Influence of the Surface Material and Illumination upon the Performance of a Microelectrode/Electrolyte Interface in Optogenetics
Abstract
:1. Introduction
2. Experiments and Methods
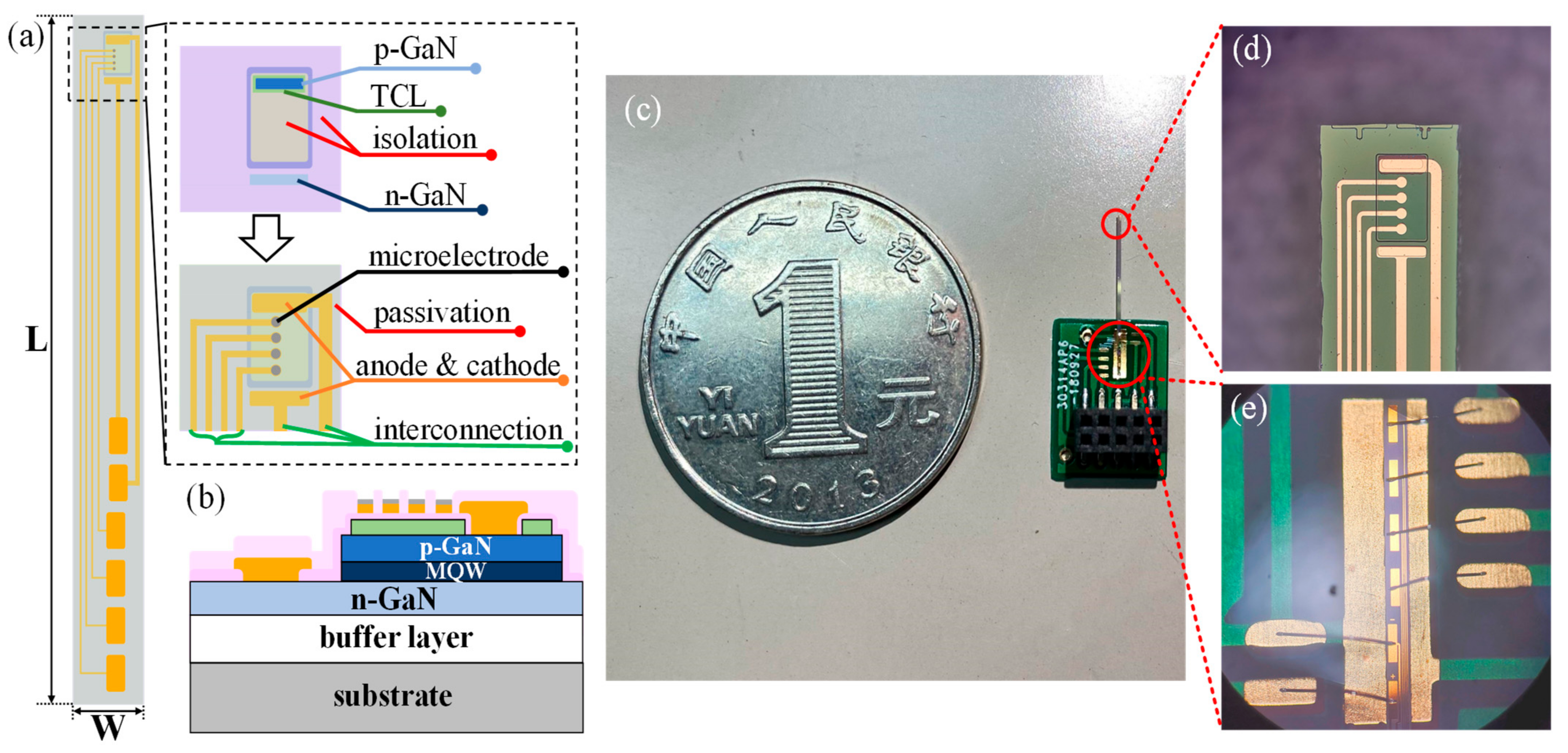
2.1. Design and Fabrication
2.2. Theoretical Model and Equivalent Circuit
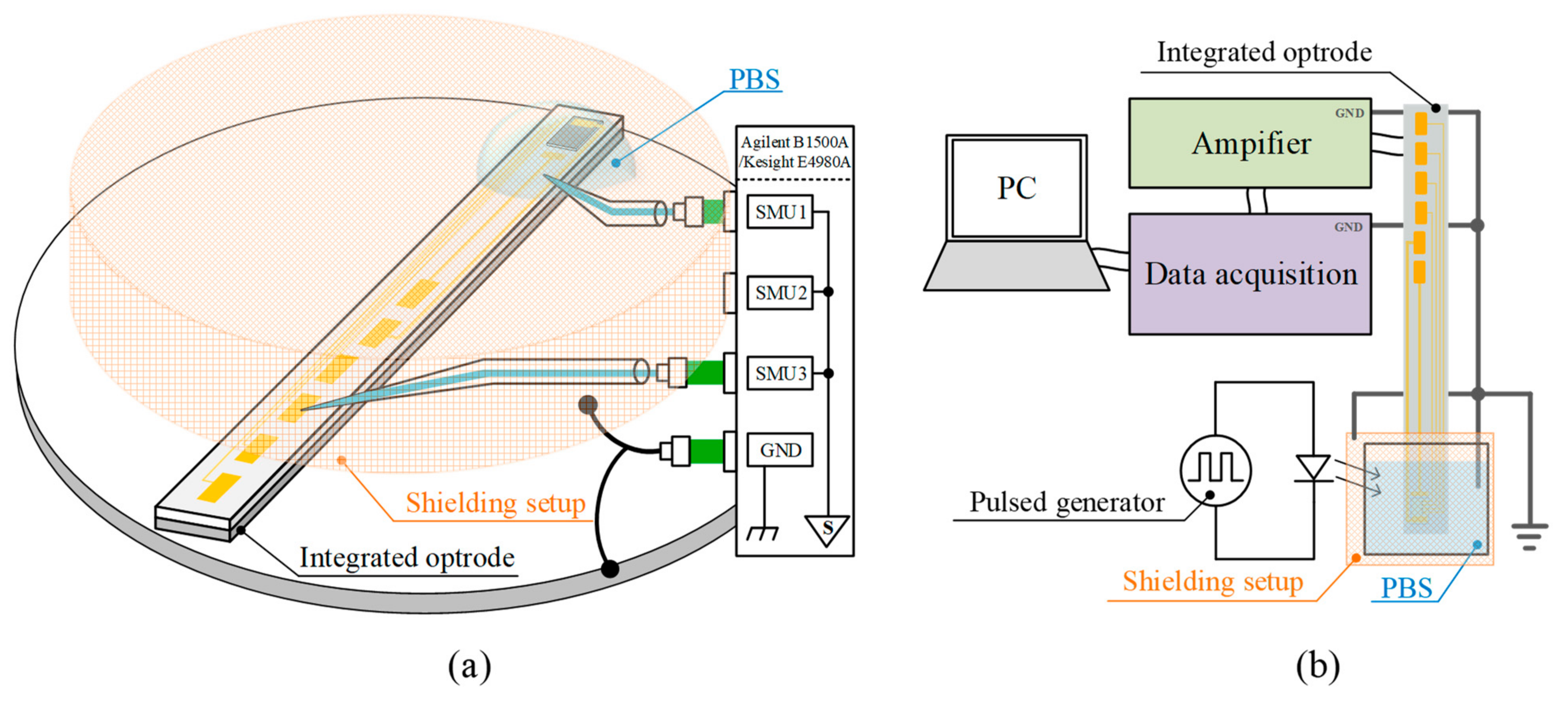
2.3. Testing System
3. Results and Discussion
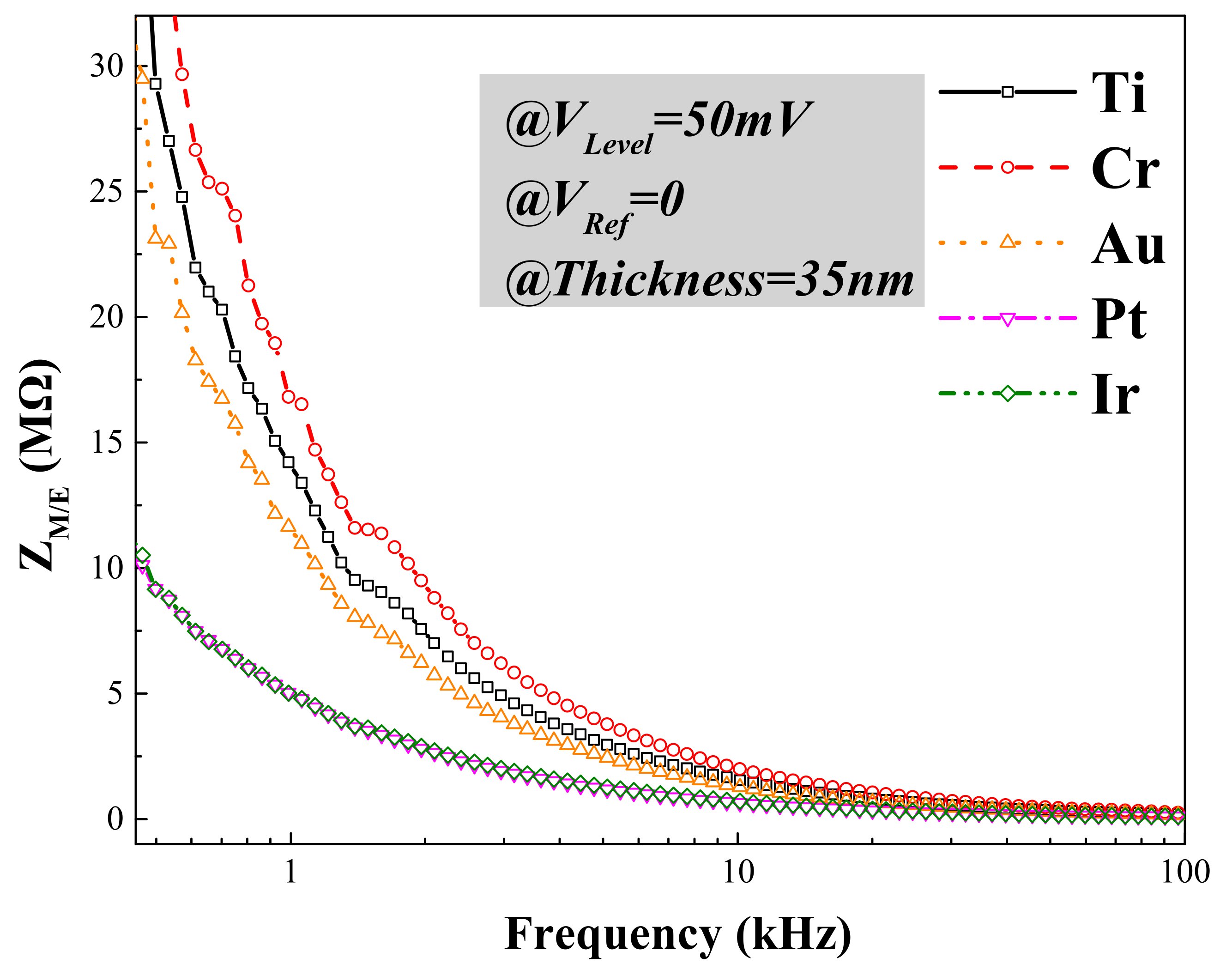
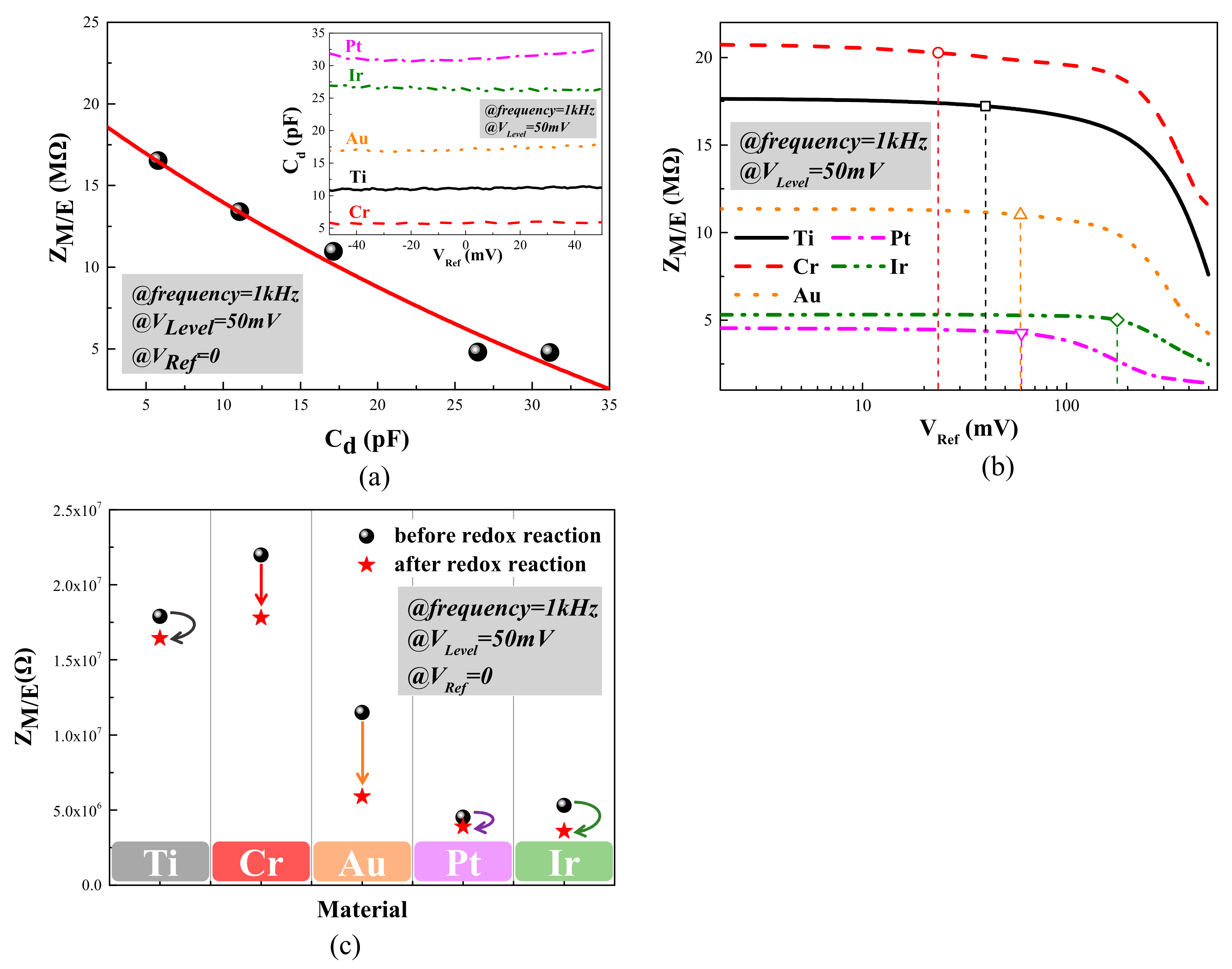
3.1. Electrical Performance Characterization
3.2. Photostability and Optical Artifacts Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in neural systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Aravanis, A.M.; Adamantidis, A.; Lecea, L.D.; Deisseroth, K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007, 8, 577–581. [Google Scholar] [CrossRef]
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef]
- Mickle, A.D.; Gereau, R.W. A bright future? Optogenetics in the periphery for pain research and therapy. Pain 2018, 159 (Suppl. 1), S65–S73. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y.; et al. An Ultra-Sensitive Step-Function Opsin for Minimally Invasive Optogenetic Stimulation in Mice and Macaques. Neuron 2020, 107, 197. [Google Scholar] [CrossRef]
- Beal, F.M. Experimental models of Parkinson’s disease. Nat. Rev. Neurosci. 2001, 2, 325–334. [Google Scholar] [CrossRef]
- Lewis, D.A. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res. Rev. 2000, 31, 270–276. [Google Scholar] [CrossRef]
