Vertical Graphene-Based Biosensor for Tumor Cell Dielectric Signature Evaluation
Abstract
:1. Introduction
2. Experimental Materials and Procedures
2.1. Materials and Instruments
2.2. Experimental Procedures
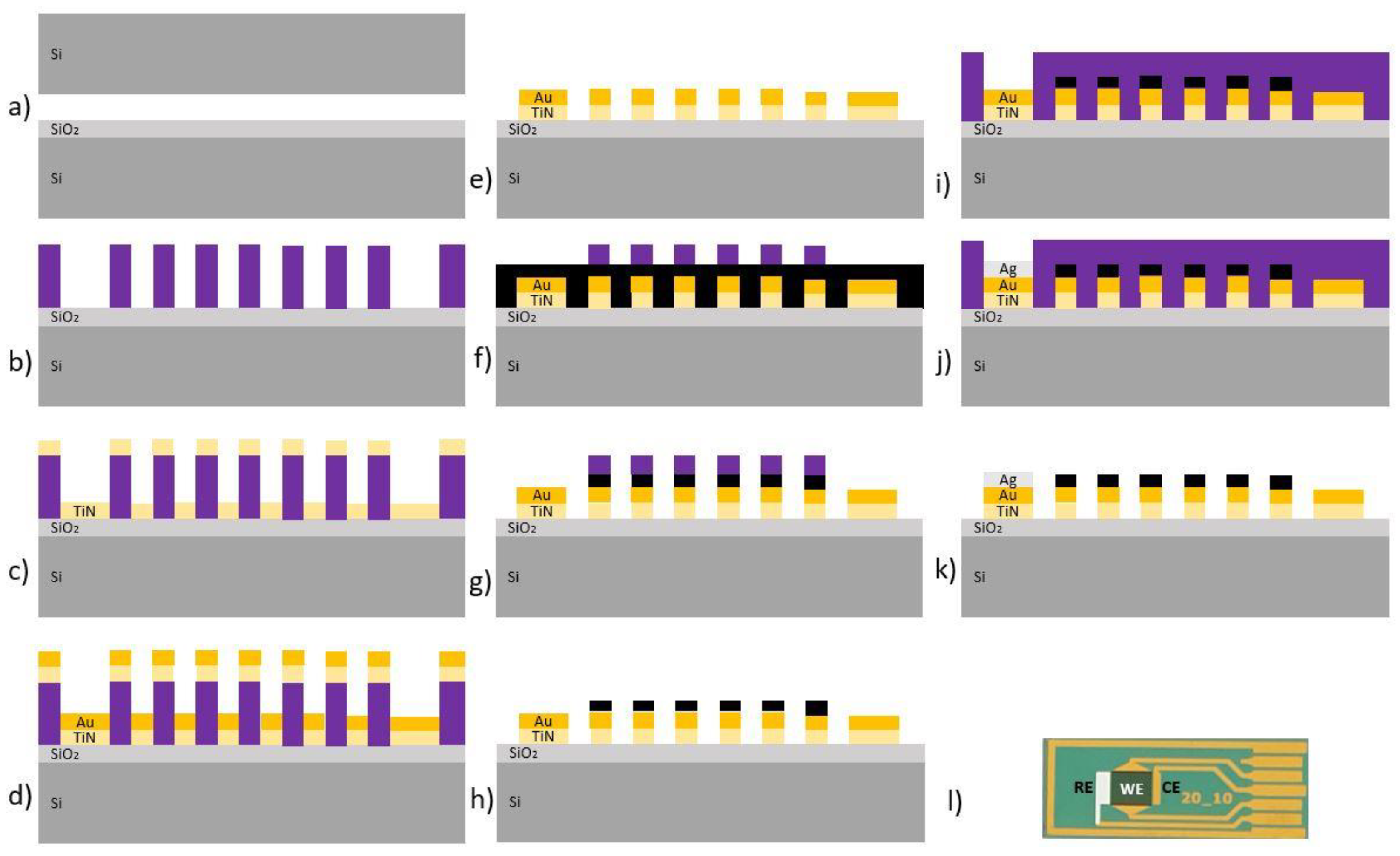
2.2.1. Fabrication of Interdigitated Electrodes Modified with Vertical Graphene
2.2.2. Functionalization of the Electrochemical Biosensor
2.2.3. Cell Capture and Detection
3. Results
3.1. Morphological Characterization of Interdigitated Microelectrodes Modified with Vertical Graphene
3.2. Structural Characterization of Interdigitated Electrodes Covered with Vertical Graphene
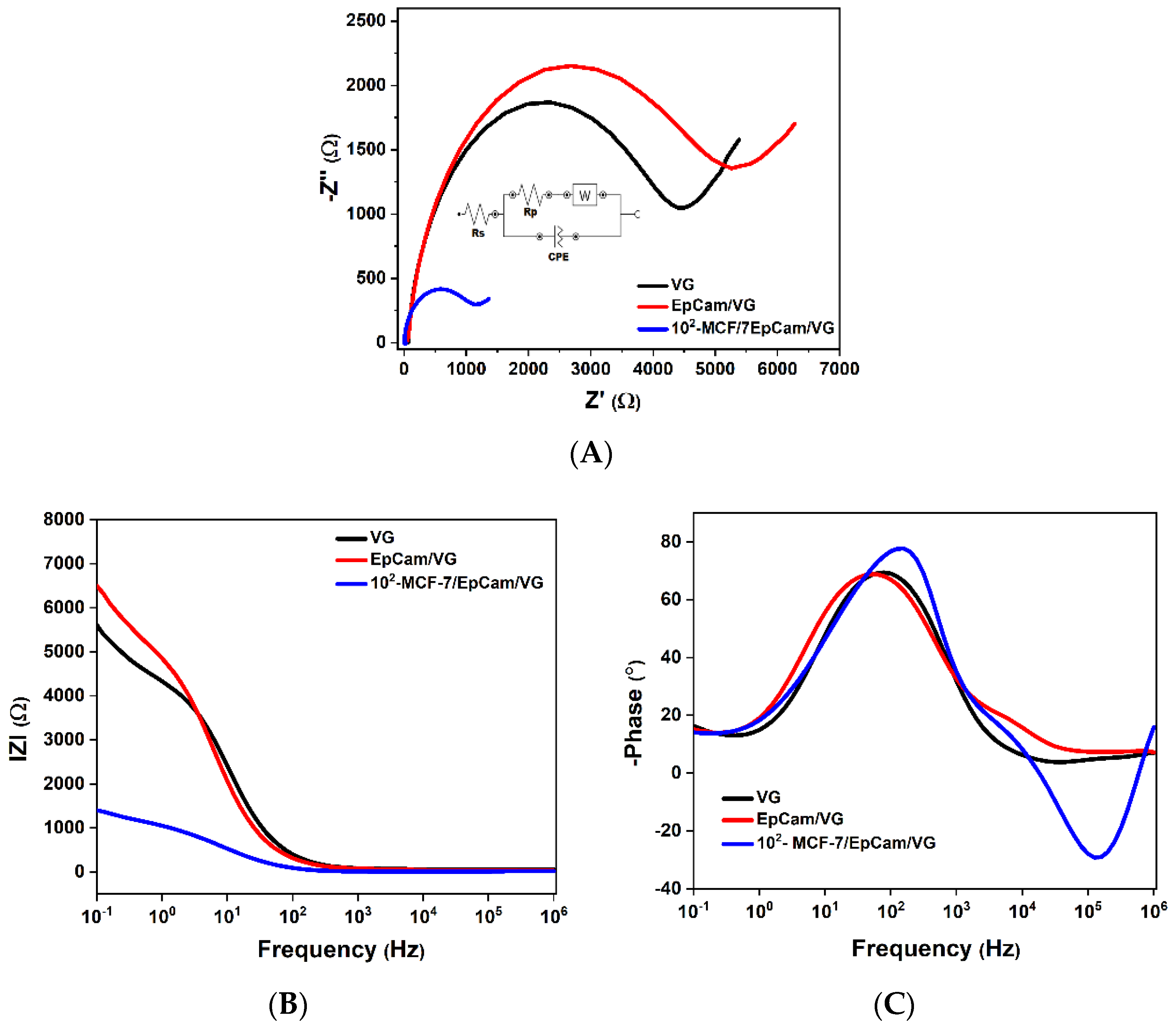
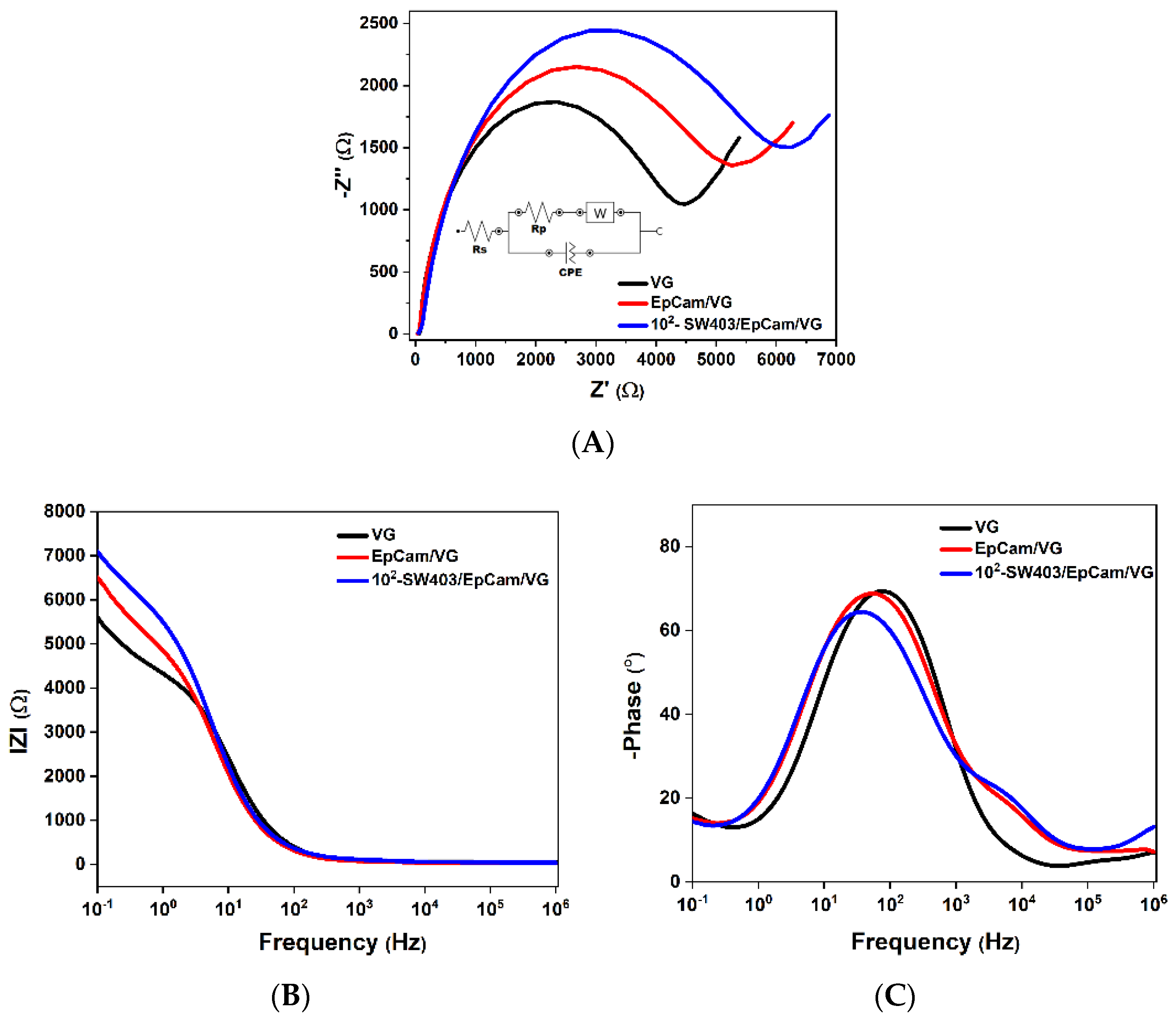
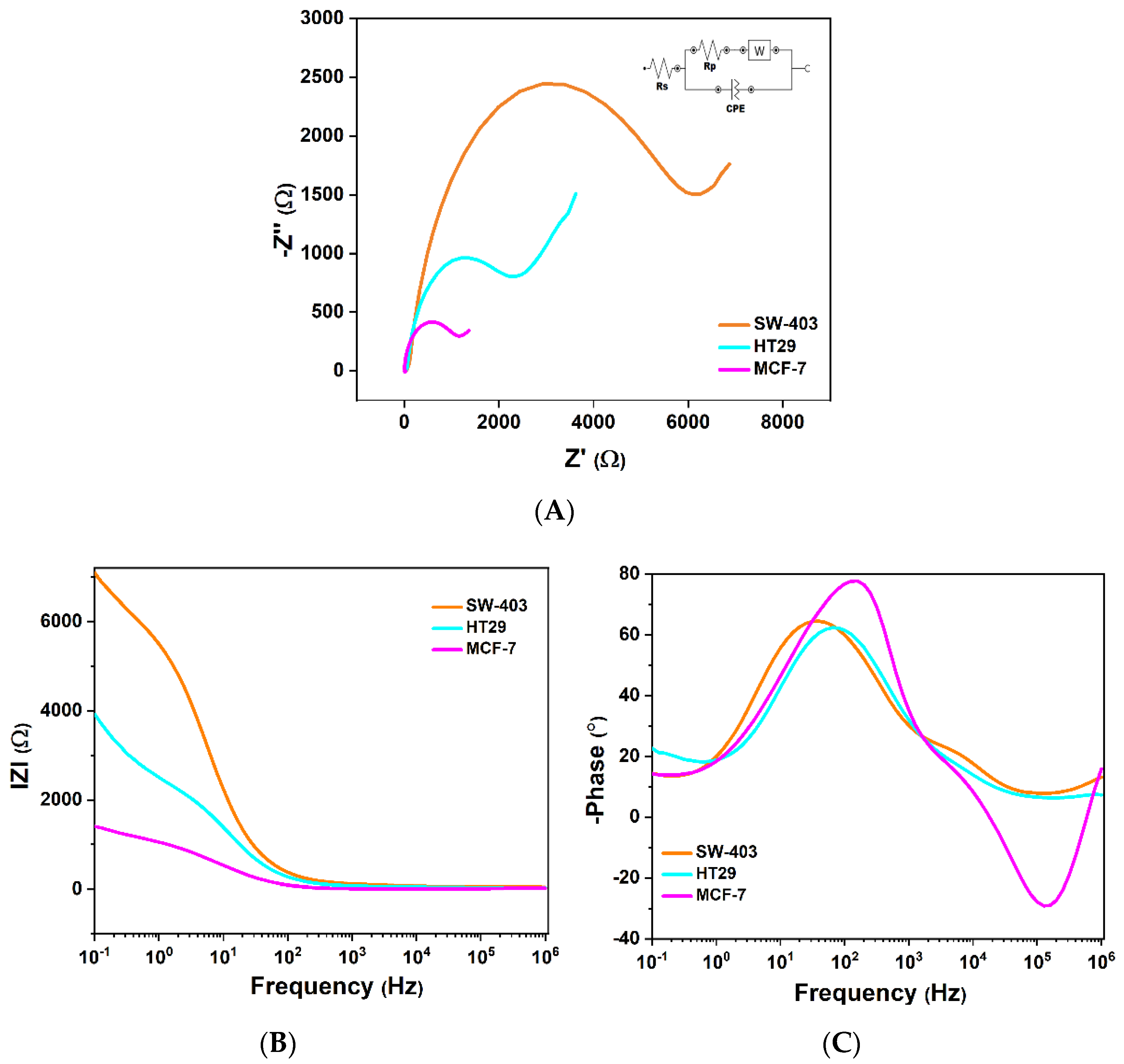
3.3. Tumor Cell Detection with Electrochemical Impedance Spectroscopy (EIS)
3.3.1. Interpretation of the Nyquist Diagram Correlated with the Bode Diagram
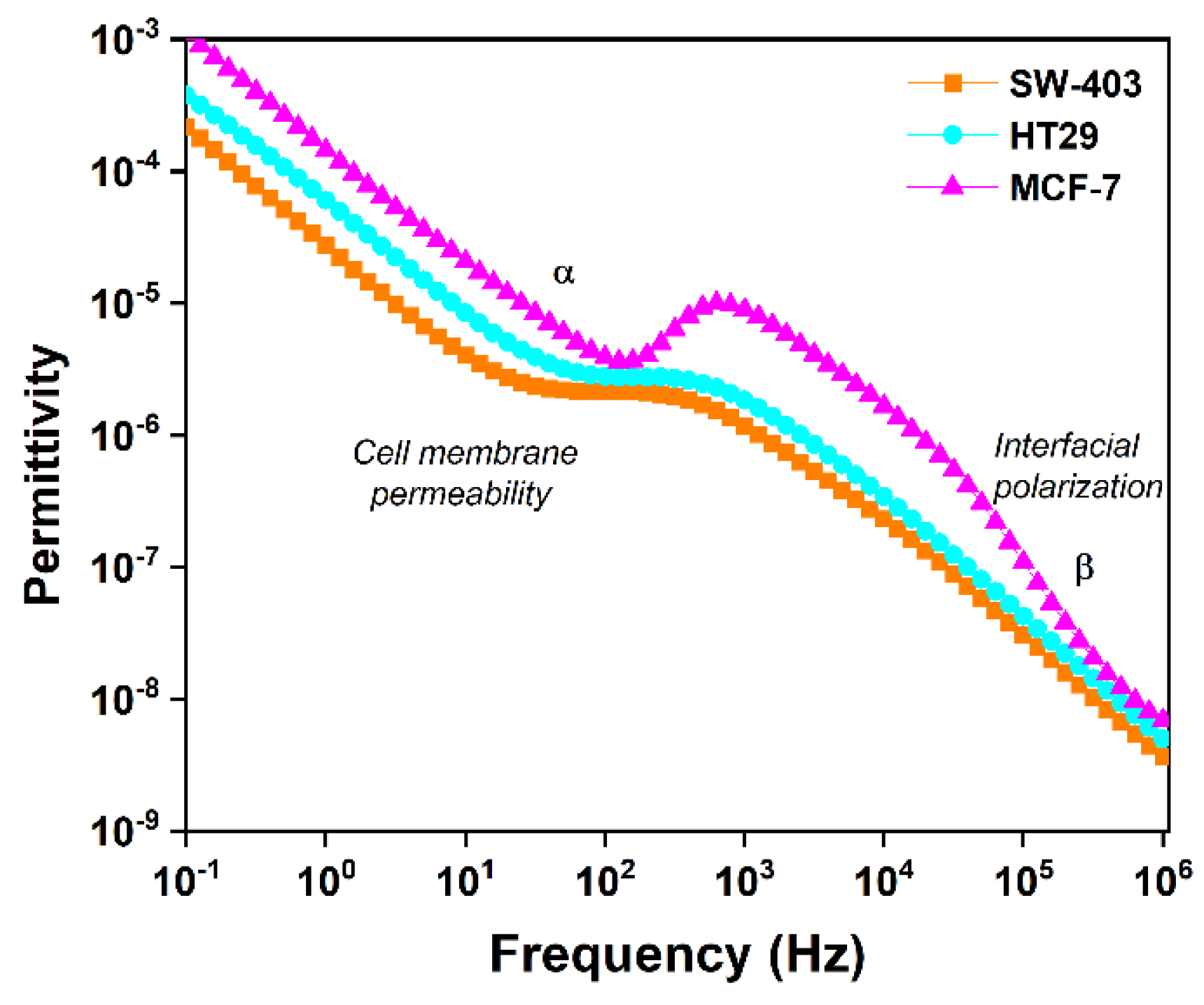
3.3.2. Interpretation of the Variation in Complex Dielectric Permittivity with Frequency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Paterlini-Brechot, P.; Benali, N.L. Circulating Tumor Cells (CTC) Detection: Clinical Impact and Future Directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Moon, J.-M.; Lee, E.S.; Kim, Y.H.; Cho, Y. An Electroactive Biotin-Doped Polypyrrole Substrate That Immobilizes and Releases EpCAM-Positive Cancer Cells. Angew. Chem. Int. Ed. 2014, 53, 4597–4602. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical Validity of Circulating Tumour Cells in Patients with Metastatic Breast Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Wan, Q.; Han, L.; Guo, Y.; Yu, H.; Tan, L.; Zheng, A.; Chen, Y. Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells. Molecules 2021, 26, 7265. [Google Scholar] [CrossRef]
