Microfluidic Gas Sensors: Detection Principle and Applications
Abstract
:1. Introduction
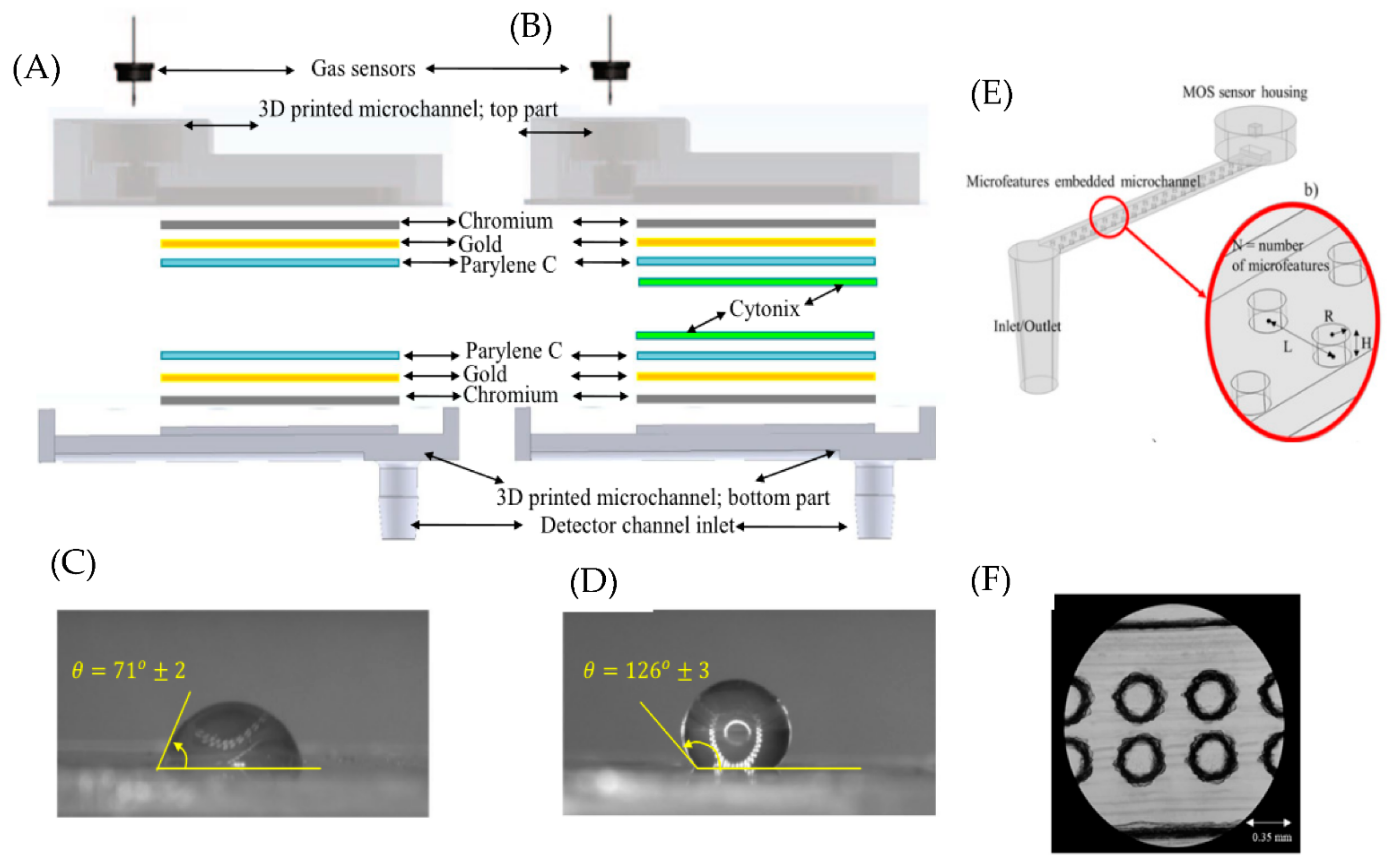
2. Microfluidic Channel Parameters
2.1. Microfluidic Channel Coatings
2.2. Geometry and Dimension of Microfluidic Channels
2.3. Surface Area of the Microfluidic Channel
3. Fabrication Materials and Methods
4. Detection Approaches
4.1. Electronic
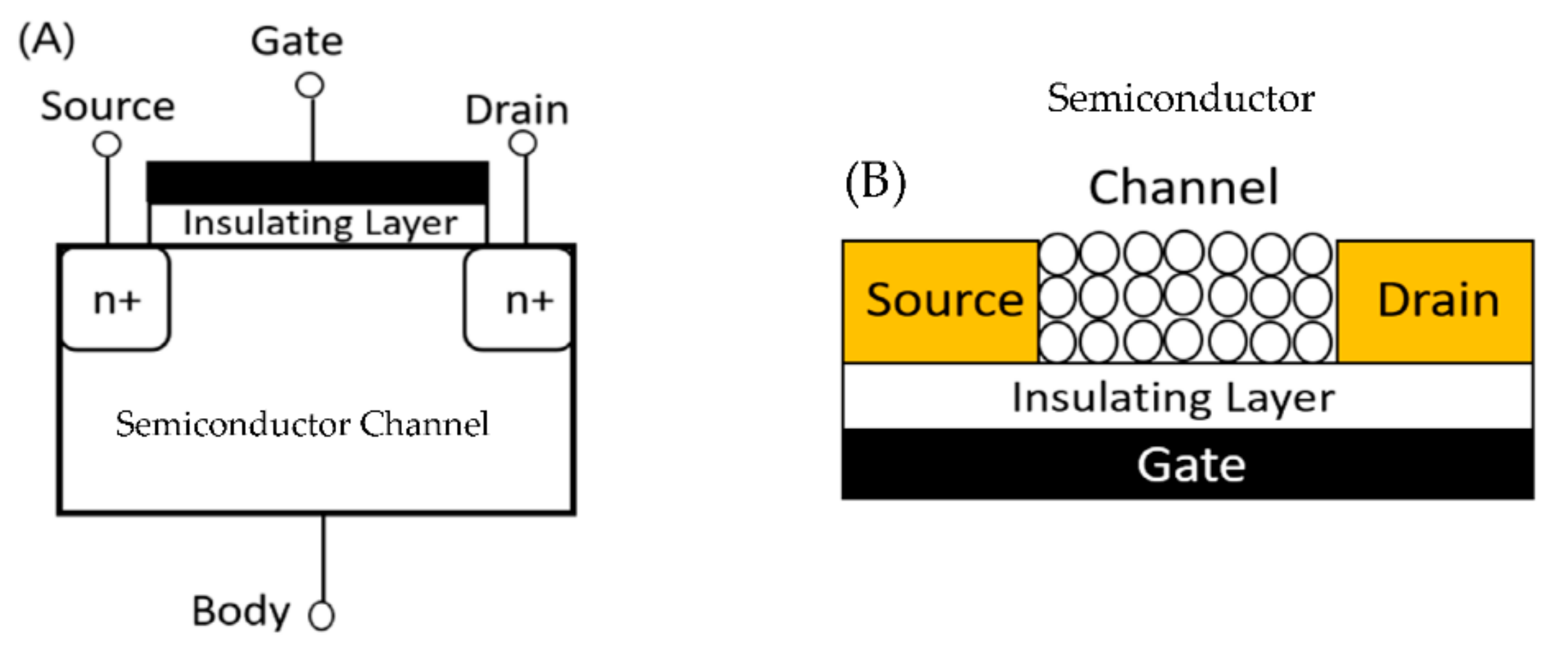
4.1.1. Metal Oxide Semiconductor
4.1.2. Field-Effect transistors (FET)
4.2. Electrochemical
Amperometry
4.3. Optical
4.3.1. Colorimetric
4.3.2. Surface-Enhanced Raman Spectroscopy (SERS)
4.3.3. Fluorescence
4.3.4. Non-Traditional Methods
5. Bubble/Droplet-Based Microfluidics
6. Applications
6.1. Air Pollutants and Particulate Matter
6.2. Airborne Pathogens
6.2.1. Bacteria
6.2.2. Viruses
6.3. Gases Released from Foods
6.4. Explosives
7. Practical Approaches toward Using Microfluidics-Based Devices in Air
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S.J. Recent Advances in Perylene Diimide-Based Active Materials in Electrical Mode Gas Sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology–gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, J.; Liu, L.; Xu, L. Application of Microfluidics in Wearable Devices. Small Methods 2019, 3, 1900688. [Google Scholar] [CrossRef]
- Martini, V.; Bernardini, S.; Bendahan, M.; Aguir, K.; Perrier, P.; Graur, I. Microfluidic gas sensor with integrated pumping system. Sens. Actuators B Chem. 2012, 170, 45–50. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [Green Version]
- Meckes, A.; Behrens, J.; Kayser, O.; Benecke, W.; Becker, T.; Müller, G. Microfluidic system for the integration and cyclic operation of gas sensors. Sens. Actuators A Phys. 1999, 76, 478–483. [Google Scholar] [CrossRef]
- Paknahad, M.; Ghafarinia, V.; Hossein-Babaei, F. A microfluidic gas analyzer for selective detection of biomarker gases. In Proceedings of the 2012 IEEE Sensors Applications Symposium Proceedings, Brescia, Italy, 7–9 February 2012; pp. 1–5. [Google Scholar]
- Lee, S.H.; Lim, J.H.; Park, J.; Hong, S.; Park, T.H. Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron. 2015, 71, 179–185. [Google Scholar] [CrossRef]