- LeChasseur, Y.; Dufour, S.; Lavertu, G.; Bories, C.; Deschenes, M.; Vallee, R.; De Koninck, Y. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 2011, 8, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Lavertu, G.; Dufour-Beausejour, S.; Juneau-Fecteau, A.; Calakos, N.; Deschenes, M.; Vallee, R.; De Koninck, Y. A multimodal micro-optrode combining field and single unit recording, multispectral detection and photolabeling capabilities. PLoS ONE 2013, 8, e57703. [Google Scholar] [CrossRef] [Green Version]
- Canales, A.; Jia, X.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y.; et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Sirowatka, B.; Li, W. Opto-μECoG array: Transparent μECoG electrode array and integrated LEDs for optogenetics. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Hsinchu, Taiwan, 28–30 November 2013. [Google Scholar]
- Ji, B.; Guo, Z.; Wang, M.; Yang, B.; Wang, X.; Li, W.; Liu, J. Flexible polyimide-based hybrid opto-electric neural interface with 16 channels of micro-LEDs and electrodes. Microsyst. Nanoeng. 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickgauer, J.P.; Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 2009, 106, 15025–15030. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.Y.; Lee, H.M.; Ghovanloo, M.; Weber, A.; Li, W. Design, fabrication, and packaging of an integrated, wirelessly-powered optrode array for optogenetics application. Front. Syst. Neurosci. 2015, 9, 69. [Google Scholar] [CrossRef]
- Lee, J.; Ozden, I.; Song, Y.K.; Nurmikko, A.V. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording. Nat. Methods 2015, 12, 1157–1162. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, Y.; Ryu, D.; Cho, Y.K.; Lee, Y.; Jun, S.B.; Ji, C.H. Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording. Micromachines 2021, 12, 725. [Google Scholar] [CrossRef]
