Module-Fluidics: Building Blocks for Spatio-Temporal Microenvironment Control
Abstract
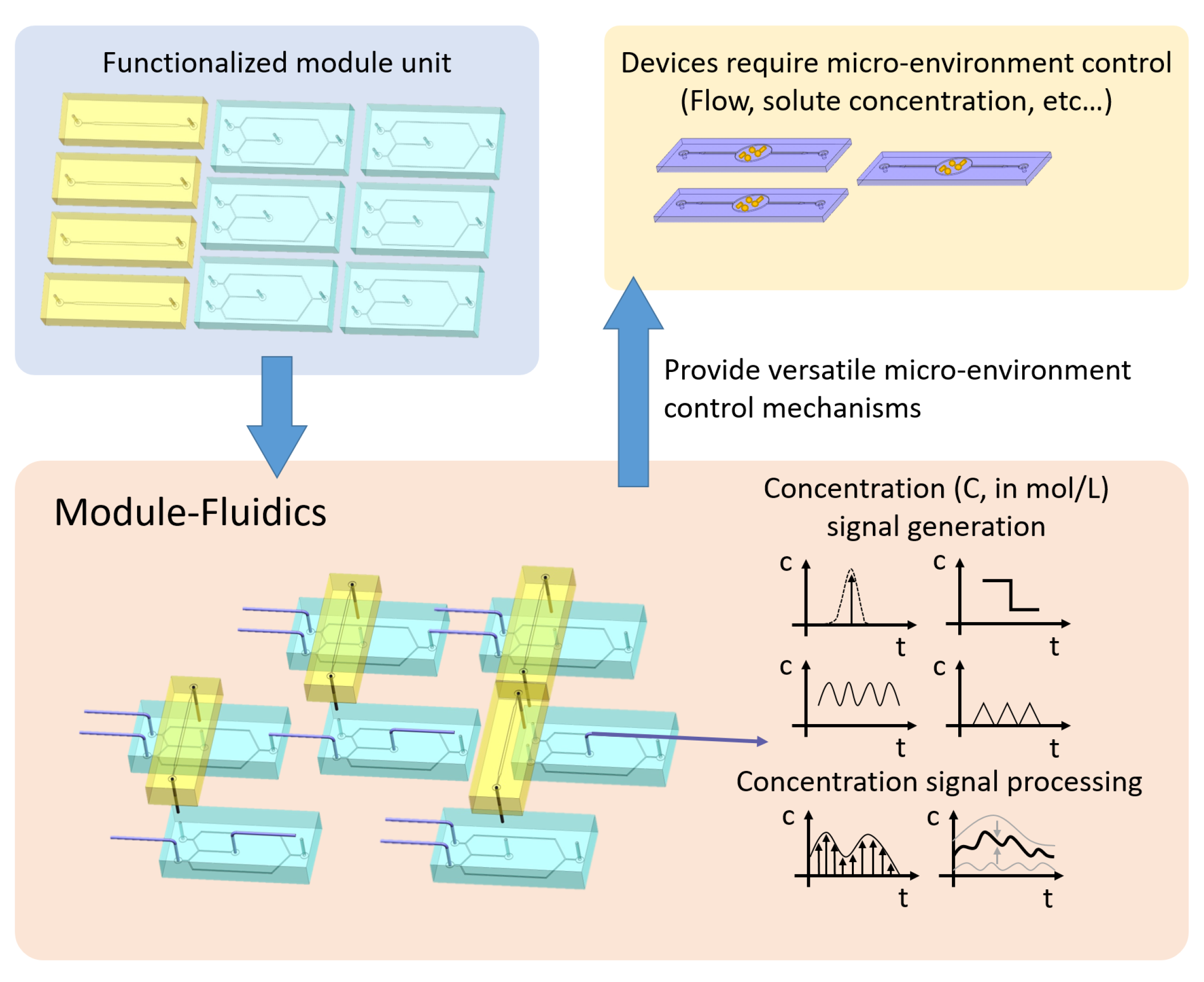
:1. Introduction
2. Materials and Methods
2.1. Signal Generator Design: Oscillator
2.2. Modularization: Integrator
2.3. System Integration: Sampling and Superposition
3. Results and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, A.C. Polymer Data Handbook; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Ling, B.; Khan, H.J.; Druhan, J.L.; Battiato, I. Multi-Scale Microfluidics for Transport in Shale Fabric. Energies 2020, 14, 21. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Maayani, S.; Martin, L.L.; Carmon, T. Water-walled microfluidics for high-optical finesse cavities. Nat. Commun. 2016, 7, 10435. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, I.; Aubry, N. Enhancement of microfluidic mixing using time pulsing. Lab Chip 2003, 3, 114–120. [Google Scholar] [CrossRef]
- Bahl, G.; Kim, K.H.; Lee, W.; Liu, J.; Fan, X.; Carmon, T. Brillouin cavity optomechanics with microfluidic devices. Nat. Commun. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Li, C.W.; Yang, J. Cell docking and on-chip monitoring of cellular reactions with a controlled concentration gradient on a microfluidic device. Anal. Chem. 2002, 74, 3991–4001. [Google Scholar] [CrossRef]
- Yandrapalli, N.; Petit, J.; Bumchen, O.; Robinson, T. Surfactant-free production of biomimetic giant unilamellar vesicles using PDMS-based microfluidics. Commun. Chem. 2021. [Google Scholar] [CrossRef]
- Song, J.; Ryu, H.; Chung, M.; Kim, Y.; Blum, Y.; Lee, S.S.; Pertz, O.; Jeon, N.L. Microfluidic platform for single cell analysis under dynamic spatial and temporal stimulation. Biosens. Bioelectron. 2018, 104, 58–64. [Google Scholar] [CrossRef]
- Gambin, Y.; VanDelinder, V.; Ferreon, A.C.M.; Lemke, E.A.; Groisman, A.; Deniz, A.A. Visualizing a one-way protein encounter complex by ultrafast single-molecule mixing. Nat. Methods 2011, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Ober, T.J.; Foresti, D.; Lewis, J.A. Active mixing of complex fluids at the microscale. Proc. Natl. Acad. Sci. USA 2015, 112, 12293–12298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foote, R.S.; Khandurina, J.; Jacobson, S.C.; Ramsey, J.M. Preconcentration of proteins on microfluidic devices using porous silica membranes. Anal. Chem. 2005, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Hjort, K. High-resolution particle separation by inertial focusing in high aspect ratio curved microfluidics. