1. Introduction
The use of microfluidic chips in biological research and drug discovery is an evolving technology that requires interdisciplinary innovation across the sciences and engineering. The PDMS microfluidic chip has enabled the development of human cellular models, commonly referred to as organs-on-chips, which replicate elements of in vivo physiology absent in traditional, static, well-plate based in vitro models [
1]. They have the potential to better represent the in vivo condition, with applications ranging from drug screening to disease modeling [
2,
3]. Additionally, microfluidic systems can integrate a plethora of microsensors for continuous monitoring of multiple culture metrics—from excretion of soluble biomarkers to trans-epithelial electrical resistance [
4,
5]. The continued development and refinement of microfluidic systems is well positioned to make a valuable contribution to drug discovery.
Microfluidic chips for drug discovery applications often require multi-layer patterning, as well as functionality under a flow-based environment. In organ-on-chip applications, multi-layer patterning is useful for developing a more physiologically relevant cellular environment, such as a co-culture separated by a porous membrane [
6,
7,
8]. In addition, the inclusion of fluid flow in the in vitro model allows for replicating the shear stress exerted by blood flow onto the cell layer or airflow over lung epithelia [
9,
10]. Traditionally, multi-layered microfluidic chips are manufactured in high throughput using hot embossing [
11,
12] or injection molding [
13,
14]. For lower throughput, research-based applications, soft lithography is often preferred, as it can leverage the low cost, biocompatible, and oxygen permeable heat-cured polymer polydimethylsiloxane (PDMS) [
15,
16]. Recently, a transition to forming master molds using rapid prototyping techniques has gained prominence [
17,
18], as the traditional method of photolithography is costly and requires a clean room facility—a factor partially responsible for the lower adoption levels PDMS has seen outside of academic environments [
19]. PDMS can also easily form multi-layered patterns due to its ability to be irreversibly bonded to PDMS or to glass using air plasma, partial curing, or corona discharge bonding [
20,
21,
22]. Commercially available microfluidic chips, on the other hand, include those made from glass, and polymers including polymethyl methacrylate, polystyrene, cyclic olefin copolymer, polycarbonate. After designs are optimized and are set for commercial production, thermoplastic polymeric chips have been manufactured using hot embossing or injection molding. Glass chips, while historically made from wet chemical etching or laser ablation [
23], can also be made using newer, more rapid methods including ultrafast laser inscription [
24] and glass imprinting [
25].
The flow required by organ-on-chip models can be achieved passively, through gravity driven or capillary action, or actively, through an external pump. However, there remains challenges with connecting micro-sized channels with macro-sized commercially available connectors and tubing, commonly known as fluidic interconnects [
26,
27,
28]. At these interfaces, creating leak-proof and durable connections is integral to the overall robustness of the chip during potentially long operation periods; the small-airway-on-chip reported by Benam et al., for example, was cultured for over three weeks [
29]. There have been many attempts to overcome this challenge, such as using a compressing tubing within a microfluidic chip [
30], using threaded inlets [
31] or using adhesive-based strategies such as an epoxy to augment, for example, a pressure-fit PDMS-steel needle seal [
32]. These methods are widely used, though contain some challenges. Using compression of tubing within the channel requires that the user does not dent the flexible PDMS channel, potentially forming a nucleus for bubble formation (detrimental to culture health and imaging capabilities). Additionally, threading inlets at the interface of a microfluidic chip can be cumbersome and become unwieldy when many inputs/outputs (I/O) are required. Lastly, epoxy can dissolve and delaminate from PDMS when exposed to alcohols used for disinfection such as ethanol; it, furthermore, has the potential to occlude the channel during hardening. There are many considerations when designing fluidic systems for organ-on-chip applications. For example, side-loaded ports for the fluid line may be desirable as they allow the microfluidic chip to be flipped for inverted imaging, especially useful for multi-compartment devices with multiple cell types. Side-loading, however, complicates the conventional technique of biopsy-punching to create fluidic interfaces. Compression of PDMS is commonly employed to augment fluidic seals and improve the robustness of fluidics; however, conventional fabrication methods often lead to non-uniformities in PDMS thickness, which have the potential to deform channels under said compression, undermining the original objective [
33]. Additionally, some organ-on-chip applications require long-term culture durations; for example, the differentiation period of primary airway epithelia is on the order of weeks [
34]. As such, protocols, and strategies for improving the ease of handling while minimizing contamination risk are essential.
In this work, we introduce key fabrication steps and strategies to improve both fabrication yield and device performance for applications in organs-on-chips development. These key steps include increasing the uniformity of PDMS thickness produced from master molds, employing a digital-light-processing 3D printer purpose-built for rapid production of PDMS master molds, as well as exploring methods for reinforcing PDMS bonding and fluidic integration. Selected aspects pertaining to the latter were applied to two separate organ-on-chip designs: an airway-on-chip model and a blood–brain barrier (BBB) model. Differences and advantages to the fabrication strategies are summarized in
Table 1. We show how these rapid prototyping strategies can improve important organ-on-chip performance metrics such as incidents of leakage, confinement of culture growth to microfluidic channels, and overall success rate of producing morphologically typical cultures. We also characterize the surface finish obtained.