- McAlinden, N.; Cheng, Y.; Scharf, R.; Xie, E.; Gu, E.; Reiche, C.F.; Sharma, R.; Tathireddy, P.; Dawson, M.D.; Rieth, L.; et al. Multisite microLED optrode array for neural interfacing. Neurophotonics 2019, 6, 035010. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Stark, E.; Ku, P.C.; Wise, K.D.; Buzsaki, G.; Yoon, E. Monolithically Integrated muLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals. Neuron 2015, 88, 1136–1148. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Gu, L.; Mohanty, S.K.; Chiao, J.C. An integrated muLED optrode for optogenetic stimulation and electrical recording. IEEE Trans. Biomed. Eng. 2013, 60, 225–229. [Google Scholar] [CrossRef]
- Goncalves, S.B.; Palha, J.M.; Fernandes, H.C.; Souto, M.R.; Pimenta, S.; Dong, T.; Yang, Z.; Ribeiro, J.F.; Correia, J.H. LED Optrode with Integrated Temperature Sensing for Optogenetics. Micromachines 2018, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Mendrela, A.E.; Kim, K.; English, D.; McKenzie, S.; Seymour, J.P.; Buzsáki, G.; Yoon, E. A High-Resolution Opto-Electrophysiology System With a Miniature Integrated Headstage. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Voroslakos, M.; Seymour, J.P.; Wise, K.D.; Buzsaki, G.; Yoon, E. Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes. Nat. Commun. 2020, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Kampasi, K.; English, D.F.; Seymour, J.; Stark, E.; McKenzie, S.; Voroslakos, M.; Buzsaki, G.; Wise, K.D.; Yoon, E. Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes. Microsyst. Nanoeng. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayling, O.G.; Harrison, T.C.; Boyd, J.D.; Goroshkov, A.; Murphy, T.H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat. Methods 2009, 6, 219–224. [Google Scholar] [CrossRef]