- Shen, F.; Tan, M.; Wang, Z.; Yao, M.; Xu, Z.; Wu, Y.; Wang, J.; Guo, X.; Zhu, T. Integrating Silicon Nanowire Field Effect Transistor, Microfluidics and Air Sampling Techniques For Real-Time Monitoring Biological Aerosols. Environ. Sci. Technol. 2011, 45, 7473–7480. [Google Scholar] [CrossRef]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of channel coating and dimensions of microfluidic-based gas detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Hooshyar Zare, A. The selective flow of volatile organic compounds in conductive polymer-coated microchannels. Sci. Rep. 2017, 7, 42299. [Google Scholar] [CrossRef]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective detection of volatile organic compounds in microfluidic gas detectors based on “like dissolves like”. Sci. Rep. 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Nidetz, R.; Zhou, M.; Lee, J.; Buggaveeti, S.; Kurabayashi, K.; Fan, X. Flow-through microfluidic photoionization detectors for rapid and highly sensitive vapor detection. Lab. Chip. 2015, 15, 3021–3029. [Google Scholar] [CrossRef]
- Jing, W.; Zhao, W.; Liu, S.; Li, L.; Tsai, C.-T.; Fan, X.; Wu, W.; Li, J.; Yang, X.; Sui, G. Microfluidic Device for Efficient Airborne Bacteria Capture and Enrichment. Anal. Chem. 2013, 85, 5255–5262. [Google Scholar] [CrossRef]
- Yang, K.; Zong, S.; Zhang, Y.; Qian, Z.; Liu, Y.; Zhu, K.; Li, L.; Li, N.; Wang, Z.; Cui, Y. Array-Assisted SERS Microfluidic Chips for Highly Sensitive and Multiplex Gas Sensing. ACS Appl. Mater. Interfaces 2020, 12, 1395–1403. [Google Scholar] [CrossRef]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective detection of VOCs using microfluidic gas sensor with embedded cylindrical microfeatures coated with graphene oxide. J. Hazard. Mater. 2022, 424, 127566. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, modification, and application of poly(methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef]
- Wang, R.; Prabhakar, A.; Iglesias, R.A.; Xian, X.; Shan, X.; Tsow, F.; Forzani, E.S.; Tao, N. A Microfluidic-Colorimetric Sensor for Continuous Monitoring of Reactive Environmental Chemicals. IEEE Sens. J. 2012, 12, 1529–1535. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Dong, H.; Fan, L.; Zheng, J. Multiplex quantification of metals in airborne particulate matter via smartphone and paper-based microfluidics. Anal. Chim. Acta 2018, 1044, 110–118. [Google Scholar] [CrossRef]
- Li, Y.; Fu, M.; Pang, W.; Chang, Y.; Duan, X. A combined virtual impactor and field-effect transistor microsystem for particulate matter separation and detection. Nanotechnol. Precis. Eng. 2021, 4, 013003. [Google Scholar] [CrossRef]
- Kuznetsov, A.E.; Komarova, N.V.; Kuznetsov, E.V.; Andrianova, M.S.; Grudtsov, V.P.; Rybachek, E.N.; Puchnin, K.V.; Ryazantsev, D.V.; Saurov, A.N. Integration of a field effect transistor-based aptasensor under a hydrophobic membrane for bioelectronic nose applications. Biosens. Bioelectron. 2019, 129, 29–35. [Google Scholar] [CrossRef]
