1. Introduction
Microbial activities reveal environmental conditions; for example, Caribbean reefs are over-run with infectious diseases caused by microbes, which stresses the urgent need to evaluate microbial activities in a timely manner [
1]. To detect microbial activity with high accuracy, microbial adenosine is measured using a somewhat complex and expensive kit [
2]. However, there are other indicators of microbiomes. The fascinating fact is that bacteria themselves are sensitive to oxidative stress, which can impact the metabolic system [
3]. Therefore, it is imperative to gain adequate knowledge of how environmental factors can interact with the bacterial redox sensor system. In other words, rapid detection of redox changes in microbiomes in environmental niches provides a means to rapidly detect dysbiosis.
Aptamers are nucleic acid fragments that grow in vitro and are sensitive to various molecules, including biological molecules and environmental toxins. Using the Systematic Evolution of Ligands by Exponential Enrichment (SELEX) protocol, aptamers can be selected for molecules of interest [
4], such as thrombin, a critical protease of blood coagulation [
5]. Many taxa of bacteria identify membrane proteins as receptors that have a strong affinity to aptamers. Thus, aptamers are great tools for identifying bacteria, such as
Staphylococcus aureus [
6,
7]. In addition, aptamers are a great tool for detection because of their sharp color differentiation [
6].
RNA aptamers have been developed as “light-up” probes whose sharp fluorescence signal can facilitate many applications, and the susceptibility of RNA restricts its implementation in vitro [
8]. However, DNA aptamers are more stable and photochemically detectable when coupled with instruments such as a flow cytometer [
9]. Additionally, its unique structure—one strand as a probe and another as an optical site—allows it to illuminate targets [
10]. An adenosine aptamer associated with gold nanoparticles has been found to illuminate samples containing adenosine and cocaine [
11]. In particular, these intricate designs have inspired new routes to simplify the assembly of aptamers and fluorophores; for example, a single-strand DNA aptamer was applied to detect different redox environments [
12]. MB was connected to the intra sites of DNA grooves when it was in an oxidized environment; it was off the DNA loop when it was in a reduced environment. However, this study did not consider the changes in the fluorescence of MB. Hypothetically, fluorescence changes when there is a conformational change in the fluorophore. The fluorescence change, either intensity change or emission spectra change, can manifest the environmental condition; for example, high redox indicates adequate oxidative reactive species (ORS) level, which is accompanied by respiratory virus infection [
13]. To enhance and stabilize the signal, 2D nanomaterials, such as graphene [
14], carbon nanotubes [
15], and gold [
16,
17], have been applied as the core for biosensors. Hexagonal boron nitride—the “white graphene”—is structurally identical to graphene, and it has already been found to have tunable electrical properties and excellent fabrication capacities [
18]. BN nanosheets have been applied to synthesize the “sandwich” immunosensor with gold and graphene [
19]. pBN is another form of BN nanofiber, which can be regarded as a good candidate for a fluorophore quencher. Upon reviewing the literature to date, we found no report of the use of pBN in the fabrication of biosensors.
In the present study, we established a correlation between the fluorescence and redox potential. The sensitivity and selectivity of this approach makes it possible to detect dysbiosis.
2. Materials and Methods
The ingredients used to synthesize pBN, namely melamine (C3N6H6) and boric acid (H3BO3), were obtained from Aladdin Biochemical Technology Co., Ltd.
A series of reagents were used to synthesize the biosensor assembly. The DNA aptamer (
Figure 1) was obtained from Shanghai Sangon Biotech Co. (Shanghai, China) Adenosine 5-triphosphate disodium salt (ATP) was obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). Poly (diallyldimethylammonium chloride) (PDDA) (20 wt.% in H
2O) and MES to prepare buffer solution (10 mM) were purchased from Rhawn Co. Ltd. HEPES (Shanghai, China) was obtained from Solarbio Ltd. (Beijing, China) to prepare a 10 mM buffer solution. 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) was purchased from J&K Chemicals Co. Ltd. (Shanghai, China). In addition, 1-hydroxypyrrolidine-2,5-dione (NHS) was purchased from Bidepharm Co. Ltd. MB (Shanghai, China) was obtained from Fuchen Chemical Co, Ltd. (Tianjin, China).
Two reagents, FeCl3·6H2O (Damao Chemical Factory, Tianjin, China) and ascorbic acid (J&K Scientific, Beijing, China), were used for the redox potential sensitivity study.