2. Materials and Methods
2.1. Fabrication and Design of the Master Molds
2.1.1. Fabrication of the Master Molds
Master molds were fabricated using a digital light processing (DLP) printer (MiiCraft Ultra 50, Distributor: Creative CadWorks) or a stereolithography (SLA) printer (Form2, Formlabs). Mold design was completed via CAD (computer-assisted design) software (SOLIDWORKS Dassault Systèmes, Vélizy, France) and resulting parts were exported into file formats accepted by each 3D printer’s instrumentation software. Specifics pertaining to mold print settings and post-processing can be found in the
Supplementary Materials.
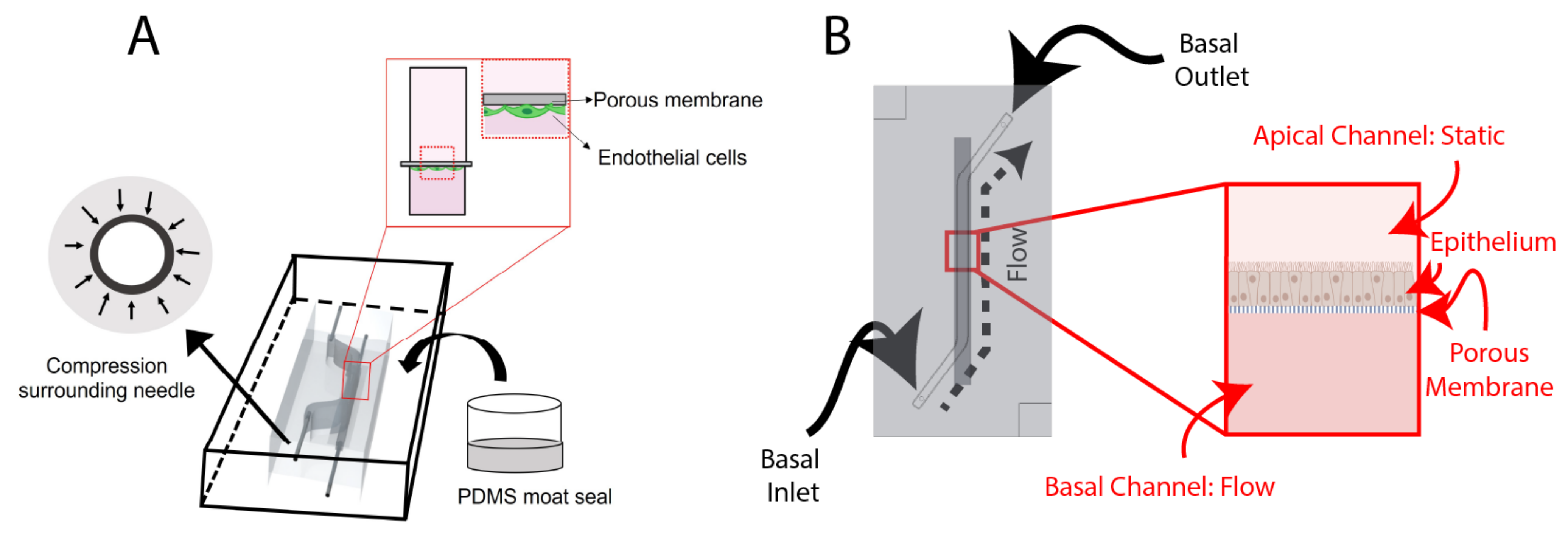
The organ-on-chips consist of two layers of PDMS that are separated by a porous, transparent polyester membrane with a pore size of 0.4 µm, a porosity of 2.0 × 106 cm2 and a thickness of 12 µm (it4ip, cat. No. 200M12/620N403/47). This structure of microfluidic chip allows for potential to include cell-to-cell interactions as well as the ability to apply physiological shear stress on the cell layer. Two organ-on-chips designs are used, a blood–brain barrier (BBB) chip and an airway-on-chip.
Design of the BBB Chip
The BBB chip (
Figure 1A) includes a static top reservoir of 1 mm width, 18 mm length (straight), (5 mm slanted), and 5 mm height. The inlet and outlets (I/O) are inserted from the side of the PDMS. Before casting the PDMS, 25G I/O needles McMaster-Carr) are manually inserted into the mold to sacrificially mold fluidic port features. Employing needles that are of a higher gauge (lower external diameter) than the needle that is intended to be used as a fluidic interconnect creates compression surrounding the needle, improving fluidic seal integrity. Finally, after removing the 25G needles, ½” 22G straight needles (McMaster-Carr) were inserted into the molded fluidic ports for the top and bottom channels.