- Kozai, T.D.; Vazquez, A.L. Photoelectric artefact from optogenetics and imaging on microelectrodes and bioelectronics: New Challenges and Opportunities. J. Mater. Chem. B 2015, 3, 4965–4978. [Google Scholar] [CrossRef]
- Kim, K.; English, D.; Mckenzie, S.; Wu, F.; Stark, E.; Seymour, J.; Ku, P.C.; Wise, K.; Buzsaki, G.; Yoon, E. GaN-on-Si μLED optoelectrodes for high-spatiotemporal-accuracy optogenetics in freely behaving animals. In Proceedings of the IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2017. [Google Scholar]
- Wang, L.; Ge, C.; Wang, M.; Ji, B.; Guo, Z.; Wang, X.; Yang, B.; Li, C.; Liu, J. An artefact-resist optrode with internal shielding structure for low-noise neural modulation. J. Neural Eng. 2020, 17, 046024. [Google Scholar] [CrossRef]
- Ji, B.; Ge, C.; Guo, Z.; Wang, L.; Wang, M.; Xie, Z.; Xu, Y.; Li, H.; Yang, B.; Wang, X.; et al. Flexible and stretchable opto-electric neural interface for low-noise electrocorticogram recordings and neuromodulation in vivo. Biosens. Bioelectron. 2020, 153, 112009. [Google Scholar] [CrossRef]
- Guo, Z.; Ji, B.; Wang, M.; Ge, C.; Wang, L.; Gu, X.; Yang, B.; Wang, X.; Li, C.; Liu, J. A Polyimide-Based Flexible Optoelectrodes for Low-Noise Neural Recording. IEEE Electron Device Lett. 2019, 40, 1190–1193. [Google Scholar] [CrossRef]
- Al-Juboori, S.I.; Dondzillo, A.; Stubblefield, E.A.; Felsen, G.; Lei, T.C.; Klug, A. Light scattering properties vary across different regions of the adult mouse brain. PLoS ONE 2013, 8, e67626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Lu, Y.; Iseri, E.; Shi, Y.; Kuzum, D. A Compact Closed-Loop Optogenetics System Based on Artifact-Free Transparent Graphene Electrodes. Front. Neurosci. 2018, 12, 132. [Google Scholar] [CrossRef]