- Gerges, N.; Rak, J.; Jabado, N. New Technologies for the Detection of Circulating Tumour Cells. Br. Med. Bull. 2010, 94, 49–64. [Google Scholar] [CrossRef]
- Adams, A.A.; Okagbare, P.I.; Feng, J.; Hupert, M.L.; Patterson, D.; Göttert, J.; McCarley, R.L.; Nikitopoulos, D.; Murphy, M.C.; Soper, S.A. Highly Efficient Circulating Tumor Cell Isolation from Whole Blood and Label-Free Enumeration Using Polymer-Based Microfluidics with an Integrated Conductivity Sensor. J. Am. Chem. Soc. 2008, 130, 8633–8641. [Google Scholar] [CrossRef]
- Shen, H.; Yang, J.; Chen, Z.; Chen, X.; Wang, L.; Hu, J.; Ji, F.; Xie, G.; Feng, W. A Novel Label-Free and Reusable Electrochemical Cytosensor for Highly Sensitive Detection and Specific Collection of CTCs. Biosens. Bioelectron. 2016, 81, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-A.; Lee, T.Y.; Lee, S.H.; Jung, H.-I. Two-Stage Microfluidic Chip for Selective Isolation of Circulating Tumor Cells (CTCs). Biosens. Bioelectron. 2015, 67, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, X.; Chen, J.; Zhan, Q.; Hua, Y.; Xu, S.; Li, Z.; Wang, Z.; Dong, Y.; Zuo, D. Hexokinase 2 Discerns a Novel Circulating Tumor Cell Population Associated with Poor Prognosis in Lung Cancer Patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2012228118. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wei, J.; Ding, L.; Yang, X.; Wu, Y. State-of-the-Arts Techniques and Current Evolving Approaches in the Separation and Detection of Circulating Tumor Cell. Talanta 2021, 239, 123024. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Das, D.; Kamil, F.A.; Biswas, K.; Das, S. Evaluation of Single Cell Electrical Parameters from Bioimpedance of a Cell Suspension. RSC Adv. 2014, 4, 18178–18185. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Deng, D.; He, H.; Yan, X.; Wang, Z.; Fan, C.; Luo, L. Effective Immobilization of Au Nanoparticles on TiO2 Loaded Graphene for a Novel Sandwich-Type Immunosensor. Biosens. Bioelectron. 2018, 102, 301–306. [Google Scholar] [CrossRef]
- Dharuman, V.; Hahn, J.H.; Jayakumar, K.; Teng, W. Electrochemically Reduced Graphene–Gold Nano Particle Composite on Indium Tin Oxide for Label Free Immuno Sensing of Estradiol. Electrochim. Acta 2013, 114, 590–597. [Google Scholar]
- Khatayevich, D.; Page, T.; Gresswell, C.; Hayamizu, Y.; Grady, W.; Sarikaya, M. Selective Detection of Target Proteins by Peptide-enabled Graphene Biosensor. Small 2014, 10, 1505–1513. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wu, A.; Wei, G. Designed Graphene-Peptide Nanocomposites for Biosensor Applications: A Review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar]
- Lu, C.-H.; Li, J.; Zhang, X.-L.; Zheng, A.-X.; Yang, H.-H.; Chen, X.; Chen, G.-N. General Approach for Monitoring Peptide–Protein Interactions Based on Graphene–Peptide Complex. Anal. Chem. 2011, 83, 7276–7282. [Google Scholar] [CrossRef]