- Cha, W.; Tung, Y.-C.; Meyerhoff, M.E.; Takayama, S. Patterned Electrode-Based Amperometric Gas Sensor for Direct Nitric Oxide Detection within Microfluidic Devices. Anal. Chem. 2010, 82, 3300–3305. [Google Scholar] [CrossRef] [Green Version]
- Hussain, G.; Ge, M.; Zhao, C.; Silvester, D.S. Fast responding hydrogen gas sensors using platinum nanoparticle modified microchannels and ionic liquids. Anal. Chim. Acta 2019, 1072, 35–45. [Google Scholar] [CrossRef]
- Guo, X.-L.; Chen, Y.; Jiang, H.-L.; Qiu, X.-B.; Yu, D.-L. Smartphone-Based Microfluidic Colorimetric Sensor for Gaseous Formaldehyde Determination with High Sensitivity and Selectivity. Sensors 2018, 18, 3141. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kang, M.; Jung, J.H. Integrated micro-optofluidic platform for real-time detection of airborne microorganisms. Sci. Rep. 2015, 5, 15983. [Google Scholar] [CrossRef] [Green Version]
- Kim, S. Direct capture and smartphone quantification of airborne SARS-CoV-2 on a paper microfluidic chip. Biosens. Bioelectron. 2022, 200, 113912. [Google Scholar] [CrossRef]
- Xiong, H.; Ye, X.; Li, Y.; Qi, J.; Fang, X.; Kong, J. Efficient Microfluidic-Based Air Sampling/Monitoring Platform for Detection of Aerosol SARS-CoV-2 On-site. Anal. Chem. 2021, 93, 4270–4276. [Google Scholar] [CrossRef]
- Jiang, X.; Jing, W.; Sun, X.; Liu, Q.; Yang, C.; Liu, S.; Qin, K.; Sui, G. High-Throughput Microfluidic Device for LAMP Analysis of Airborne Bacteria. ACS Sens. 2016, 1, 958–962. [Google Scholar] [CrossRef]
- Piorek, B.D.; Lee, S.J.; Santiago, J.G.; Moskovits, M.; Banerjee, S.; Meinhart, C.D. Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 18898–18901. [Google Scholar] [CrossRef] [Green Version]
- Piorek, B.D.; Lee, S.J.; Moskovits, M.; Meinhart, C.D. Free-Surface Microfluidics/Surface-Enhanced Raman Spectroscopy for Real-Time Trace Vapor Detection of Explosives. Anal. Chem. 2012, 84, 9700–9705. [Google Scholar] [CrossRef]
- Lee, M.R.; Lee, H.K.; Yang, Y.; Koh, C.S.L.; Lay, C.L.; Lee, Y.H.; Phang, I.Y.; Ling, X.Y. Direct Metal Writing and Precise Positioning of Gold Nanoparticles within Microfluidic Channels for SERS Sensing of Gaseous Analytes. ACS Appl. Mater. Interfaces 2017, 9, 39584–39593. [Google Scholar] [CrossRef]
- Lafuente, M.; Almazán, F.; Bernad, E.; Urbiztondo, M.A.; Santamaría, J.; Mallada, R.; Pina, M.P. SERS Detection of Neurotoxic Agents in Gas Phase Using Microfluidic Chips Containing Gold-Mesoporous Silica as Plasmonic-Sorbent. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1313–1316. [Google Scholar]