Design of the Airway-On-Chip
The airway-on-chip (
Figure 1B) channel measures 1.3 mm wide, 0.75 mm high, and 25 mm long; these dimensions were chosen to achieve overall culture areas equivalent to commonly used 24-well Transwell
® inserts while maintaining a hydraulic diameter under 1 mm to better replicate small airway physiology [
36,
37].
The airway-on-chip is composed of a monolayer of airway epithelia; as such, it does not benefit from the ability for dual-side imaging (or, flipping the chip under a microscope) enabled by side-loaded fluidics. As such, it employed top-loaded fluidic ports, which are partially defined by raised columnar features in the top-layer master mold and hollowing is completed using a tissue biopsy punch (TedPella, 0.5 mm). Importantly, both the diameter of these features in the master mold as well as the diameter of the biopsy punch is undersized, relative to the 22G needle which is pressure-fit into the PDMS. Additional considerations regarding fluidic port design and integration are provided in the
Supplementary Materials.
The mold edge heights define the thickness of the PDMS, which is 6 mm; this value, while large relative to other microfluidic organs-on-chip, was found to impart greater stability to the stainless-steel needles used to interface fluidics. Alignment features, consisting of recessed and protruding squares on the top and bottom chip halves with a 100 µm margin, are also included at two corners to assist with manual alignment during fabrication.
2.2. Fabrication and Assembly of the Microfluidic Chips
2.2.1. PDMS Preparation and Bonding
A 10:1 mixture of PDMS elastomer base and curing agent (Dow Sylgard
® 184 Silicon Elastomer) was mixed in a planetary centrifugal mixer (Thinky, cat. No. ARE 310) for 90 s at 2000 RPM followed by 60 s at 2200 RPM. The PDMS mixture was casted into the molds and placed into a vacuum chamber for removal of air bubbles in the PDMS mixture for 30–60 min. Following degassing, a transparency film (Apollo, purchased from
Amazon.ca (accessed on 9 December 2021)), cut to fit the mold’s footprint with an excess 1–2 cm border, was placed over top of the mold. In order to minimize the formation of bubbles (or air-pockets) forming at the transparency-PDMS surface, a tweezer was used to enable gradual laying of the film from one side to the other of the mold (
Figure 2A–D). After placing the transparency, a flat piece of 3/32” thick acrylic was placed on top of the film to enable even compression by an aluminum weight (total weight 200–300 g). The molds were transferred to an oven (65 °C) to cure for at least four hours. Then, the PDMS layers were removed from the molds using an Exacto-knife, scalpel, or razor blade and excess debris were removed from the surfaces using Scotch™ Magic tape and compressed air.
To identify the bonding performance of the PDMS-PDMS or PDMS-glass bonds, first, a visual inspection was made, to ensure all areas of the chip were transparent, then a leak-test was performed by manually pushing food-colored dyed PBS through tubing attached to the chip.
Oxygen Plasma Bonding—BBB Chip
For the BBB organ-on-chip application, using tweezers, a membrane (it4ip, cat. No. 200M12/620N403/47) was carefully set into position to cover the channel. The two layers of PDMS were treated with air plasma (Harrick plasma cleaner) at ~600 mTorr for 1 min before manually aligning the channels and compressing the PDMS layers together for approximately 30 s. The plasma-bonded PDMS layers were then transferred to a 65 °C oven for at least 2 h to complete the bonding procedure.
Partial Curing—Airway-On-Chip
The airway-on-chip leveraged PDMS-to-PDMS bonding through partial curing, as characterized by Eddings et al. [
22]. This process comprised an initial, partial cure of PDMS-filled molds for 60 min at 65 °C following which molds were removed from the oven for membrane alignment, punching, and conjoining the two chip halves. The chips were then covered with Scotch™ tape and returned to the oven under compression for an additional three hours to allow polymerization to complete, yielding a bonded and complete microfluidic device.
2.2.2. Fluidic Integration and Reinforcement
BBB Chip
In order to augment the fluidic interface in the side-loading BBB chip, a PDMS encasement (“moat”) (
Figure 3) was prepared. This encasement was created by taping the tubing in position, then using a flat, smooth bottom dish into which chips were set. To preserve PDMS, the chips were placed side-by-side and tape was used to keep them together. Then, liquid PDMS was poured around the chips, such that the PDMS surrounded the inlet and outlet tubing and fully encased the system. Additionally, a weight was placed on top of the chips to keep them on the bottom of the dish. Finally, the moat was cured at 65 °C overnight.
Airway-On-Chip
To improve the robustness of fluidic interfaces and operation of the airway-on-chip over long (10 day) culture durations, two approaches were explored and ultimately included. These consist of: (1) mechanical reinforcement of the reversible PDMS-to-PET-membrane bond through a rigid plastic clamp and (2) fluidic seal enhancement via dispensing of uncured PDMS around fluidic interfaces.