- Khurram, A.; Seymour, J.P. Investigation of the photoelectrochemical effect in optoelectrodes and potential uses for implantable electrode characterization*. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; pp. 3032–3035. [Google Scholar]
- Rui, Y.F.; Liu, J.Q.; Yang, B.; Li, K.Y.; Yang, C.S. Parylene-based implantable platinum-black coated wire microelectrode for orbicularis oculi muscle electrical stimulation. Biomed. Microdevices 2012, 14, 367–373. [Google Scholar] [CrossRef]
- Du, J.; Roukes, M.L.; Masmanidis, S.C. Dual-side and three-dimensional microelectrode arrays fabricated from ultra-thin silicon substrates. J. Micromech. Microeng. 2009, 19, 1403–1407. [Google Scholar] [CrossRef]
- Wang, M.H.; Ji, B.W.; Gu, X.W.; Tian, H.C.; Kang, X.Y.; Yang, B.; Wang, X.L.; Chen, X.; Li, C.Y.; Liu, J.Q. Direct electrodeposition of Graphene enhanced conductive polymer on microelectrode for biosensing application. Biosens. Bioelectron. 2018, 99, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Wang, M.H.; Ge, C.F.; Ji, B.W.; Guo, Z.J.; Wang, X.L.; Yang, B.; Li, C.Y.; Liu, J.Q. The use of a double-layer platinum black-conducting polymer coating for improvement of neural recording and mitigation of photoelectric artifact. Biosens. Bioelectron. 2019, 145, 111661. [Google Scholar] [CrossRef]
- Brosch, M.; Deckert, M.; Rathi, S.; Takagaki, K.; Lippert, M.T. An optically transparent multi-electrode array for combined electrophysiology and optophysiology at the mesoscopic scale. J. Neural Eng. 2020, 17, 046014. [Google Scholar] [CrossRef]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. Electrochemical