- Yang, K.; Zhu, K.; Wang, Y.; Qian, Z.; Zhang, Y.; Yang, Z.; Wang, Z.; Wu, L.; Zong, S.; Cui, Y. Ti3C2Tx MXene-Loaded 3D Substrate toward On-Chip Multi-Gas Sensing with Surface-Enhanced Raman Spectroscopy (SERS) Barcode Readout. ACS Nano 2021, 15, 12996–13006. [Google Scholar] [CrossRef] [PubMed]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. Polymer Composite Optically Integrated Lab on Chip for the Detection of Ammonia. J. Electrochem. Soc. 2018, 165, B3078–B3083. [Google Scholar] [CrossRef] [Green Version]
- Bulbul, A.; Kim, H. A bubble-based microfluidic gas sensor for gas chromatographs. Lab A Chip 2015, 15, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Damit, B. Droplet-based microfluidics detector for bioaerosol detection. Aerosol Sci. Technol. 2017, 51, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Tirandazi, P.; Hidrovo, C.H. An integrated gas-liquid droplet microfluidic platform for digital sampling and detection of airborne targets. Sens. Actuators B Chem. 2018, 267, 279–293. [Google Scholar] [CrossRef]
- Huang, S.; Connolly, J.; Khlystov, A.; Fair, R.B. Digital Microfluidics for the Detection of Selected Inorganic Ions in Aerosols. Sensors 2020, 20, 1281. [Google Scholar] [CrossRef] [Green Version]
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Rebordão, G.; Palma, S.I.C.J.; Roque, A.C.A. Sensors|Free Full-Text|Microfluidics in Gas Sensing and Artificial Olfaction|HTML. Available online: https://www.mdpi.com/1424-8220/20/20/5742/htm (accessed on 15 July 2022).
- Lee, S.-H.; Galstyan, V.; Ponzoni, A.; Gonzalo-Juan, I.; Riedel, R.; Dourges, M.-A.; Nicolas, Y.; Toupance, T. Finely Tuned SnO 2 Nanoparticles for Efficient Detection of Reducing and Oxidizing Gases: The Influence of Alkali Metal Cation on Gas-Sensing Properties. ACS Appl. Mater. Interfaces 2018, 10, 10173–10184. [Google Scholar] [CrossRef]
- Bao, N.; Gold, J.I.; Szilvasi, T.; Yu, H.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Designing chemically selective liquid crystalline materials that respond to oxidizing gases. J. Mater. Chem. C 2021, 9, 6507–6517. [Google Scholar] [CrossRef]