The airway-on-chip model features cells cultured on a PET membrane that is sandwiched between two PDMS microfluidic channels. If the PDMS is not bonded well and there is a gap between the PDMS and the membrane, cells can grow into the thin gap. This can lead to unpredictable culture sizes and cell numbers, which may undermine reproducibility from chip to chip. Mechanical reinforcement of the reversible PDMS-to-PET-membrane bond to restrict cell-growth to the microfluidic channel was found to be necessary and achieved through an acrylic clamp. Quarter-inch cast acrylic (McMaster-Carr, Elmhurst, IL, USA) was purchased in 12″-by-12″ sheets and cut with a VLS 2.30 (Universal Laser Systems, Scottsdale, AZ, USA) CO2 laser cutter; outer dimensions of the clamp measured 73 mm-by-48 mm, 20 mm larger than the chip along both the length and width. A square cutout (4 mm-by-4 mm) was centered over each fluidic inlet/outlet port to enable access, and two circular cut-outs sized for 8–32 threaded rods (McMaster-Carr) were made collinear with the top channel. Compression was achieved utilizing 8–32 thumb nuts (McMaster-Carr) coupled with washers for improved uniformity of force distribution. Following assembly of the chip-clamp unit, 22-gauge stainless steel needles (McMaster-Carr) with a 1″ 90-degree bend were inserted into the fluidic access ports with the other end of the needle coupled to a 10 cm length of 0.02″ ID microbore Tygon tubing (VWR). The other end of this tubing length was coupled to a 0.5″ 22GA straight dispensing tip with a luer-lock connection for ease of fluidic interfacing and handling. A strip of tape was placed on the clamp, overtop of the fluidic lines for added stability during handling. Liquid PDMS was, then, dispensed via pipette (with the tip scissor-cut to increase the opening size, due to the fluid’s viscosity) into the clamp’s cut-outs to augment the compression fit seal between the needles and the PDMS, and cured at room temperature for 48 h. The thickness of the clamp as well as the PDMS of the top chip layer is sized such that the bent stainless steel needle rests on the acrylic when the bent portion penetrates approximately 3.5 mm into the 5.25 mm thick (with the channel depth subtracted from the overall layer thickness) PDMS (not too deep so as to risk piercing the PDMS of the bottom layer while retaining a stable pressure-fit seal).
2.3. Microfluidic Cell Culture
2.3.1. BBB Chip
For the BBB chip, human brain microvascular endothelial cells (HBMECs) (Lonza, Basel, Switzerland, lot# 376.01.03.01.2F) were thawed from passage 6 and used between passage 6–9. HBMECs were cultured onto fibronectin-coated plates and passaged at 80% confluency. Cells were lifted using 0.05% trypsin and cultured with EGM-2 bullet kit containing 2% v/v FBS (Lonza, cat. no. CC-3162).
A 1 mL syringe with a 22G dispensing tip was inserted into chip tubing to perform all liquid exchanges. For the BBB chip, a 10 min 70% ethanol rinse, followed by two PBS washes, and an overnight equilibration using EGM-2 media was used to prepare the chips for ECM-coating. For the airway-on-chip, a 20 min ethanol incubation preceded coating.
For the BBB chip, a coating of 0.4 mg/mL collagen IV (Sigma Aldrich, St. Louis, MO, USA, cat. no. c5533), 0.1 mg/mL fibronectin (Sigma Aldrich, cat. no. F1141), and 0.1 mg/mL laminin (Sigma Aldrich, cat. no. L2020) was applied the day after equilibration and left overnight in the fridge. Lastly, the chip was washed twice with PBS. Then, HBMECs were seeded into the chip at a density of 5 million cells/mL and then attached to a pump for at least 5 days to allow for perfusion through the endothelial channel. A syringe pump was used to provide media perfusion resulting in shear stress values of ~0.001 dyne/cm
2 within the channel. The top channel media was exchanged every two days. The profile of the flow rate delivered by the syringe pump was measured using microfluidic flow sensors, as described in the
Supplementary Materials.
2.3.2. Airway-On-Chip
Three vials of Calu-3 human lung adenocarcinoma airway epithelial cell line were obtained from ATCC and expanded and maintained in T-75 flasks using Eagle’s Minimum Essential Medium (Corning, Corning, NY, USA) supplemented with fetal bovine serum (MilliporeSigma, St. Louis, MO, USA, Cat: F1051, Lot: 19D019), and Antibiotic-Antimycotic (Thermo, Waltham, MA, USA, Cat: 15240-062, Lot: 2441423). Cells were passaged at 80% confluence and used for experiments between Passages 20–25.
Microfluidic chips were filled with a 100μg/mL rat-tail-collagen (Corning, Cat: 354236, Lot: 1049001) solution in PBS and incubated in a biosafety cabinet overnight to complete membrane coating. Following flushing and equilibration with complete culture medium, chips were seeded via syringe-infusion with a suspension containing six-million cells per milliliter. After the suspension was loaded, luer caps (McMaster-Carr) were coupled to each fluidic line and the chips were transferred to an incubator to allow cells to attach for five hours.