- Farid, S.; Meshik, X.; Choi, M.; Mukherjee, S.; Lan, Y.; Parikh, D.; Poduri, S.; Baterdene, U.; Huang, C.-E.; Wang, Y.Y. Detection of Interferon Gamma Using Graphene and Aptamer Based FET-like Electrochemical Biosensor. Biosens. Bioelectron. 2015, 71, 294–299. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Soleimani Dinani, H.; Saeidi Tabar, F.; Khassi, K.; Janfaza, S.; Tasnim, N.; Hoorfar, M. Properties and Applications of Graphene and Its Derivatives in Biosensors for Cancer Detection: A Comprehensive Review. Biosensors 2022, 12, 269. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Askari, E.; Haghiralsadat, F.; Tofighi, D. A Highly Sensitive Nanobiosensor Based on Aptamer-Conjugated Graphene-Decorated Rhodium Nanoparticles for Detection of HER2-Positive Circulating Tumor Cells. Nanotechnol. Rev. 2022, 11, 793–810. [Google Scholar] [CrossRef]
- Oliveira, M.E.; Lopes, B.V.; Rossato, J.H.H.; Maron, G.K.; Gallo, B.B.; la Rosa, A.B.; Balboni, R.D.C.; Alves, M.L.F.; Ferreira, M.R.A.; da Silva Pinto, L. Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies. Surfaces 2022, 5, 187–201. [Google Scholar] [CrossRef]
- El-Said, W.A.; Al-Bogami, A.S.; Alshitari, W. Synthesis of Gold Nanoparticles@ Reduced Porous Graphene-Modified ITO Electrode for Spectroelectrochemical Detection of SARS-CoV-2 Spike Protein. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120237. [Google Scholar] [CrossRef]
- Tincu, B.; Demetrescu, I.; Avram, A.; Tucureanu, V.; Matei, A.; Tutunaru, O.; Burinaru, T.; Comanescu, F.; Voitincu, C.; Avram, M. Performance of Single Layer Graphene Obtain by Chemical Vapor Deposition on Gold Electrodes. Diam. Relat. Mater. 2019, 98, 107510. [Google Scholar] [CrossRef]