- Zhang, P. Highly sensitive gas sensing platforms based on field effect Transistor-A review. Anal. Chim. Acta 2021, 1172, 338575. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Kim, S.K.; Kim, M. Ion-Sensitive Field-Effect Transistor for Biological Sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2018, 60, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical impedimetric biosensors, featuring the use of Room Temperature Ionic Liquids (RTILs): Special focus on non-faradaic sensing. Biosens. Bioelectron. 2021, 177, 112940. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Methods and approach of utilizing ionic liquids as gas sensing materials. RSC Adv. 2015, 5, 58371–58392. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-H.; Kargupta, R.; Ghoshal, D.; Zhenglong, L.; Chande, C.; Feng, L.; Chatterjee, S.; Koratkar, N.; Motkuri, R.K.; Basuray, S. ESSENCE–A rapid, shear-enhanced, flow-through, capacitive electrochemical platform for rapid detection of biomolecules | Elsevier Enhanced Reader. Biosens. Bioelectron. 2021, 182, 113163. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–Organic Framework-Based Microfluidic Impedance Sensor Platform for Ultrasensitive Detection of Perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514. [Google Scholar] [CrossRef]
- Stiles, P.; Dieringer, J.; Shah, N.; Duyne, R. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. Palo Alto Calif. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Kneipp, K. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Itou, T.; Markotter, W.; Nel, L.H. Chapter Eight–Reverse Transcription-Loop-Mediated Isothermal Amplification System for the Detection of Rabies Virus. In Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention; Rupprecht, C., Nagarajan, T., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; Volume 1, pp. 85–95. ISBN 978-0-12-800014-4. [Google Scholar]
- Mansour, S.M.G.; Ali, H.; Chase, C.C.L.; Cepica, A. Loop-mediated isothermal amplification for diagnosis of 18 World Organization for Animal Health (OIE) notifiable viral diseases of ruminants, swine and poultry. Anim. Health Res. Rev. 2015, 16, 89–106. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. SYTO probes in the cytometry of tumor cell death–Wlodkowic–2008–Cytometry Part A–Wiley Online Library. J. Quant. Cell Sci. 2008, 73A, 496–507. [Google Scholar] [CrossRef]
- Ven, K.; Vanspauwen, B.; Pérez-Ruiz, E.; Leirs, K.; Decrop, D.; Gerstmans, H.; Spasic, D.; Lammertyn, J. Target Confinement in Small Reaction Volumes Using Microfluidic Technologies: A Smart Approach for Single-Entity Detection and Analysis. ACS Sens. 2018, 3, 264–284. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective photocatalytic oxidation of gaseous ammonia at ppb level over Pt and F modified TiO2. Appl. Catal. B Environ. 2022, 300, 120688. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 21 August 2022).