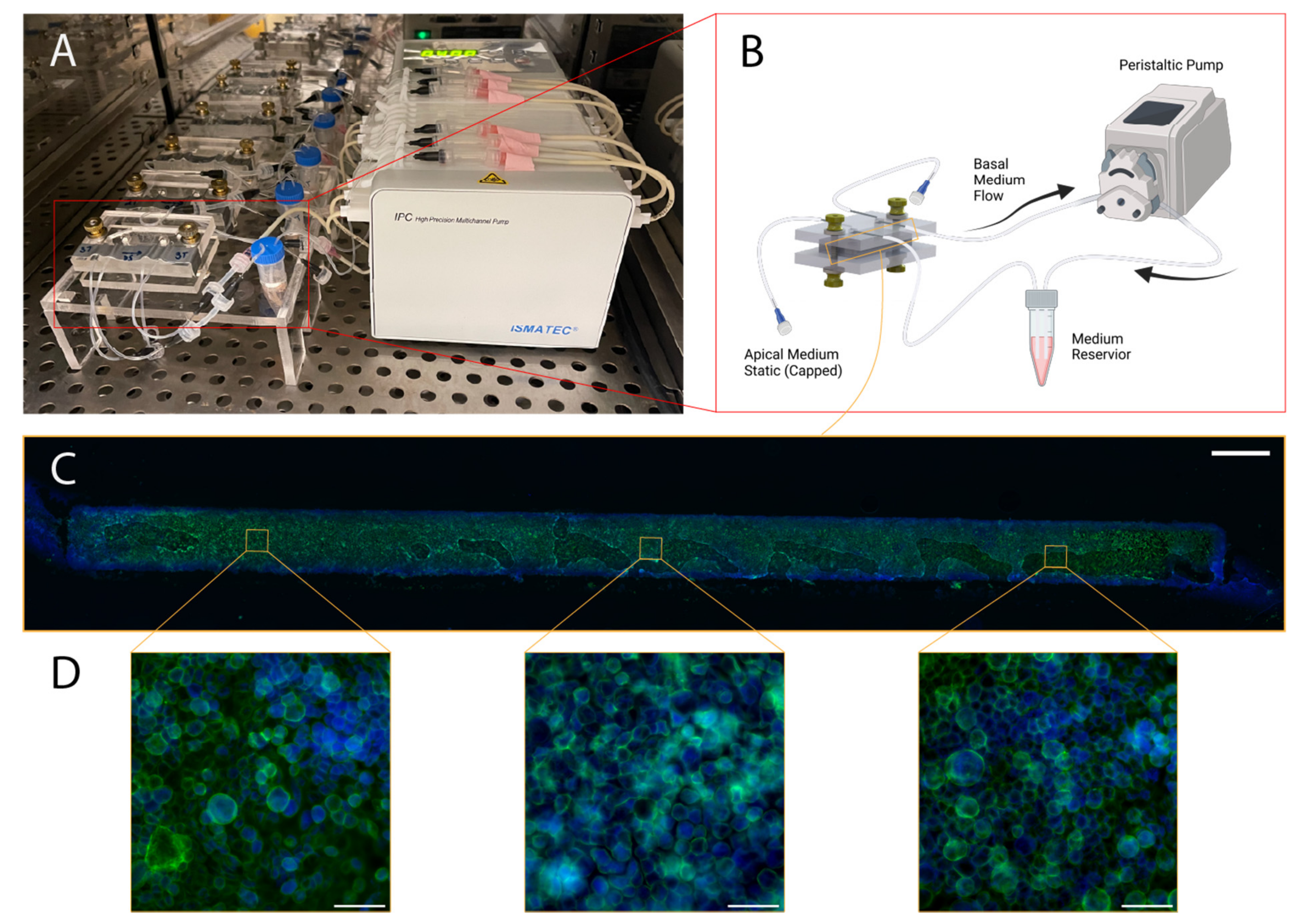
Following the five-hour attachment period, luer caps were removed from the basal channel inlet and outlet lines. They were, then, connected to a recirculating flow system, wherein a peristaltic pump was positioned to pull culture medium through the basal channel and into a 2 mL medium reservoir containing the same medium used for culture maintenance. The recirculating flow regime enables the application of physiologically relevant shear stresses while minimizing reagent consumption. Reservoir culture medium was contained in five milliliter screw-cap MacroTubes™ (FroggaBio, Toronto, ON, Canada); their caps were punctured with 16-gauge needles (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), creating an opening just large enough to accommodate the insertion of the microfluidic tubing employed. The tubing expands in the warmer environment of the CO2 cell culture incubator, creating a tighter seal for the duration of the experiment. The following day, the apical channel was washed with fresh complete medium, and maintained in a submerged state for the duration of the experiment.