- Tincu, B.; Avram, M.; Avram, A.; Tutunaru, O.; Tucureanu, V.; Matei, A.; Burinaru, T.; Comanescu, F.; Demetrescu, I. Progress and Control in Development of Single Layer Graphene Membranes. Vacuum 2020, 175, 109269. [Google Scholar] [CrossRef]
- Arshad, F.; Nabi, F.; Iqbal, S.; Khan, R.H. Applications of Graphene-Based Electrochemical and Optical Biosensors in Early Detection of Cancer Biomarkers. Colloids Surf. B Biointerfaces 2022, 212, 112356. [Google Scholar] [CrossRef]
- Pandey, R.K.; Kapoor, D.; Kumar, D.; Tonk, R.; Kalikeri, S.; Rao, S.; Jayaprakash, G.K. Recent Progress in the Graphene Functionalized Nanomaterial-Based Electrochemical Sensors. In Functionalized Nanomaterial-Based Electrochemical Sensors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–38. [Google Scholar]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel Enzymatic Graphene Oxide Based Biosensor for the Detection of Glutathione in Biological Body Fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef]
- Sharma, P.; Tuteja, S.K.; Bhalla, V.; Shekhawat, G.; Dravid, V.P.; Suri, C.R. Bio-Functionalized Graphene–Graphene Oxide Nanocomposite Based Electrochemical Immunosensing. Biosens. Bioelectron. 2013, 39, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, A.; Xing, Y.; Duan, H.; Xu, W.; Zhou, Q.; Wu, H.; Chen, C.; Chen, B. Three-Dimensional Graphene Biointerface with Extremely High Sensitivity to Single Cancer Cell Monitoring. Biosens. Bioelectron. 2018, 105, 22–28. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Wang, X. Applications of Graphene-Based Materials in Sensors: A Review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Wang, Z.; Ogata, H.; Morimoto, S.; Hashimoto, Y.; Endo, M. Vertical Graphene for Biosensors. In Graphene Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–56. [Google Scholar]