- Effenhauser, C.S.; Harttig, H.; Krämer, P. An Evaporation-Based Disposable Micropump Concept for Continuous Monitoring Applications. Biomed. Microdevices 2002, 4, 27–32. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Khadheer Pasha, S.K. State of the art recent progress in two dimensional MXenes based gas sensors and biosensors: A comprehensive review. Coord. Chem. Rev. 2020, 424, 213514. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Shafiei, M.; Langford, S.J. Nanoporous naphthalene diimide surface enhances humidity and ammonia sensing at room temperature. Sens. Actuators B Chem. 2022, 351, 130972. [Google Scholar] [CrossRef]







| Detection Technique | Target Analyte(s) | Transducer Material | LOD/ Lowest Concentration Tested | Selectivity | Merits | Limitations | Ref |
|---|---|---|---|---|---|---|---|
| ELECTRONIC | |||||||
| Metal oxide semiconductor (MOS) | Ammonia | Tungsten trioxide film | 10 ppm | Poor selectivity due to the use of MOS sensors. No techniques are employed to improve selectivity. | Integrated sampling system by thermal creep. | Poor selectivity. Need to maintain a constant working temp of 473 K | [4] |
| methanol, ethanol, propanol, pentanol, hexanal, and toluene | Commercially available MOS Sensor | 100 ppm | Can differentiate between 6 Volatile organic compounds (VOCs) | High surface area channel to improve selectivity. Good response and recovery time. | Fails to detect gas in gas mixture selectively. | [16] | |
| methanol, ethanol, 1-propanol, 2-pentanol, acetone, pentane, and hexane | Commercially available MOS Sensor (FIGARO, TGS 2602) | Not reported | Can differentiate between 7 VOCs | Channel with a coating to improve selectivity. | It needs 4 layers of coating to get good selectivity. Fails to detect gas in gas mixture selectively. | [12] | |
| Carbon monoxide, hexane and benzene | Commercially available MOS Sensor | Not reported | It can distinguish between carbon monoxide, hexane, and benzene. Filters out methanol, ethanol, and acetone. | Filters out ketones. | Fails to detect gas in gas mixture selectively. | [11] | |
| Field effect transistor (FET) | Airborne influenza A H3N2 viruses | Silicon nanowires functionalized with influenza A H3N2 subtype antibodies | 104 viruses/uL | Selective against H1N1 viruses and house dust allergens. | Real-time continuous detection. Very low response time. | Absence of integrated (on-chip) sampling system. | [9] |
| Trimethylamine (TMA) | Single-walled carbon nanotubes (SWNTs) functionalized with olfactory receptor-derived peptides (ORPs) | 10 ppt | Response of other odorants is very low compared to TMA | Real-time sensing with high selectivity and sensitivity. Successfully used for on-site testing. | No integrated sampling system. It may not be suitable for continuous sensing. | [8] | |
| Silicon dioxide (SiO2) particles (model for PM) | Gold auxiliary electrode | Not reported | It can discriminate between 2, 5, and 10 um SiO2 particles. | Sensor material can differentiate between different sizes of particles. | Sensor response is highly dependent on generation velocity. | [20] | |
| Vanillin | Aptamer-modified ion selective FETs | 2.7 ppt | The response generated by the gaseous vanillin is much stronger than that generated by the other odorant molecules. | High selectivity and sensitivity. | Very noisy response. Extensive sample preparation is needed. | [21] | |
| ELECTROCHEMICAL | |||||||
| Amperometric | Nitrogen oxide | Catalytic gold-hexacyanoferrate (Au-HCF) working electrode | ~1 nM | Selective against common interfering agents (nitrite, ascorbate, ammonia, etc.) | Real-time sensing and reversible detection of NO. | Sensors are polarized for at least 4 h before use. | [22] |