The reservoir volume was chosen based on manufacturer’s recommendations for Transwell
® 24-well culture inserts; this recommendation stipulates a culture volume of 600 µL to support a culture area of 0.33 square centimeters, equivalent to the airway-on-chip. Exchange of medium in a Transwell plate every two days is a common culture regime that has been demonstrated to produce functional cultures [
38,
39]. Direct communication with ATCC yielded an upper bound on culture medium stability in a cell-culture incubator of four days, and this exchange interval has been employed in previously reported organ-on-chip cultures; as such, the 600 µL value was doubled to 1.2 mL and a factor of safety was added to arrive at the 2 mL reservoir volume, with reservoir exchanges and apical channel washes taking place every three to four days [
40]. The initial flow rate employed was 300 μL/min, which was ramped up following the second media reservoir exchange and apical channel wash to 540 μL/min, corresponding to a wall shear stress between 0.6 and 0.7 dyne per square centimeter exerted basally at the higher flow rate—small airway epithelia experience a range of 0.5–3 dynes per square centimeter at rest due to airflow in vivo [
10,
41].
Cultures were maintained for eleven days, at which point Calu-3 epithelia cultured on Transwell inserts reach peak barrier integrity (measured based on apparent permeability to fluorescein isothiocyanate-conjugated dextrans) [
39].
2.4. Cell Culture Analysis
2.4.1. BBB Chip
HBMECs were stained to visualize f-actin and nuclei. All staining was performed in-chip. A 1 mL syringe with a 22G dispensing tip was inserted into the chip tubing to perform all liquid exchanges. For f-actin staining, samples were fixed, with 4% paraformaldehyde in PBS and left at room temperature for 15 min. Samples were washed twice with PBS for 10 min. After washing the fixative 2 × 5 min with PBS, samples were incubated with the 66 µM dimethyl sulfoxide (DMSO) Alexa-Fluor-488 Phalloidin stock solution at a 1:400 dilution in PBS for 1 h at room temperature. Samples were left in a dark, covered container to prevent photobleaching and evaporation while staining. Then, samples are washed 2 × 5 min with PBS (1×). For nuclei staining, cells were either stained live or fixed, with Hoechst (Thermofisher Scientific, Waltham, MA, USA, cat. no. H3570) at a 1:1000 dilution in EGM-2 or PBS. Live cells were washed with EGM-2 and fixed cells were washed 2 × 5 min with PBS (1×).
BBB chips were disassembled using an Exacto knife and pliers, and the membrane was removed and placed onto a glass slide with tweezers. The membrane was placed with the cell side facing upwards. ProLong® gold Anti-Fade containing 4′,6-diamidino-2-phenylindole (DAPI) was used to mount the membrane and a cover slip was placed onto the membrane; because the cells were stained with Hoechst, the DAPI was not necessary but was included in the commercial formulation. The edges of the coverslip were sealed using clear nail polish to prevent evaporation. The mounted samples were stored at 4 °C.
2.4.2. Airway-On-Chip
On Day 11, the airways-on-chip were fixed in situ for preservation and staining. All steps involving manual syringe-infusion of chips are conducted separately for each channel, while the other is occluded; for example, when washing with PBS, the apical channel is washed first while the basal channel is closed with luer-caps. Infusion of a channel is confirmed both visually in the channel, for example by tracking small bubbles that may be introduced during injection, as well as via confirmation of three droplets forming at the outlet of the channel being infused (chosen conservatively, based on the internal volume of the channel). The single-channel infusion with the second channel blocked minimized the chance of crossflow, which is especially important earlier in the experiment when cells have not adhered or formed a barrier tighter than the porous membrane itself.
Chips were washed with PBS three times; following this, a 4% methanol-free formaldehyde solution in PBS (Thermofisher, Pierce) was infused to fill each channel. This solution was incubated for 15 min at room temperature, following which an additional three PBS washes were performed. Cells were then permeabilized by injection of a 0.02% Tween-20 solution in PBS, which was incubated for 15 min followed by an additional three PBS washes. Chips were then blocked with a 1% BSA solution for 90 min before loading the working concentration of Alexa-Fluor 488 Phalloidin (Thermofisher) stain (165 nM in PBS containing 1% BSA), which was incubated for 45 min. Chips were, then, washed a final three times before fluidic and clamp disassembly.
Four incisions along each edge of the membrane, through the PDMS, were made to extract the cell-laden membrane from the chip. Extracted membranes were promptly laid onto a microscope slide with two drops of ProLong Gold antifade reagent with DAPI (Thermofisher); a coverslip was carefully placed atop the membrane and the mounting media was cured under a coverslip at room temperature in the dark overnight. The following morning, slides were transferred to a slide-box for storage at 4 °C.