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haswell, S.J.; Watts, P. Green chemistry: Synthesis in micro reactors. Green Chem. 2003, 5, 240–249. [Google Scholar] [CrossRef]
- Morissette, S.L.; Lewis, J.A.; Cesarano, J.; Dimos, D.B.; Baer, T. Solid freeform fabrication of aqueous alumina–poly (vinyl alcohol) gelcasting suspensions. J. Am. Ceram. Soc. 2000, 83, 2409–2416. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wu, Z. Micromixers—A review. J. Microm. Microengin. 2004, 15, R1. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Lin, F.; Saadi, W.; Rhee, S.W.; Wang, S.J.; Mittal, S.; Jeon, N.L. Generation of dynamic temporal and spatial concentration gradients using microfluidic devices. Lab Chip 2004, 4, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Broyles, B.S.; Jacobson, S.C.; Ramsey, J.M. Sample filtration, concentration, and separation integrated on microfluidic devices. Anal. Chem. 2003, 75, 2761–2767. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid microfluidic mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, B.; Bao, J.; Oostrom, M.; Battiato, I.; Tartakovsky, A.M. Modeling variability in porescale multiphase flow experiments. Adv. Water Resour. 2017, 105, 29–38. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, B.; Battiato, I. Module-Fluidics: Building Blocks for Spatio-Temporal Microenvironment Control. Micromachines 2022, 13, 774. https://doi.org/10.3390/mi13050774
Ling B, Battiato I. Module-Fluidics: Building Blocks for Spatio-Temporal Microenvironment Control. Micromachines. 2022; 13(5):774. https://doi.org/10.3390/mi13050774
Chicago/Turabian StyleLing, Bowen, and Ilenia Battiato. 2022. "Module-Fluidics: Building Blocks for Spatio-Temporal Microenvironment Control" Micromachines 13, no. 5: 774. https://doi.org/10.3390/mi13050774
APA StyleLing, B., & Battiato, I. (2022). Module-Fluidics: Building Blocks for Spatio-Temporal Microenvironment Control. Micromachines, 13(5), 774. https://doi.org/10.3390/mi13050774