For the microbial study, the bacterial strain Escherichia coli (E. coli) and Bacillus subtilis were obtained from Beijing Sanyao Science & Technology Development Co (Beijing, China). In addition, Flavobacterium sp. in a frozen tube was obtained from the China Centre for Type Culture Collection (Wuhan, China). To prepare the culture media, R2A liquid medium and nutrient broth were purchased from Qingdao Hope Biotechnology Co., Ltd. (Qingdao, China).
To prepare the biofilm, anhydrous calcium chloride (CaCl2) was obtained from Kermel Chemical Co., and sodium alginate ((C6H7NaO6)n) was obtained from Tianjin Fengchuan Chemical Reagent Technologies Co. Ltd. (Tianjin, China).
The heavy metals used to induce toxicity in the microbial community were Zn (CH3COO)2 (Fengchuan Chemical Reagent Technology Co. Ltd., Tianjin, China), PbCl2 (Damas-beta Chemical Co. Ltd., Shanghai, China), Cs2CO3 (Macklin Biochemical Co. Ltd., Shanghai, China), and CoCl2·6H2O (Rhawn Co. Ltd., Shanghai, China).
To test the redox potential, a portable ORP device (ORP-2, Beijing Shunkeda Technology Co. Ltd., Beijing, China) was used.
The multipurpose microplate reader, Molecular Devices i3x, was used to examine fluorescence when the biosensor was added to the opaque 96-well microplate.
IR spectra were collected using a Bruker TENSOR 27 FTIR spectrometer. The zeta potential was examined using a PSS-Nicomp 380 particle sizer. A laser scanning confocal microscope (OLYMPUS OLS5000-LAF) was used to obtain images of the biosensor. Flow cytometry was performed using a FACSAria SORP (Becton Dickinson, Franklin Lakes, NJ, USA). 16S rRNA sequencing was performed by Berry Genomics, China, Ltd. (Beijing, China).
The synthetic route for pBN was based on Song’s 2020 study [
20]. Melamine, measured to 21 g, along with 20.6 g boric acid, were added to 1 L deionized water. The mixture was then stirred and heated at 80 °C for 4 h, after which it was allowed to naturally cool and crystallized. The product was then vacuum dried, and the precursor was obtained. Next, in a tubing furnace, the precursor was placed and heated in N
2 atmosphere to 1050 °C for 240 min, and then kept for 4 h. After natural cooling to room temperature, the product became pBN.
PDDA (0.6 mL, 20 wt% in H
2O), and pBN (40 mg) were added to 100 mL of ultrapure H
2O, and the mixture was sonicated for 5 h [
19]. The suspension was then centrifuged at 50,000×
g for 30 min to obtain the white precipitate (the hydrolyzed pBN), which was dried at 80 °C for 4 h [
21]. Next, 30 mg of the as-prepared pBN was added to 200 mL of HEPES under sonication for 30 min. A 5-mL volume of the suspension was then measured and labeled as A. Next, the ATP-aptamer/dye assembly was constructed. In a 10-mL centrifuge tube (labeled as B) with 5 mL MES buffer (10 mM), 100 µL aptamer (
Figure 1), 100 µM ATP, 200 µM MB, 500 µM EDC, and 500 µM NHS were added. Finally, A and B were mixed and swirled in a rotator for 40 min, thus producing the cross-linking form of the aptamer.
To test how the aptamer responded to different redox environments, a simplified biosensor without integrating pBN was used. This biosensor solution was added to 1 mL of ascorbic acid or Fe (III) solution (0.00001 M, 0.0001 M, 0.001 M, and 0.01 M), which were then mixed well and tested using Fluorolog Horiba (FL3-22) to obtain the fluorescence intensity.
Flavobacteria sp. were grown in R2A liquid culture for 18 h at 18 °C and E. coli and Bacillus subtilis were grown in nutrient broth for 18 h at 37 °C. When the OD630 reached approximately 0.4 for each species, the following procedures were performed. A volume of 3 mL of Flavobacteria sp. fluid was added to each of 22 testing tubes. Subsequently, volumes of 0, 100, 200, 300, and up to 1000 µL E. coli or Bacillus subtilis fluids were added to each test tube with two replicates for each sample. Afterwards, all samples were placed on a shaker for another 18 h at 18 °C. The OD630, pH, and ORP were then measured for each sample.