Methods Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Chen, C.H.; Pun, S.H.; Mak, P.U.; Vai, M.I.; Klug, A.; Lei, T.C. Circuit Models and Experimental Noise Measurements of Micropipette Amplifiers for Extracellular Neural Recordings from Live Animals. BioMed Res. Int. 2014, 2014, 135026. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Allred, A.L.; Rochow, E.G. Electronegativities of carbon, silicon, germanium, tin and lead. J. Inorg. Nucl. Chem. 1958, 20, 167–170. [Google Scholar] [CrossRef]
- Yu, D. A new set of electronegativities of elements in valence states. Chin. J. Inorg. Chem. 2005, 21, 955–959. [Google Scholar]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 15007. [Google Scholar] [CrossRef] [PubMed]
- Schwaerzle, M.; Seidl, K.; Schwarz, U.T.; Paul, O.; Ruther, P. Ultracompact optrode with integrated laser diode chips and SU-8 waveguides for optogenetic applications. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems, Taipei, Taiwan, 20–24 January 2013. [Google Scholar]
- Fan, B.; Kwon, K.Y.; Rechenberg, R.; Khomenko, A.; Li, W. A polycrystalline diamond-based, hybrid neural interfacing probe for optogenetics. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems, Estoril, Portugal, 18–22 January 2015. [Google Scholar]
- Wang, M.; Guo, B.; Ji, B.; Fan, Y.; Wang, G. Controlled Electrodeposition of Graphene Oxide doped Conductive polymer on Microelectrodes for Low-Noise Optogenetics. IEEE Electron Device Lett. 2021, 42, 418–421. [Google Scholar] [CrossRef]