- Wang, M.; Guo, H.; Wu, N.; Zhang, J.; Zhang, T.; Liu, B.; Pan, Z.; Peng, L.; Yang, W. A Novel Triazine-Based Covalent Organic Framework Combined with AuNPs and Reduced Graphene Oxide as an Electrochemical Sensing Platform for the Simultaneous Detection of Uric Acid, Dopamine and Ascorbic Acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127928. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-Dimensional Macroscopic Graphene Supported Vertically-Ordered Mesoporous Silica-Nanochannel Film for Direct and Ultrasensitive Detection of Uric Acid in Serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Y.; Qu, Y.; Chen, C.-H.; Chen, C.-M.; Chuang, C.-H.; Zhu, Y. Enhanced Light–Matter Interaction of Graphene–Gold Nanoparticle Hybrid Films for High-Performance SERS Detection. J. Mater. Chem. C 2014, 2, 4683–4691. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Song, X.; Ran, Q.; Yu, C.; Yang, J.; Feng, H.; Yu, L.; Wei, D. Flexible Electrochemical Biosensors Based on Graphene Nanowalls for the Real-Time Measurement of Lactate. Nanotechnology 2017, 28, 315501. [Google Scholar] [CrossRef]
- Tincu, B.; Avram, M.; Tucureanu, V.; Mihailescu, C.; Tutunaru, O.; Avram, A.; Anghel, E. Single Layer Graphene and Vertical Graphene as a Promising Candidate for Electrochemical Biosensors. Revista de Chimie (Rev. Chim.) 2020, 71, 24–29. [Google Scholar] [CrossRef]