2.5. Imaging
Images of HBMECs were taken on an inverted microscope (Zeiss, Observer.Z1, Oberkochen, Germany). Images of Calu-3s were taken on an inverted microscope with epifluorescent capabilities and intermediate magnification switching from 1.0× to 1.5× (Nikon Eclipse Ti2-E (Nikon, Tokyo, Japan)); 10×/NA 0.3, dry (Nikon: MRH10105) and 40×/NA 0.6, dry (Nikon: MRH48430) objectives were used in conjunction with 432 nm (Semrock, Rochester, NY, USA, 36 nm bandwidth, cat: FF01-432/36) and 515 nm (Semrock, 30 nm bandwidth, cat: FF01-515/30) single bandpass emission filters for DAPI and Alexa-Fluor-488-Phalloidin microscopy, respectively).
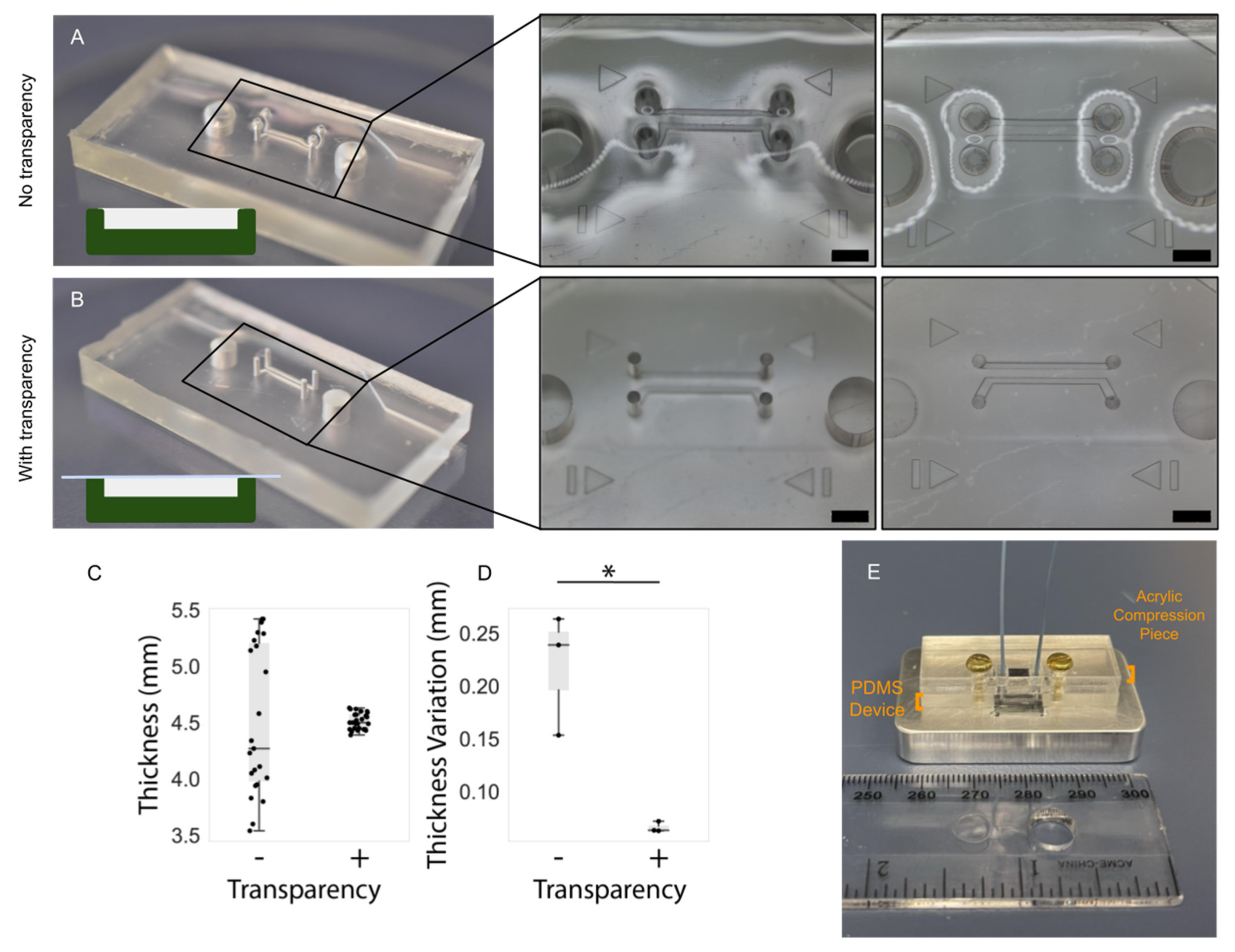
2.6. PDMS Thickness Uniformity Characterisation
In microfluidic devices employing pressure clamps to aid in sealing (such as that depicted in
Figure 4), the thickness uniformity of the PDMS critically impacts both the quality of the seal as well as channel integrity. Thickness nonuniformity can lead to uneven compression across the device, in turn leading to leakage in regions of low compression or channel deformation in regions of higher compression. We introduced the use of transparency films during curing to improve thickness uniformity and thus device performance. In order to characterize the impact of employing transparency films during PDMS curing, a separate PDMS microfluidic device with two, smaller, microfluidic channels was fabricated that would better highlight channel integrity under compression. Master molds were created on the same printers as for the organ-on-chip devices and fabrication methods were identical to the organs-on-chips. Three devices were fabricated without the use of a transparency film to quantify its effects on the flatness of a cured device, and three devices were fabricated with a transparency film for comparison.