| Hydrogen (H2) | Platinum nanoparticle-modified gold microchannel electrode | 3.4 vol% | No selectivity experiments were reported. | Ultra-fast response time of 2 s. | Background gases might interfere with the oxidation of H2 | [23] | |
| OPTICAL | |||||||
| Colorimetric | Nitrogen oxide | o-Phenylenediamine (PDA) | 50 ppb/min | A molybdenum oxide-based filter is highly selective against common interfering gases and ozone interference. | Prolongs the lifetime of the colorimetric sensor without sacrificing its sensitivity. | The lifetime of the sensor is still short compared to microfluidic sensors that employ other detection techniques. | [18] |
| Cobalt (Co) Copper (Cu) Iron (Fe) Manganese (Mn) Chromium (Cr) Nickel (Ni) | Chrysoidine-G Dithiooxamide 1,10-phenanthroline monohydrate 4- (2-pyridylazo) resorcinol 1,5-diphenylcarbazide Dimethylglyoxime | 8.16 ng 45.84 ng 1.86 × 102 ng 10.08 ng 1.52 × 102 ng 80.40 ng | Detection reservoirs are coated with ligands specific to the different metals | Rapid and cost-effective fabrication method—UV curing method. 48 chips made in the 30 s with a cost of $ 1.92. Use of smartphone and app for multiplex quantification. | Not a direct capture of PM particles onto the paper microfluidic chip. | [19] | |
| Formaldehyde | 4-aminohydrazine-5-mercapto- 1,2,4-triazole (AHMT) | 0.01 ppm | Unaffected by the presence of other gases such as acetaldehyde and VOCs. | Automated quantification using a smartphone-based system. | Single-use sensor. | [24] | |
| Fluorescence | Escherichia coli Bacillus subtilis Staphylococcus epidermis | SYTO82 dye | 9.9 ± 0.18/μL 7.8 ± 0.17/μL 6.5 ± 0.25/μL | Can differentiate between fluorescent bacterial cells from other residue particles. | Better detection efficiency than conventional microscopy cell counting and colony counting techniques. Gives additional information on the total particle number concentration and continuous real-time detection. | It cannot discriminate between the three bacteria. | [25] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Antibody-conjugated (Rabbit polyclonal antibody to SARS-CoV-2) submicron fluorescent particle (Yellow-green fluorescent carboxylated polystyrene particles). | 200 pg/mL | The use of polyclonal antibodies might undermine the selectivity of the device. | Direct capture of SARS-CoV-2 virus from air. | Not a real-time detection. Needs to undergo post-sampling procedures for quantification. | [26] | |
| SARS-CoV-2 | LAMP primer targeting the O gene and N gene | 10 copies/uL | Very high selectivity. 16 other viruses showed negative results. | Clinically verified. | Not a real-time detection. Needs to undergo post-sampling procedures for quantification. | [27] | |
| S. aureus, E. coli, P. aeruginosa, C. koseri, and K. pneumonia | Separate LAMP primers targeting each bacterium | 24 colony-forming units (CFU) for S. aureus | High selectivity because of the use of the LAMP technique. | Capable of detecting 5 different bacterial species. The results generated are visible to the naked eye. | We need to incubate the chip at 63 °C for 50 min. Not a real-time detection. Only qualitative analysis. | [28] | |
| Surface-enhanced Raman spectroscopy | 4-aminobenzenethiol(4-ABT) | The colloidal suspension of silver nanoparticles | 300 uM | Highly selective | Fast response time (1–3 s). | Sensors can only detect the water-soluble analyte. | [29] |
| 2,4-dinitrotoluene (DNT) | Colloidal solution of silver nanoparticles | 1 ppb | Highly selective | Continuous detection of the analyte. Quick response time (t ≈ 2 min) | Experiments performed under fixed relative humidity (40% RH) | [30] | |