- Taue, S.; Nishida, K.; Sakaue, H.; Takahagi, T. Immobilization of Gold Nanoparticles on Silanized Substrate for Sensors Based on Localized Surface Plasmon Resonance. e-J. Surf. Sci. Nanotechnol. 2007, 5, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Layqah, L.A.; Eissa, S. An Electrochemical Immunosensor for the Corona Virus Associated with the Middle East Respiratory Syndrome Using an Array of Gold Nanoparticle-Modified Carbon Electrodes. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eissa, S.; Zourob, M. Competitive Voltammetric Morphine Immunosensor Using a Gold Nanoparticle Decorated Graphene Electrode. Microchim. Acta 2017, 184, 2281–2289. [Google Scholar] [CrossRef]
- Sandoz-Rosado, E.; Page, W.; O’Brien, D.; Przepioski, J.; Mo, D.; Wang, B.; Ngo-Duc, T.-T.; Gacusan, J.; Winter, M.W.; Meyyappan, M. Vertical Graphene by Plasma-Enhanced Chemical Vapor Deposition: Correlation of Plasma Conditions and Growth Characteristics. J. Mater. Res. 2014, 29, 417–425. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Imrich, S.; Hachmeister, M.; Gires, O. EpCAM and Its Potential Role in Tumor-Initiating Cells. Cell Adhes. Migr. 2012, 6, 30–38. [Google Scholar] [CrossRef]







| Sample | Rs (Ω) | Rp (Ω) | Rct (Ω) | Z″max (Ω) | Cdl (μF) | YW (μS) |
|---|---|---|---|---|---|---|
| VG | 53.8 | 2630 | 1474 | 1237 | 5.98 | 533 |
| Functionalized VG | 62.8 | 5020 | 2650 | 2123 | 6.10 | 685 |
| SW-403 | 32.8 | 6070 | 3180 | 2740 | 6.38 | 722 |
| HT-29 | 36.3 | 2410 | 1146 | 908 | 5.66 | 735 |
| MCF-7 | 10.8 | 862 | 551 | 415 | 18.8 | 1800 |
| Sample | τ1 (s) | τ2 (s) | τr (s) | δ | tg max | d (m) | λD | De (m2/s) | Di (m2/s) |
|---|---|---|---|---|---|---|---|---|---|