Biofilm making is a way to simulate how microbial species inhabit tissues [
22]. In the current study, a biofilm-fluorescence assay was freshly prepared and tested using a multipurpose microplate reader (SpectraMax i3x). In a dark 96-well plate, 20 µL CaCl
2 (50 mM), 20 µL alginic acid (4 g·100 mL
−1 H
2O), microbes with different volumes (
Figure S1) from the last step, and 1 µL biosensor from step 2 were added to each well. The plate was then placed into a microplate reader (25 °C, orbital shaking for 30 s before testing, excitation wavelength of 565 nm, and emission wavelength of 665 nm) to test the fluorescence intensity.
In a separate experiment, the toxicity induced by heavy metals in each microbial community was examined using a microplate reader. In a dark 96-well plate, 20 µL of CaCl
2 (50 mM), 20 µL of alginic acid (4 g·100 mL
−1 H
2O), microbes including
E. coli,
Flavobacterium sp., and
Bacillus subtilis with the same volume (50 μL each) from the last step, heavy metals, namely Zn (CH
3COO)
2 (i), Cs
2CO
3 (j), CoCl
2 (k), and PbCl
2 (l) with different volumes (
Figure S2), and 1 µL biosensor from step 2 were added to each well. The plate was then placed into a microplate reader (25 °C, orbital shaking for 30 s before testing, excitation wavelength of 565 nm, and emission wavelength of 665 nm) to test the fluorescence intensity.
4. Discussion
In
Figure S3, amine (N–H bending) and aromatic amine (C–N stretching) peaks are shown, which are characteristic peaks of MB. Compared with the peaks in MB, the peaks of pBN are simpler, because of the simple bonding pattern in pBN. The spectra of the biosensor contain peaks belonging to the DNA aptamer. Imine (C=N stretching) groups were observed at ~1637 cm
−1, which represent base pairs in DNA. Sulfonyl groups (S=O) from the interaction of sulfur in MB and phosphate groups in DNA can be found at ~1385 cm
−1. Overall, the peaks from the biosensor are a combination of peaks in pBN and MB.
In comparing
Figure S5a,b, a lighter blue color in (b) is apparent, due to the addition of the aptamer. This is evidence that MB can interact with DNA grooves to change the color appearance of MB. In
Figure S5c,d, light blue is apparent in (c) and clear white in (d). As a strong oxidizer, Fe (III) interacts with MB and the aptamer, resulting in a switchable conformational change between MB and the aptamer, and ascorbic acid, which is conversely a strong reducer. This also proves that MB-ATP-aptamer can switch “on” when it is in an oxidized environment, and switch “off” when it is in a reduced environment [
12].
The higher the concentration of Fe (III), the lower the fluorescence intensity (
Figure S6). There is an antagonistic effect when MB interacts with concentrated Fe (III), which can absorb emission photons from MB [
23]. When MB interacts with the aptamer, there is a π-π stack between the MB and DNA base pairs. A conformational change in MB, the fluorescence probe, causes the shift in emission spectra, from 690 nm to approximately 696 nm [
24,
25].
In the study of biosensors in an environment with various redox potentials, the MB/aptamer biosensor can demonstrate the colorimetric difference in different redox potential environments, yet it is not sensitive enough to demonstrate the correlation between fluorescence intensity and redox potential in a strongly reduced environment. However, the living conditions for bacteria vary, and the redox potential impacted by ascorbic acid cannot fully manifest, as proved by the microbial study we did next.
Surprisingly, when
Bacillus subtilis, a Gram-positive bacterium, grew together with
Flavobacterium sp., a Gram-negative bacterium, the overall OD
630 was elevated compared with the values from
E. coli, a Gram-negative bacterium, and
Flavobacterium sp. (
Figure S8). The competition between two different Gram-positive bacteria was not pronounced. It can be explained that the reactivity of
Flavobacterium sp. was much higher than that of its competitor,
Bacillus subtilis, and as a result,
Flavobacterium sp. grew even faster.
