capsules. Tenside Surfactants Deterg. 2021, 58, 88–96. [Google Scholar] [CrossRef]
- Yang, S.B.; Kim, E.H.; Kim, S.H.; Kim, Y.H.; Oh, W.; Lee, J.T.; Jang, Y.A.; Sabina, Y.; Ji, B.C.; Yeum, J.H. Electrospinning Fabrication of Poly(Vinyl Alcohol)/Coptis Chinensis Extract Nanofibers for Antimicrobial Exploits. Nanomaterials 2018, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Crivoi, F.; Stefan, L.; Moldovan, L.; Vasile, C. Compatibility and Cytotoxicity Testing of Some Polyvinyl Alcohol and Hydrolyzated Collagen Based Blends. Pharmacology 2007, 12, 2–9. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, A.; Ghosh, G.; Meeseepong, M.; Haq Chouhdry, H.; Bag, A.; Chinnamani, M.V.; Kumar, S.; Sultan, M.J.; Yadav, A.; Lee, N.-E. A Composite Microfiber for Biodegradable Stretchable Electronics. Micromachines 2021, 12, 1036. https://doi.org/10.3390/mi12091036
Hanif A, Ghosh G, Meeseepong M, Haq Chouhdry H, Bag A, Chinnamani MV, Kumar S, Sultan MJ, Yadav A, Lee N-E. A Composite Microfiber for Biodegradable Stretchable Electronics. Micromachines. 2021; 12(9):1036. https://doi.org/10.3390/mi12091036
Chicago/Turabian StyleHanif, Adeela, Gargi Ghosh, Montri Meeseepong, Hamna Haq Chouhdry, Atanu Bag, M. V. Chinnamani, Surjeet Kumar, Muhammad Junaid Sultan, Anupama Yadav, and Nae-Eung Lee. 2021. "A Composite Microfiber for Biodegradable Stretchable Electronics" Micromachines 12, no. 9: 1036. https://doi.org/10.3390/mi12091036
APA StyleHanif, A., Ghosh, G., Meeseepong, M., Haq Chouhdry, H., Bag, A., Chinnamani, M. V., Kumar, S., Sultan, M. J., Yadav, A., & Lee, N.-E. (2021). A Composite Microfiber for Biodegradable Stretchable Electronics. Micromachines, 12(9), 1036. https://doi.org/10.3390/mi12091036