- Chen, C.H.; Mccullagh, E.A.; Pun, S.H.; Mak, P.U.; Vai, M.I.; Mak, P.I.; Klug, A.; Lei, T.C. An Integrated Circuit for Simultaneous Extracellular Electrophysiology Recording and Optogenetic Neural Manipulation. IEEE Trans. Biomed. Eng. 2017, 64, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, D.R.; Schmidt, E.M. Extracellular Single-Unit Recording Methods. In Neuromethods; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–64. [Google Scholar]
- Cohen, I.; Huang, Y.; Chen, J.; Benesty, J. Pearson Correlation Coefficient; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]

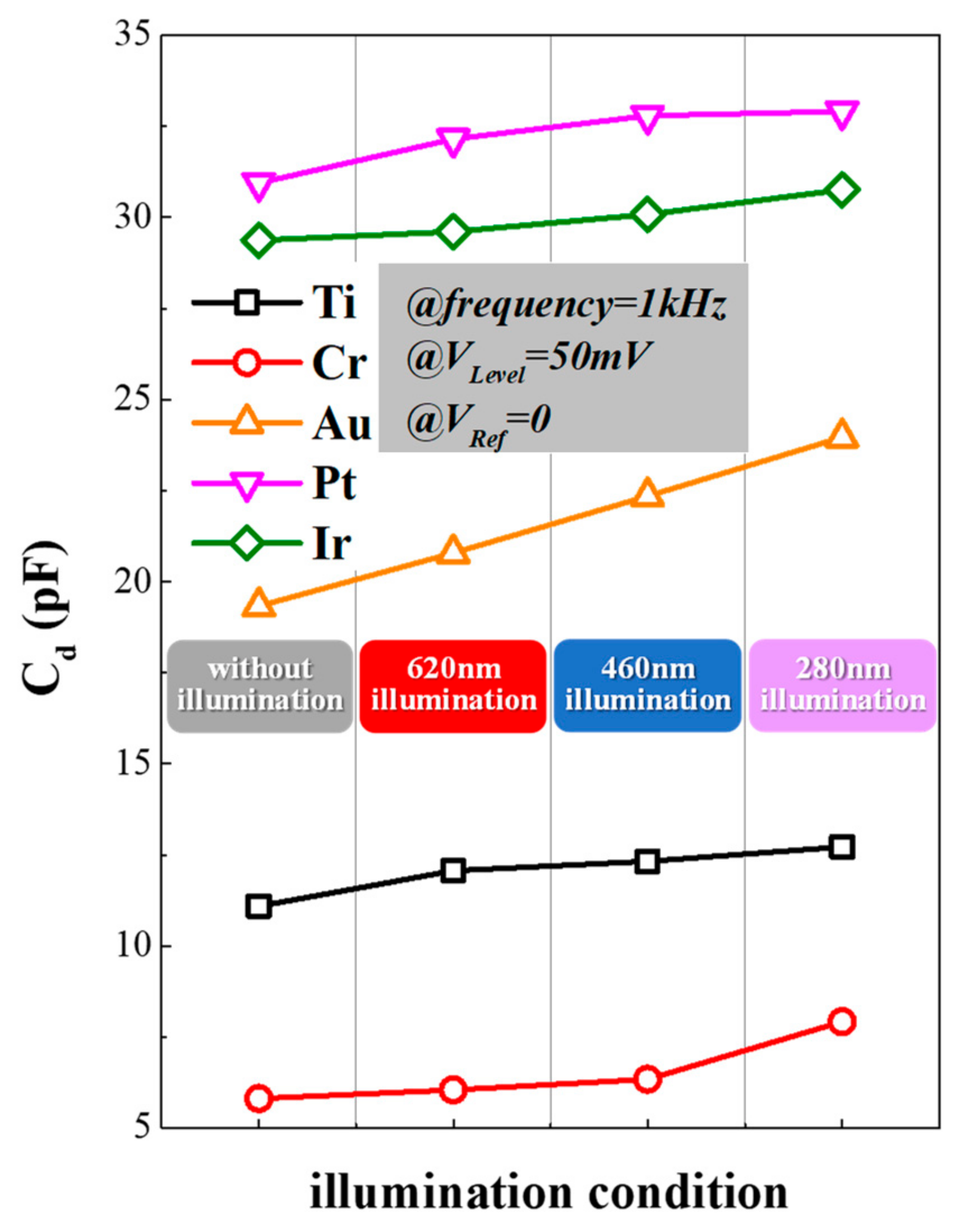
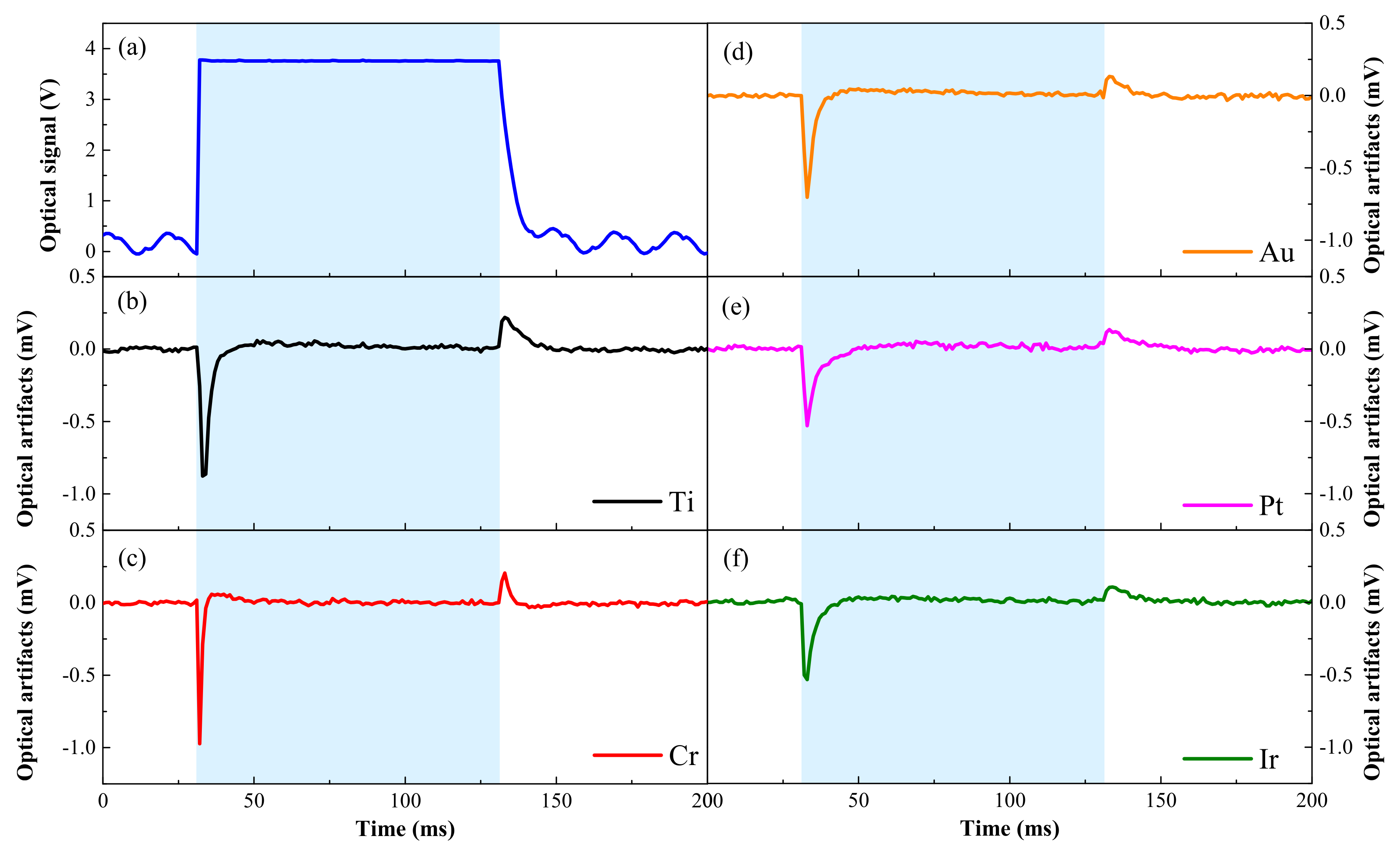
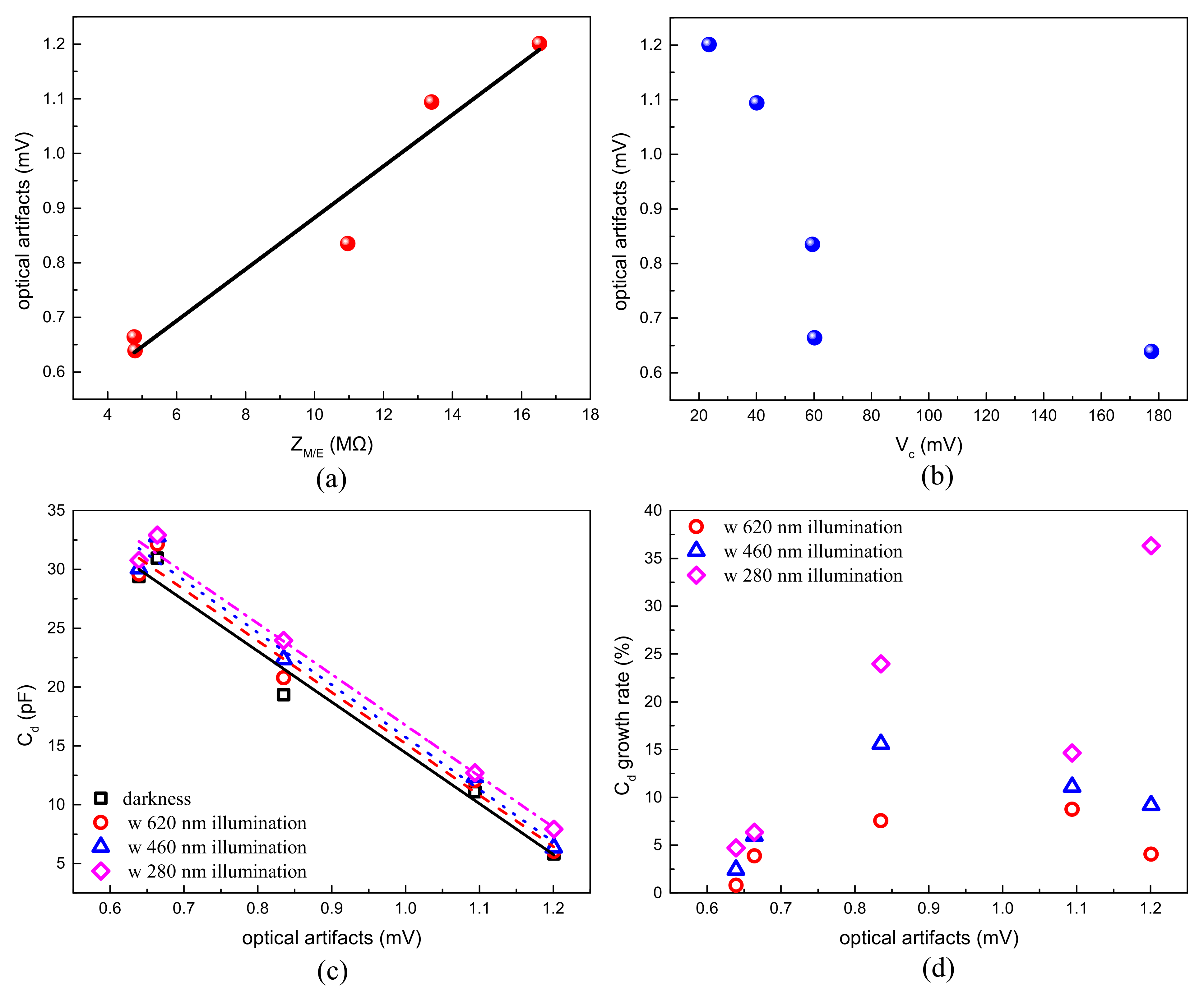
| Material | Ti | Cr | Au | Pt | Ir | |
|---|---|---|---|---|---|---|
| Electronegativity scales | Xp | 1.5 | 1.6 | 2.4 | 2.2 | 2.2 |
| Xa | 1.32 | 1.56 | 1.42 | 1.44 | 1.55 | |
| XY | 1.47 | 1.67 | 1.77 | 1.79 | 1.83 | |
| Critical value (mV) | 40.14 | 23.50 | 59.53 | 60.26 | 177.53 | |
| Material | Cr | Ti | Au | Pt | Ir | |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| ZM/E/MΩ (at 1 kHz) | 16.5211 | 13.3976 | 10.9625 | 4.7727 | 4.7996 | |
| Critical value/mV | 23.50 | 40.14 | 59.53 | 60.26 | 177.53 | |
| Electrical recoverability/MΩ (after redox reaction) | 4.1770 | 1.4597 | 5.5946 | 0.6343 | 1.7192 | |
| Cd/pF | darkness | 5.8127 | 11.0977 | 19.3344 | 30.9437 | 29.3717 |
| 620 nm | 6.0492 | 12.0689 | 20.7934 | 32.1484 | 29.6067 | |
| 460 nm | 6.3470 | 12.3297 | 22.3501 | 32.7914 | 30.0818 | |
| 280 nm | 7.9230 | 12.7229 | 23.9677 | 32.9115 | 30.7582 | |
| Cd growth rate/% | 620 nm | 4.070 | 8.751 | 7.546 | 3.893 | 0.800 |
| 460 nm | 9.193 | 11.101 | 15.597 | 5.971 | 2.418 | |
| 280 nm | 36.306 | 14.644 | 23.964 | 6.359 | 4.720 | |
| Peak-to-peak amplitude of optical artifacts/mV | 1.201 | 1.094 | 0.835 | 0.664 | 0.639 | |
| Parameter | Pearson Correlation Coefficients|r| | |