| SW-403 | 0.040 | 0.634 | 1 × 10−6 | 15.84 | 1.99 | 2.3 × 10−7 | 1.45 × 10−8 | 5.27 × 10−15 | 3.32 × 10−16 |
| HT-29 | 0.025 | 0.100 | 8 × 10−7 | 3.98 | 1.00 | 2.3 × 10−7 | 5.78 × 10−8 | 1.32 × 10−13 | 3.32 × 10−14 |
| MCF-7 | 0.032 | 0.400 | 1 × 10−5 | 12.58 | 1.77 | 2.3 × 10−7 | 1.83 × 10−8 | 1.05 × 10−14 | 8.34 × 10−16 |
| Frequency | Permittivity (F) | ||
|---|---|---|---|
| SW-403 | HT-29 | MCF-7 | |
| 102 | 2.12 × 10−6 | 2.79 × 10−6 | 3.88 × 10−6 |
| 104 | 2.37 × 10−7 | 4.42 × 10−7 | 1.66 × 10−6 |
| 106 | 3.63 × 10−9 | 4.97 × 10−9 | 6.9 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tincu, B.; Burinaru, T.; Enciu, A.-M.; Preda, P.; Chiriac, E.; Marculescu, C.; Avram, M.; Avram, A. Vertical Graphene-Based Biosensor for Tumor Cell Dielectric Signature Evaluation. Micromachines 2022, 13, 1671. https://doi.org/10.3390/mi13101671
Tincu B, Burinaru T, Enciu A-M, Preda P, Chiriac E, Marculescu C, Avram M, Avram A. Vertical Graphene-Based Biosensor for Tumor Cell Dielectric Signature Evaluation. Micromachines. 2022; 13(10):1671. https://doi.org/10.3390/mi13101671
Chicago/Turabian StyleTincu, Bianca, Tiberiu Burinaru, Ana-Maria Enciu, Petruta Preda, Eugen Chiriac, Catalin Marculescu, Marioara Avram, and Andrei Avram. 2022. "Vertical Graphene-Based Biosensor for Tumor Cell Dielectric Signature Evaluation" Micromachines 13, no. 10: 1671. https://doi.org/10.3390/mi13101671
APA StyleTincu, B., Burinaru, T., Enciu, A. -M., Preda, P., Chiriac, E., Marculescu, C., Avram, M., & Avram, A. (2022). Vertical Graphene-Based Biosensor for Tumor Cell Dielectric Signature Evaluation. Micromachines, 13(10), 1671. https://doi.org/10.3390/mi13101671