Following fabrication, the PDMS devices were measured with calipers at the same five points equally spaced along the long edge of the device. The devices were then compressed against a silicon wafer and visualized under a brightfield epi-illumination microscope (Aven MicroVue (Aven, Ann Arbor, MI, USA)) to assess the deformation of channels. Compression was achieved using 3–32 bolts and a rigid, laser-cut, cast-acrylic washer; the same individual manually screwed the bolts using fingertips to achieve comparable compression across all tested devices.
2.7. PDMS and Mold Surface Roughness Characterisation
The surface topography and surface roughness of the mold interior and the subsequent PDMS gasket was investigated with an atomic force microscope (AFM). The images of three random spots on each respective surface were obtained using a Nanosurf EasyScan 2 AFM system (Nanosurf, Liestal, Switzerland) in tapping mode with Si tips over the three regions of area 10 µm 10 µm with a 512 512 pixel resolution. The root mean square (RMS) was then calculated to quantify surface roughness.
4. Conclusions
PDMS-based organs-on-chip have enabled the development of next-generation in vitro cell culture models; this platform, however, does not come without its challenges. Leakage, flow system design and management, and PDMS bond strength are among the established difficulties associated with this platform that we have aimed to mitigate [
22,
48]. The flexibility offered by PDMS can be leveraged to quickly and inexpensively iterate on design and scale up production of more physiologically translatable in vitro models; limitations center largely on master mold production and fabrication success rates [
49,
50]. We have employed PDMS-focused 3D printing technologies that enable inexpensive (<CAD 5 in resin cost) and rapid (<2 h) mold production and described the improvements they offer over more conventional stereolithography 3D printers and resins due to PDMS cure-inhibition and surface roughness [
17]. We have, further, described the inclusion of a number of fabrication strategies focused on improving robustness and fabrication success rate of PDMS organs-on-chip that utilize inexpensive, commercially available materials such as biopsy punches, copier transparency films, and needles.
We have applied subsets of these strategies to two different organs-on-chip and demonstrated the establishment of morphologically typical cell cultures through their application, achieving fabrication success rates of over 85% and average culture formation and maintenance success rates of approximately 65%. These strategies were focused on reducing the impact of persistent failure modes such as fluidic leakage (through optimizing the creation and reinforcement of fluidic ports) and increasing the ease of fluidics management and interfacing (through the use of luer-lock for every connection in the system). Employing liquid uncured PDMS as a sealant, either as a moat (blood–brain barrier chip) or as small volumes dispensed around top-loaded ports (airway-on-chip) reinforces needle and tubing connections while remaining resistant to chemical disinfectants (a limitation of epoxy-based reinforcement methods). Incorporation of luer connections simplifies fluidic changes (such as injection of medium for washing channels or in situ staining protocols) and channel clamping or blocking (essential to minimizing the risk of crossflow across porous membranes). We have also described methods to achieve uniform compression using inexpensive, commercially available fastening tools (thumb nuts, washers, and threaded rods) in conjunction with laser-cut cast acrylic (the single unit cost of which is under CAD 5 and required less than 10 min of processing time). This enables further reinforcement of PDMS-PDMS bonding as well as adds support in areas where PDMS is in contact with another material (such as the PET membrane used as a cell-culture substrate). We have found incorporation of compression improves control over cell proliferation and better confines growth to channel borders, essential for reproducible analysis.
Additional challenges associated with the use of PDMS in the organ-on-chip include the established tendency for PDMS to absorb small hydrophobic molecules [
51]; there have been multiple strategies to combat this reported in the literature. These include the incorporation of coating solutions [
52] as well as surface modifications [
53]. While the strategies described in this article do not directly address these challenges, they can be used in conjunction with existing methods reported and cited above.
The methods described are a compromise between increasing the reproducibility of organs-on-chip fabrication and assembly, while providing straightforward procedures that allow for wider platform adoption by non-specialized users. Dependence on manual fabrication and assembly is less desirable, where variability can be introduced by the end user. However, more automated and standardized industrial processes can have larger overhead costs, may require complex protocols for specialized equipment, and may require users to spend a significant amount of time initially on device design, all of which can be prohibitive to the uptake and application of organs-on-chip for research. Fabrication tools such as 3D printers and laser cutters are becoming more common in research laboratories. The use of less time-consuming techniques with low-cost and ubiquitous consumables (such as the use of transparency films to improve PDMS flatness or using a PDMS moat for reinforcing the inlet and outlet ports), can be more easily and readily transferable to other applications for PDMS-based microfluidic devices and organ-on-chip development.