This inversely proportional relationship (
Figure 2) between the redox potential and fluorescence intensity corresponds with the results shown in
Figure S7. Carbon nanotubes [
26], nano C60 [
27], and magnetic nanomaterials [
28] have been developed as fluorescence quenchers. Technically, pBN can be regarded as a fluorescence quencher. Nucleic acids, such as DNA, were tightly adsorbed onto pBN [
29]. Furthermore, aptamers are prone to structural switching when encountering any specific target so that the DNA/DNA duplex is converted to a DNA/target complex [
10,
30], which enhances the selectivity of our biosensor. RNA aptamers can specifically bind to malachite green, a tag, to produce fluorescence [
31]; hence, MB is a legitimate option as a tag to release fluorescence in our study. In an oxidized environment, MB was bound to the DNA base-pair zone, which resulted in its close vicinity to pBN; however, in a reduced environment, MB was removed from the aptamer, which furthered its distance to pBN, and consequently, the quenching ability of pBN was reduced. This explains why the lower the redox potential, the higher the fluorescence intensity of this biosensor.
The significant indicator (
Figure 3) indicated that the biosensor could detect changes in the microbial community. When the proportion of
Bacillus subtilis is within 5–20%, the fluorescence levels off, and the fluorescence rises rapidly when the proportion is higher than 20%. In an actual aqueous environment,
Flavobacterium sp. is usually the major microbial species in fish mucus. In addition,
E. coli and
Bacillus subtilis may also be present. As environmental factors change, the composition of each microbial community changes as well. Evidently, using the method in this study, we can detect the changing trend of the microbial community with a micro amount of bacterial fluid, ranging from 100 to 200 µL. A similar method requires a small amount of sample to examine glucosidase activity using a biochar-enhanced enzyme kit [
32], which is not cost-effective for long-term monitoring. In addition, operating a potentiometric meter can measure pH or redox potential (Eh) of a microbial culture; however, a high volume of a sample must be taken into account [