|---|---|---|
| ZM/E/MΩ (at 1 kHz) | 0.97589 | |
| Critical Value/mV | 0.72762 | |
| Electrical Recoverability/MΩ | 0.36151 | |
| Cd/pF | Darkness | 0.98977 |
| 620 nm | 0.98919 | |
| 460 nm | 0.99148 | |
| 280 nm | 0.99455 | |
| Cd growth rate/% | 620 nm | 0.51144 |
| 460 nm | 0.48589 | |
| 280 nm | 0.80052 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Xu, Y.; Xiao, Z.; Liu, Y.; Liu, H.; Wang, F.; Yao, W.; Yan, Z.; Zhang, M.; Wu, Z.; et al. Influence of the Surface Material and Illumination upon the Performance of a Microelectrode/Electrolyte Interface in Optogenetics. Micromachines 2021, 12, 1061. https://doi.org/10.3390/mi12091061
Shen J, Xu Y, Xiao Z, Liu Y, Liu H, Wang F, Yao W, Yan Z, Zhang M, Wu Z, et al. Influence of the Surface Material and Illumination upon the Performance of a Microelectrode/Electrolyte Interface in Optogenetics. Micromachines. 2021; 12(9):1061. https://doi.org/10.3390/mi12091061
Chicago/Turabian StyleShen, Junyu, Yanyan Xu, Zhengwen Xiao, Yuebo Liu, Honghui Liu, Fengge Wang, Wanqing Yao, Zhaokun Yan, Minjie Zhang, Zhisheng Wu, and et al. 2021. "Influence of the Surface Material and Illumination upon the Performance of a Microelectrode/Electrolyte Interface in Optogenetics" Micromachines 12, no. 9: 1061. https://doi.org/10.3390/mi12091061
APA StyleShen, J., Xu, Y., Xiao, Z., Liu, Y., Liu, H., Wang, F., Yao, W., Yan, Z., Zhang, M., Wu, Z., Liu, Y., Pun, S. H., Lei, T. C., Vai, M. I., Mak, P. U., Chen, C., & Zhang, B. (2021). Influence of the Surface Material and Illumination upon the Performance of a Microelectrode/Electrolyte Interface in Optogenetics. Micromachines, 12(9), 1061. https://doi.org/10.3390/mi12091061