| 4-methylbenzenethiol (4-BMT) | Glass + Au microstructures | 0.5 M 4-MBT | Highly selective | Direct metal writing using two-photon lithography fabricates microfluidic channels with densely packed AuNP. Very low response time (2 s). | Use of expensive instruments for fabrication of sensors. | [31] | |
| Dimethyl methylphosphonate (DMMP) | Gold-mesoporous silica nanoparticles | 2.5 ppmV | Highly selective | Regeneration of SERS substrate is possible by desorption at 200 °C. | Complete regeneration of substrate takes 60 min. | [32] | |
| Benzaldehyde 3-ethylbenzaldehyde glutaraldehyde | Zeolitic Imidazolate framework-8 (ZIF-8) and cysteamine (CA)-coated silver nanotubes (AgNCs) | 1 ppb 1 ppb Not reported | High selectivity for aldehydes. Acetone, ethanol, and toluene showed very low SERS intensity. | Very high sensitivity and selectivity. | No reusability test is reported. | [15] | |
| DNT Odoriferous benzaldehyde Indole | Polystyrene microspheres with nano pits containing bimetallic nanocubes (gold core of 30 nm, silver nanocube of 54 nm, and a 2 nm gold shell) | 10 ppb 10 ppb 50 ppb | Highly selective | Excellent multiplexing ability with high sensitivity. | Elaborate sample preparation is required. | [33] | |
| Absorbance spectrum | Ammonia | Ninhydrin—PDMS composite | 2 ppm | Highly selective | Excellent response time (5–10 s) | We may need to replace the sensing material after every run. Not suitable for continuous sensing. | [34] |
| Photoionization | Benzene Toluene Ethyl benzene m-Xylene Hexane | Two electrodes | 1.4 ppt 1.2 ppt 1.3 ppt 1.2 ppt 8.8 ppt | No selectivity | Excellent sensitivity. The ideal candidate is the detector in gas chromatography systems. | It cannot be used as a standalone gas sensor. | [13] |
| Bubble/Droplet-Based Techniques | |||||||
|---|---|---|---|---|---|---|---|
| Detection Technique | Target Analyte | Transducer Material | LOD/Lowest Concentration REPORTED | Selectivity | Merits | Limitations | Ref |
| Bubble-based | Carbon dioxide (CO2) Helium (He) Hydrogen gas (H2) Nitrogen gas (N2) Methane (CH4) | No transducer material. Detection based on the size of individual bubbles | Not reported | Can selectively detect 5 different gases (CO2, He, H2, N2, and CH4) | It can be used for continuous real-time detection. | Uses a conventional chromatographic column. | [35] |
| Fluorescence | Escherichia coli (E. coli) | Droplets containing propidium iodide (PI.) | Not reported | Can differentiate between non-bioaerosol and bioaerosol | Detection happens within 20 s. | Not portable due to the use of a conventional fluorescence microscope | [36] |
| Colorimetric | Ammonia | Nessler’s reagent | 500 ppm | Selectively detects ammonia | Suitable for continuous real-time detection | Poor sensitivity. | [37] |
| Sulfate Ammonium | Methylthymol blue (MTB) | 11 ppm 0.256 ppm | The reaction is selective. | Sampling, manipulation, and detection are integrated into a single chip. | Complex fabrication procedure. | [38] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaaliveetil, S.; Yang, J.; Alssaidy, S.; Li, Z.; Cheng, Y.-H.; Menon, N.H.; Chande, C.; Basuray, S. Microfluidic Gas Sensors: Detection Principle and Applications. Micromachines 2022, 13, 1716. https://doi.org/10.3390/mi13101716
Kaaliveetil S, Yang J, Alssaidy S, Li Z, Cheng Y-H, Menon NH, Chande C, Basuray S. Microfluidic Gas Sensors: Detection Principle and Applications. Micromachines. 2022; 13(10):1716. https://doi.org/10.3390/mi13101716
Chicago/Turabian StyleKaaliveetil, Sreerag, Juliana Yang, Saud Alssaidy, Zhenglong Li, Yu-Hsuan Cheng, Niranjan Haridas Menon, Charmi Chande, and Sagnik Basuray. 2022. "Microfluidic Gas Sensors: Detection Principle and Applications" Micromachines 13, no. 10: 1716. https://doi.org/10.3390/mi13101716