33]. Even without using expensive methods such as 16S rRNA, we can still have a general idea about the health conditions of microbial communities.
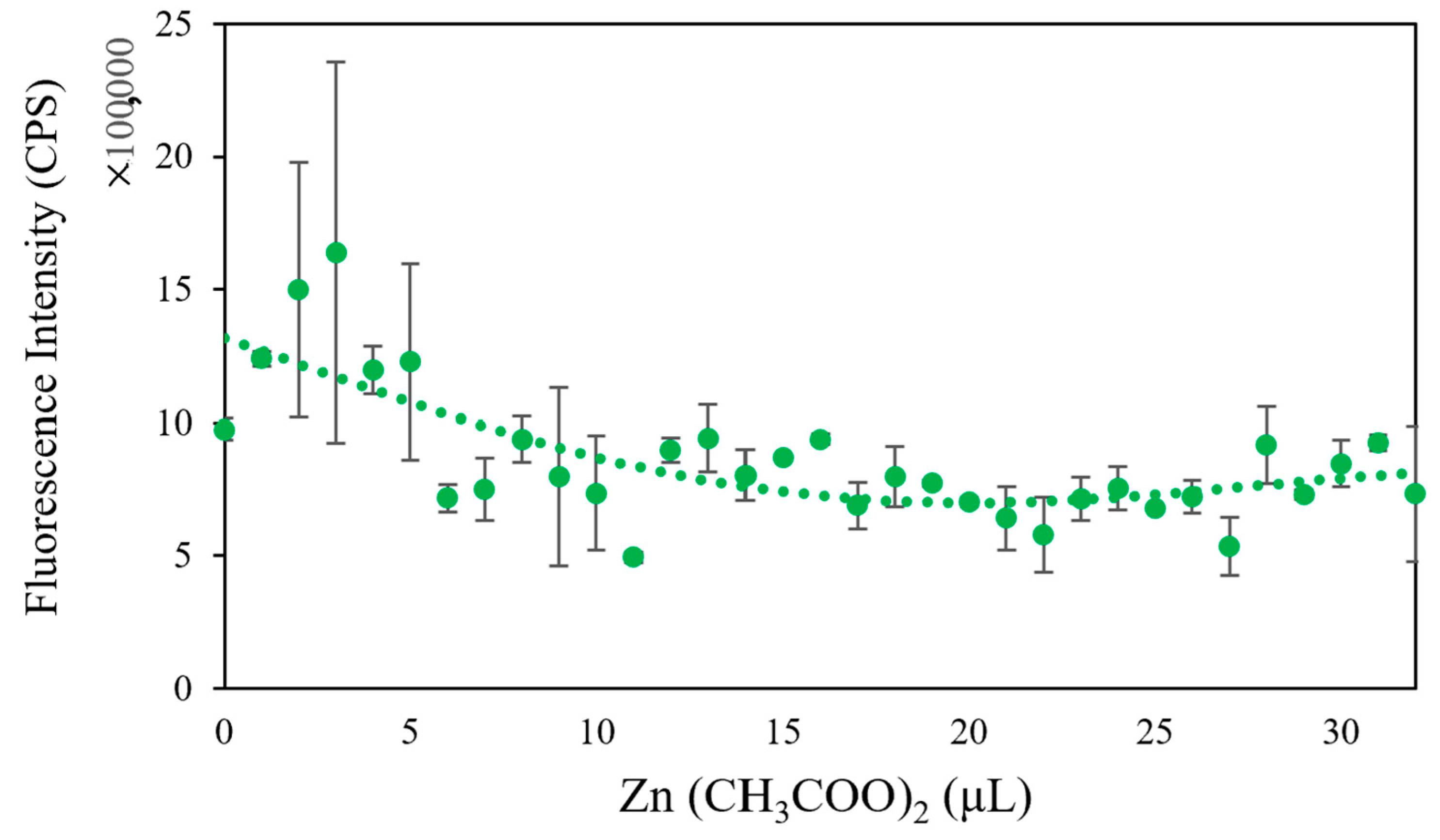
Heavy metals can induce the production of ROS in microbial communities, which helps to elevate the redox potential of microbial communities. Aptamers can sensitively probe foreign substances, such as bisphenol A, in cells when cooperating with gold nanoparticles, a fluorescence quencher [
34]. Thus, we tested whether our biosensor could provide optimal signals when various heavy metals invade a microbial community. From the evidence, we already have in the study, the higher the redox potential, the lower the fluorescence intensity. The results indicated that the designed biosensor could track the induced toxicity level in a microbial community. In addition, the biosensor exhibited good selectivity for various heavy metals. When Zn (CH
3COO)
2 was added to the microbial community from low amount to high amount, the fluorescence intensity declined from 2.0 × 10
6 CPS to 1.0 × 10
6 CPS (
(
Figure 4); with the same amount of Cs
2CO
3 added, the fluorescence intensity declined with increasing Cs
2CO
3, from 1.2 × 10
6 CPS to 6.0 × 10
5 CPS (
(
Figure 5); yet, the fluorescence intensity declined from 2.0 × 10
6 CPS to 8.0 × 10
5 CPS for PbCl
2 (
(
Figure 6). The changing range of fluorescence intensity (
Figure 7) was maintained at a similar scale.
According to flow cytometry analysis, as the proportion of
Flavobacterium sp. declines, the collected ROS shifts more to the right, which indicates that as other bacterial species join the community,
Flavobacterium sp. must compete with them to produce more ROS (
Figure 8a). In another set of studies, Zn (CH
3COO)
2 was added to the microbial community. The ROS content was shifted to the right as more Zn (CH
3COO)
2 was added, which is a clear indicator that Zn (CH
3COO)
2 can induce toxicity to the microbial community (
Figure 8b). The results confirm those in
Figure 4, where the fluorescence intensity declines as the redox potential increases.
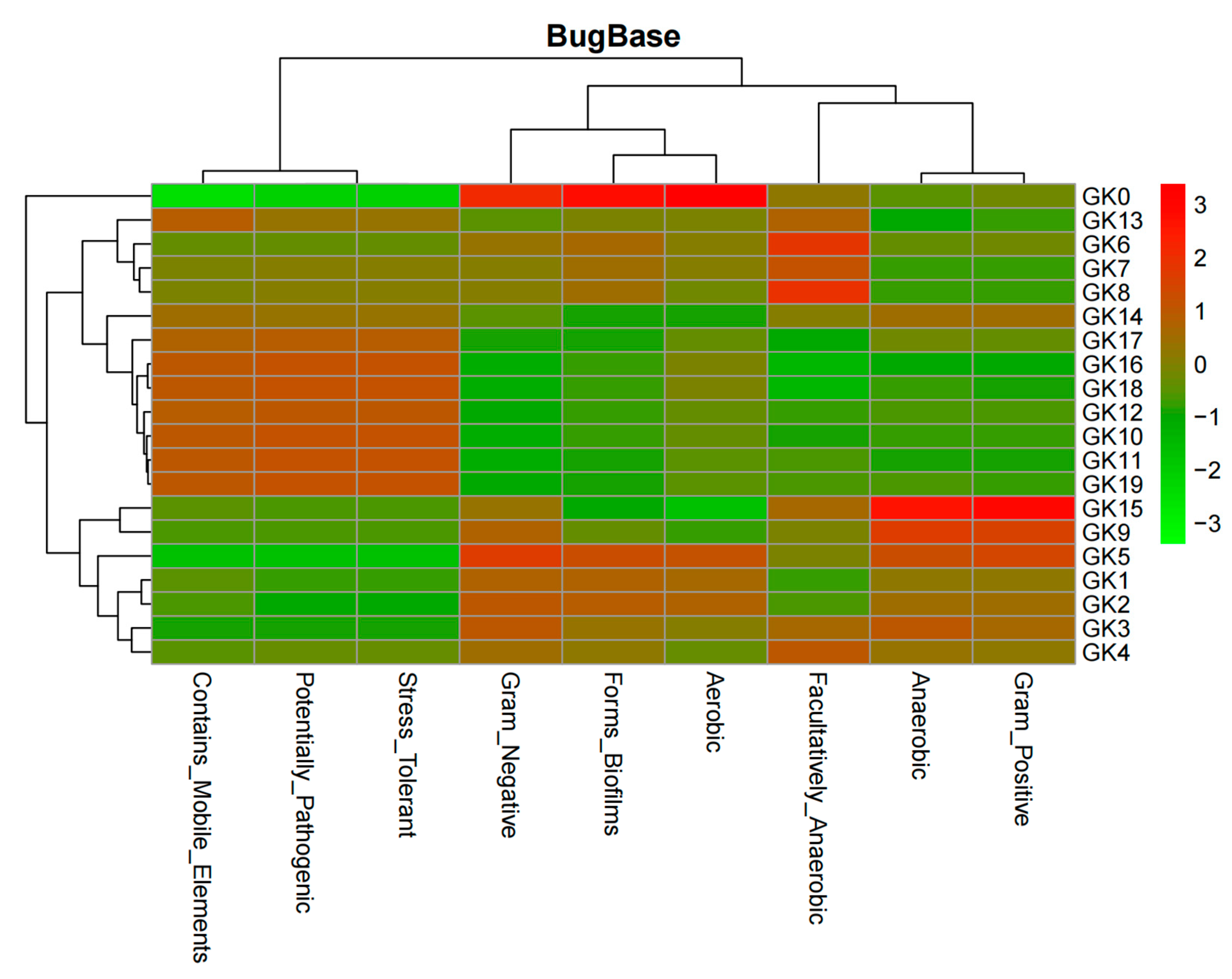
Lastly, the 16S rRNA technique (
Figure 9,
Figure 10,
Figure 11 and
Figure 12) further confirmed the accuracy of our biosensor. GK0-GK9 contains
Flavobacteria sp., the major species, and
Bacillus subtilis, the competition species, which occupies an increasing amount of space in the microbial colony. In addition, GK10-GK19 stands for the colony filled with the same species, but different amounts of Zn (CH
3COOH)
2. Because the ratio of
Flavobacteria sp. was compressed and that of
Bacillus subtilis was inflated from GK0-GK9, the bar representing Gram-negative bacteria contracts, which represents the expansion of Gram-positive bacteria. The pinkish-orange bar denoting the stress tolerance is distinctively longer for samples GK10-GK19 owing to their stimulation by Zn (CH
3COOH)
2. GK0 represents the purest colony so that it tends to form the most stabilized biofilm, which is represented by the brightest red color. From GK1-GK9, more competitive bacterial species were added, which allowed the biofilm to become more fragile, as can be seen that the colder color deepens. The greenish color of samples GK10-GK19 indicates that it is too difficult for microbial biofilms to form, since the heavy metal intake is increasing owing to the distinctive reddish color grids representing mobile elements. The biosensor we synthesized can intricately predict the metabolic and physiological activities of a microbial colony.
To date, 16S rRNA is still the best tool for predicting and elucidating various metabolic activities. The growing trend for the ATP-binding cassette (ABC) transporters (ko02010) pathway is a result of the intensified stimulators to a microbial community. The drawback of this method is that cross-contamination within a microbial community with low biomass is inevitable [
35]. However, the advantage of our biosensor is that the abnormal and alarming activities of a microbial community can be detected rapidly. The information we report here provides a foundation for further study, in which a more comprehensive database will be established. We aim to provide practical results that are of value in both environmental and medical applications, especially where microbial activity is a research focus.